Peer-review started: May 24, 2015
First decision: September 28, 2015
Revised: November 27, 2015
Accepted: January 5, 2016
Article in press: January 7, 2016
Published online: March 28, 2016
Processing time: 315 Days and 1.4 Hours
AIM: To investigate predictors of incident stroke in a large epidemiological sample of cognitively healthy individuals in their early 60’s.
METHODS: Cardiovascular (systolic and diastolic blood pressure, hypertension status and medication, body mass index, lung forced vital capacity), lifestyle (alcohol intake, smoking, physical activity), mental health (anxiety and depression status, medication and symptomatology), cognition (executive function, processing speed, working memory, sensorimotor skills), and personality measures (behavioural inhibition and activation, positive and negative affect, neuroticism, psychoticism, extraversion) were investigated as predictors of incident stroke in 1774 participants from the Personality and Total Health Through Life Project over an 8-year follow-up. Logistic regression analyses controlled for age, gender, and education were conducted in the whole cohort as well as in case-control sub-analyses including precisely matched controls to identify factors associated with stroke incidence.
RESULTS: The cohort selected had a mean age of 62.5 years (SD = 1.5) and was 48.6% female with an average of 14.1 years of education (SD = 2.6). When 28 individuals with incident stroke were compared to 1746 cognitively healthy individuals in multivariate logistic regression analyses the only significant predictors of stroke across the five domains considered (cardiovascular, lifestyle, mental health, cognition, personality) and after controlling for gender, age, and education were systolic blood pressure (per unit above 140 mmHg: OR = 1.04, 95%CI: 1.01-1.07, P = 0.002), smoking (trend OR = 2.28, 95%CI: 0.99-5.24, P = 0.052), and sensorimotor skills (purdue pegboard: OR = 0.80, 95%CI: 0.62-0.96, P = 0.037). Similarly, in matched-control analyses significant group differences were found for systolic blood pressure (P = 0.001), smoking (P = 0.036), and sensorimotor skills (P = 0.028).
CONCLUSION: Identified predictors of incident stroke in community-living individuals included high systolic blood pressure and smoking - but also, sensorimotor performance, a measure which has not yet been reported in the literature.
Core tip: An investigation of incident stroke predictors in community-living individuals in their 60’s revealed that systolic blood pressure, smoking, and impaired sensorimotor skills were most predictive of future stoke. Every 10 mmHg above 140 was associated with a 40% increased risk and smoking was associated with a more than two-fold increased risk. While both blood pressure and smoking are known risk factors for stroke, impaired sensorimotor skills is a promising novel bio-marker which needs further investigation and may be useful in identifying those at risk.
- Citation: Cherbuin N, Carey L, Mortby M, Anstey KJ. Predictors of future stroke in adults 60-64 years living in the community. World J Neurol 2016; 6(1): 14-22
- URL: https://www.wjgnet.com/2218-6212/full/v6/i1/14.htm
- DOI: https://dx.doi.org/10.5316/wjn.v6.i1.14
Large multi-country studies have found that certain risk factors are consistently associated with an increased risk of stroke[1,2]. These include a history of hypertension, coronary heart disease, atrial fibrillation, smoking, waist-to-hip ratio, sedentary lifestyle, excessive alcohol intake, diabetes, psychosocial stress and depression. In addition, a number of biomarkers related to inflammatory processes and blood coagulation have been associated with stroke[2]. Older individuals are at higher risk of stroke with more than two-third of strokes occurring above the age of 65 years. Men are somewhat more likely to experience a stroke than women. For example, Marrugat et al[3] reported incidence rates per 100000 people of 218 in men and 127 in women in Spain which is consistent with Australian estimates (157 for men and 123 for women)[4] and those of other western countries with a recent meta-analysis reporting an incidence rate 33% higher in men than women[5]. However, this effect does not appear to be present in the young or the very old[4].
Most risk factors have been determined retrospectively in individuals who have already suffered a stroke. However, the extent to which these risk factors may be present in prospective population based studies needs to be considered in the context of population attributable risks. Some prospective studies have been undertaken and confirm the influence of risk factors already identified retrospectively, such as cardiovascular disease, diabetes, depression[6], anxiety[7], smoking[8], and reduced physical activity[9]. The significance of some risk factors such as alcohol intake vs abstinence has not been confirmed although dose response analyses suggest intake of less than one drink per day to be associated with a lower mortality risk due to stroke[10]. In addition, a few new risk factors have been more clearly characterised by prospective investigations. For example, body mass index (BMI) was found to be associated with an increased risk of ischemic and to a lesser extent hemorrhagic stroke[11,12] while the protective effect of physical activity was more clearly quantified[9]. Of substantial interest, the association between cognitive function and incident stroke which cannot be reliably investigated retrospectively has been clarified in a recent meta-analysis of prospective studies which provided evidence of a dose-response protective effect of cognitive abilities and particularly in relation to attention, memory and language function[13].
Some important questions which remain unanswered include the extent to which the effect of the identified risk factors reviewed above can be detected in a specific population, whether they apply to a specific age-range, for example, particularly in the early 60’s when the greatest incidence of stroke occurs, and whether currently under-researched factors such as personality traits may contribute to stroke risk. The useful time-window in which risk factors operate also need to be further investigated. With these factors in mind the aim of the present study was to investigate risk factors for incident stroke in five domains, cardio-vascular, lifestyle, mental health, cognition and personality, in a large epidemiological sample of individuals in their early 60’s and living in the community.
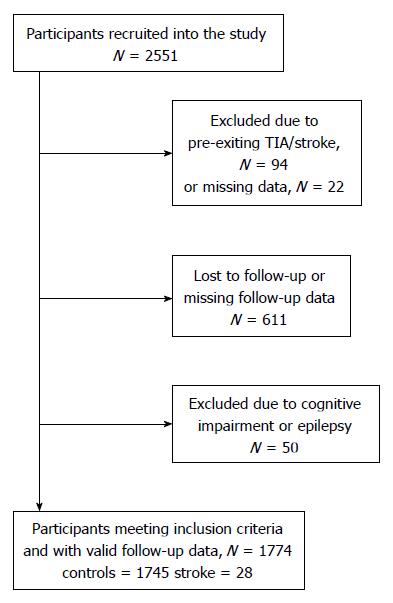
Participants taking part in the Personality and Total Health (PATH) Through Life Project, a large longitudinal study of ageing, were considered for inclusion in this investigation. The design of the PATH Project has been described elsewhere[14]. Briefly, we recruited participants who were residents of the city of Canberra and the adjacent town of Queanbeyan, Australia, randomly through the electoral roll to participate in a study interested in the risk and protective factors for common mental disorders, normal ageing, and dementia. Enrolment to vote is compulsory for Australian citizens. The Australian National University Ethics Committee approved the study and all participants gave written informed consent to be included in this study. While the PATH study surveys three age groups in a narrow-age cohort design of participants in their 20’s, 40’s, and 60’s, the present investigation focuses on the older age group only. Participants were aged 60-64 years at wave 1 (i.e., initial testing time, 2001-2002), 65-69 at wave 2 (2005-2006), and 69-72 at wave 3 (2009-2010). The baseline participation rate was 58.3% (2551 out of 4376 participants invited). From 2551 participants recruited into the study, 116 were excluded due to pre-existing TIA (n = 62) or stroke (n = 32) or missing TIA/stroke data (n = 22) at wave 1. Another 661 participants were excluded due to dropout or missing follow-up data (n = 611), epilepsy (n = 13), or cognitive impairment (MMSE < 26, n = 37) leaving 1774 participants for inclusion in analyses; including 28 participants who experienced an incident stroke (confirmed by a medical specialist) in the eight-year follow-up (Figure 1). Compared to 777 individuals who were not included, the selected participants did not differ in age or gender but had higher levels of education (14.08 vs 13.04 years; P < 0.001).
Stroke was assessed by self-report at each assessment point. Stroke status was considered valid only if it had been confirmed by a doctor. Those who reported “not being sure” or who reported a stroke which had not been verified by a doctor were excluded from the analyses. As at the first assessment, participants were asked about stroke and transient ischemic attack (TIA) in a single question “Have you ever suffered a stroke or a TIA?” Both those with a stroke or TIA at baseline were excluded from analyses to ensure only incident cases were included in analyses.
Demographic: Total years of education and race were assessed by self-report.
Cardio-vascular: Systolic/diastolic blood pressures (BP) (upper arm) were measured twice (standard/large cuff as appropriate) while seated after a rest of at least 5 min with an Omron M4 monitor by experienced interviewers. Participants were classified as hypertensive if their mean diastolic or systolic blood pressure measures were higher than 90 and 140 mmHg respectively or if they reported taking anti-hypertensive medication. Diabetes, height and weight were assessed by self-report. BMI was computed with the formula weight (kg)/height × height (m2) based on self-report of weight and height. Cardio-vascular fitness was assessed with forced vital capacity measured with a Spirometer (Micro Spirometer, Micro Medical Ltd., Rochester, Kent, United Kingdom) using the best of 3 attempts.
Lifestyle: Alcohol intake was assessed with the Alcohol Use Disorders Identification Test[15]. Smoking history was assessed by self-report and included questions on the timing of smoking initiation and cessation as well as the quantity of cigarettes typically smoked in a month which were used to classify participants into non-smokers or “ever” smokers (i.e., who had a current or past history of smoking) and to compute a measure of cigarettes smoked over the lifetime (pack/years). Physical activity (PA) was assessed by self-report. Hours of average weekly PA were reported for three intensity categories: (1) mild (e.g., walking, weeding); (2) moderate (e.g., dancing, cycling); and (3) vigorous (e.g., running, squash). To provide an intensity-sensitive continuous score of PA, the three activity levels were combined using a weighting system such that hours of mild PA were multiplied by 1, hours of moderate PA multiplied by 2, and hours of vigorous PA multiplied by 3[16].
Mental health: Depression and anxiety symptomatology were assessed with the Goldberg Depression and Anxiety Inventory[17]. Depression status was assessed with the brief patient health questionnaire based on responses to ten questions assessing depressive symptomatology. Major and minor depression status was computed with a validated coding algorithm which has been shown to have good psychometric characteristics against clinical diagnosis[18]. Anti-depressive and anxiety medication use was assessed by self-report.
Cognition/sensorimotor: General cognitive health was screened with the mini-mental state examination. Executive function and processing speed were assessed using the Symbol Digit Modalities Test[19]. Working memory was assessed using the Digits-Span Backwards Task[20]. Delayed recall of the first list of the California Verbal Learning Test[21] was used as a measure of episodic memory. The Spot-the-Word task[22] was used as a measure of verbal ability. Sensorimotor skill, involving manual dexterity and bimanual coordination, were assessed with the Purdue Pegboard both hands task which involves placing as many pegs as possible on a board with 2 columns of holes for the pegs in 30 s[23]. For all measures, higher scores should be interpreted as better performance.
Personality: Personality measures were selected based on their relationship to mechanisms involved in psychological stress, depression, and addictive traits. The Behavioural Inhibition Scale was used to assess behavioural inhibition. Negative affect was assessed with the Positive and Negative Affect Schedule[24]. Neuroticism, psychoticism, and extraversion were assessed with the short form of the Eysenck Personality Questionnaire - Revised[25].
In addition to analyses contrasting non-stroke to stroke participants in the whole sample further analyses were planned based on a case-control design. Each participant who met the inclusion criteria and was found to have suffered a stroke at follow-up was closely matched with five stroke-free participants meeting inclusion criteria. The matching procedure was conducted in SPSS and was based on sex (exact), race (exact), age (closest), height (closest), and education (closest) after sorting the dataset according to these fields. Participants were matched on height to avoid the inadvertent introduction of spurious associations due to body size.
Descriptive analyses were conducted using χ2 tests for categorical data and t-tests to compare groups on continuous variables. Logistic regression analyses were conducted to investigate stroke predictors in the whole sample while controlling for age, gender, and education. It is noteworthy that Cox regression analyses could not be used here because participants were followed-up at a fixed interval. Variables considered for each domain investigated (cardiovascular, lifestyle, mental health, cognition/sensorimotor and personality) were entered together in multivariate analyses. Diabetes, anxiety and depression medication were excluded from analyses due to small cell sizes. Significant predictors were further investigated in univariate analyses. ANOVAs were conducted to test for differences in the case-control analyses. Sex interactions were also tested. Significance is reported at α 0.05 and 0.01 levels.
One important question to consider when assessing the results of an investigation of this kind is whether analyses were sufficiently powered to detect meaningful differences. Despite the complexity of accurately assessing statistical power in logistic regression, it is expected that given the population sample size and the relatively small number of stroke cases, these analyses are likely to be under-powered to detect all but large differences. However, a priori estimates computed with G*Power 3.1 showed that the case-control analyses had a power of 0.8 to detect effects of medium size at alpha 0.05 two-tailed and of large size at alpha 0.01.
After exclusions, 1774 participants were available for analysis including 28 participants with incident stroke, 18 of whom experienced a stroke between wave 1 and wave 2 and 10 between wave 2 and wave 3. This is equivalent to an annual incidence rate of 197 (161 for women and 230 for men) per 100000 individuals. Table 1 presents the demographic and health characteristics for participants who remained free of stroke over the 8-year follow-up and for participants who suffered a stroke. Significant differences (unadjusted) between groups were present for diastolic and systolic blood pressure, hypertension, and smoking. Importantly, no difference was detected on demographic variables including sex, age, gender, race or education.
| Comparison groups | ||||
| Controls (n = 1746) | Stroke (n = 28) | |||
| Mean/count | SD (%) | Mean/count | SD (%) | |
| Age | 62.52 | 1.51 | 62.50 | 1.58 |
| Female | 851 | 48.70 | 11 | 39.30 |
| Caucasian | 1686 | 96.60 | 28 | 100.00 |
| MMSE | 29.38 | 0.85 | 29.25 | 0.80 |
| Education | 14.08 | 2.62 | 13.77 | 2.90 |
| Hypertension | 1095 | 62.70 | 241 | 85.70 |
| Diastolic BP | 82.85 | 10.5 | 88.041 | 10.81 |
| Systolic BP | 139.14 | 18.58 | 155.251 | 24.46 |
| BP medication | 525 | 30.10 | 14 | 50.00 |
| FVC | 3.29 | 0.79 | 3.22 | 0.79 |
| BMI | 26.82 | 4.99 | 27.37 | 6.12 |
| Diabetes | 115 | 6.60 | 1 | 3.60 |
| Smoker (ever) | 786 | 45.00 | 191 | 68.00 |
| Alcohol | 6.96 | 8.19 | 9.46 | 13 |
| Physical activity | 13.98 | 12.92 | 11.97 | 10.76 |
| Anxiety symptoms | 2.12 | 2.23 | 2.71 | 2.57 |
| Depress symptoms | 1.62 | 1.80 | 1.75 | 2.12 |
| Depression status | 13 | 6.20 | 1 | 3.60 |
| Anxiety medication | 10 | 4.80 | 1 | 3.60 |
| Depression medication | 7 | 3.40 | 1 | 3.60 |
| SDMT | 50.85 | 8.78 | 49.21 | 9.65 |
| Delayed recall | 6.28 | 2.41 | 6.07 | 2.29 |
| Digit backward | 4.86 | 2.17 | 5.14 | 2.32 |
| Spot-the-word | 51.74 | 5.36 | 53.64 | 5.42 |
| Purdue pegboard | 10.60 | 1.57 | 9.752 | 1.29 |
| BIS | 20.18 | 3.14 | 20.32 | 2.74 |
| Negative affect | 13.64 | 4.64 | 13.75 | 4.15 |
| Neuroticism | 3.22 | 2.91 | 3.96 | 4.16 |
| Extraversion | 6.90 | 3.42 | 6.82 | 4.39 |
| Psychoticism | 1.71 | 1.32 | 1.82 | 1.59 |
Table 2 presents the results of the multivariate logistic regression analyses investigating the predictors of incident stroke in the whole sample. Across all domains investigated only higher systolic BP and poorer sensorimotor skills were associated with higher risk of stroke, with smoking and neuroticism identified as trends. The following associations were significant in univariate analyses, and after controlling for age, sex and education: Systolic BP (P < 0.001), smoking (P = 0.034) and sensorimotor skills (P = 0.027). Only systolic BP remained a significant predictor after correcting for multiple comparisons (Bonferroni). None of the sex interactions reached significance.
| Predictors | B | SE | Wald | df | Sig | Exp(B) | 95%CI for EXP(B) | |
| Lower | Upper | |||||||
| Covariates | ||||||||
| Sex | -0.431 | 0.394 | 1.195 | 1 | 0.274 | 0.650 | 0.300 | 1.407 |
| Age | -0.002 | 0.127 | 0.000 | 1 | 0.985 | 0.998 | 0.778 | 1.279 |
| Education | -0.056 | 0.071 | 0.628 | 1 | 0.428 | 0.945 | 0.823 | 1.086 |
| Cardio-vascular | ||||||||
| Hypertension | 0.321 | 0.639 | 0.252 | 1 | 0.616 | 1.378 | 0.394 | 4.824 |
| Systolic BP | 0.041 | 0.013 | 9.820 | 1 | 0.002 | 1.042 | 1.015 | 1.069 |
| Diastolic BP | -0.018 | 0.024 | 0.551 | 1 | 0.458 | 0.982 | 0.937 | 1.030 |
| FVC | -0.157 | 0.313 | 0.251 | 1 | 0.616 | 0.855 | 0.463 | 1.578 |
| BMI | -0.001 | 0.039 | 0.001 | 1 | 0.975 | 0.999 | 0.926 | 1.077 |
| Lifestyle | ||||||||
| Alcohol | 0.016 | 0.019 | 0.759 | 1 | 0.384 | 1.017 | 0.980 | 1.055 |
| Ever smoker | 0.824 | 0.424 | 3.773 | 1 | 0.052 | 2.280 | 0.993 | 5.235 |
| Physical activity | -0.015 | 0.016 | 0.865 | 1 | 0.352 | 0.985 | 0.954 | 1.017 |
| Mental health | ||||||||
| Anxiety symptoms | 0.153 | 0.104 | 2.147 | 1 | 0.143 | 1.165 | 0.950 | 1.429 |
| Depression symptoms | -0.012 | 0.142 | 0.007 | 1 | 0.932 | 0.988 | 0.748 | 1.305 |
| Depression status | -0.530 | 1.092 | 0.235 | 1 | 0.628 | 0.589 | 0.069 | 5.008 |
| Cognition/sensorimotor | ||||||||
| SDMT | -0.020 | 0.024 | 0.715 | 1 | 0.398 | 0.980 | 0.934 | 1.027 |
| Delayed recall | -0.039 | 0.089 | 0.186 | 1 | 0.666 | 0.962 | 0.808 | 1.146 |
| Digit backward | 0.024 | 0.095 | 0.066 | 1 | 0.797 | 1.025 | 0.851 | 1.233 |
| Spot-the-word | 0.083 | 0.047 | 3.122 | 1 | 0.077 | 1.087 | 0.991 | 1.192 |
| Purdue pegboard | -0.250 | 0.120 | 4.349 | 1 | 0.037 | 0.779 | 0.616 | 0.985 |
| Personality | ||||||||
| BIS | -0.017 | 0.077 | 0.052 | 1 | 0.820 | 0.983 | 0.845 | 1.143 |
| Negative affect | -0.038 | 0.051 | 0.551 | 1 | 0.458 | 0.963 | 0.870 | 1.065 |
| Neuroticism | 0.151 | 0.082 | 3.419 | 1 | 0.064 | 1.163 | 0.991 | 1.364 |
| Extraversion | 0.039 | 0.057 | 0.468 | 1 | 0.494 | 1.040 | 0.929 | 1.164 |
| Psychoticism | 0.082 | 0.143 | 0.330 | 1 | 0.566 | 1.085 | 0.821 | 1.436 |
The effectiveness of the match procedure was confirmed by analyses which showed that, in addition to the exact match on sex and race, participants with incident stroke did not differ from controls on age, education, height or MMSE. Moreover, group analyses showed that participants who experienced a stroke had a higher systolic blood pressure, were more likely to smoke, and performed less well on the sensorimotor task (Table 3).
| Predictors | Matched controls | Stroke | Fχ | P | ||
| Mean/count | SD (%) | Mean/count | S (%) | |||
| Socio-demographic | ||||||
| Age | 62.48 | 1.48 | 62.5 | 1.58 | 0.005 | 0.945 |
| Education | 13.78 | 2.54 | 13.77 | 2.90 | 0.000 | 0.984 |
| Cardio-vascular | ||||||
| Hypertension | 105 | 75 | 24 | 85.7 | 1.498 | 0.223 |
| Systolic BP | 141.23 | 19.41 | 155.25 | 24.46 | 11.103 | 0.001 |
| Diastolic BP | 84.02 | 12.06 | 87.95 | 10.62 | 2.574 | 0.111 |
| FVC | 3.37 | 0.80 | 3.22 | 0.79 | 0.743 | 0.390 |
| BMI | 26.73 | 4.98 | 27.37 | 6.12 | 0.352 | 0.554 |
| Lifestyle | ||||||
| Alcohol | 7.66 | 8.79 | 9.46 | 13.00 | 0.815 | 0.368 |
| Ever smoker | 66 | 47.1 | 19 | 67.9 | 4.054 | 0.046 |
| Physical activity | 14.82 | 14.07 | 11.97 | 10.76 | 1.026 | 0.313 |
| Mental health | ||||||
| Anxiety symptoms | 2.05 | 2.24 | 2.71 | 2.57 | 1.959 | 0.163 |
| Depression symptoms | 1.49 | 1.77 | 1.75 | 2.12 | 0.460 | 0.498 |
| Depression status | 4 | 2.9 | 1 | 3.6 | 0.041 | 0.840 |
| Cognition/sensorimotor | ||||||
| SDMT | 51.29 | 7.78 | 49.21 | 9.65 | 1.520 | 0.219 |
| Delayed recall | 6.21 | 2.28 | 6.07 | 2.29 | 0.091 | 0.763 |
| Digit backward | 4.86 | 2.07 | 5.14 | 2.32 | 0.405 | 0.525 |
| Spot-the-word | 51.94 | 5.09 | 53.64 | 5.42 | 2.546 | 0.112 |
| Purdue pegboard | 10.42 | 1.52 | 9.75 | 1.29 | 4.722 | 0.031 |
| Personality | ||||||
| BIS | 20.04 | 3.27 | 20.32 | 2.74 | 0.187 | 0.666 |
| Negative affect | 1.70 | 1.35 | 1.82 | 1.59 | 0.178 | 0.674 |
| Neuroticism | 3.02 | 2.81 | 3.96 | 4.16 | 2.205 | 0.139 |
| Extraversion | 13.56 | 4.81 | 13.75 | 4.15 | 0.036 | 0.849 |
| Psychoticism | 6.72 | 3.32 | 6.82 | 4.39 | 0.021 | 0.886 |
To further clarify associations between hypertension and stroke, we estimated the risk of stroke for those who had high systolic blood pressure and were or were not on anti-hypertensive treatment. A more than three-fold increased risk was associated with having systolic hypertension while being on anti-hypertensive treatment in the whole sample (OR = 3.36, 95%CI: 1.57-7.19, P = 0.002) and in the case-control sub-sample (OR = 2.88, 95%CI: 1.27-6.54, P = 0.012). In contrast, no increased risk was associated with being hypertensive and on medication but with a normal systolic BP in the whole sample (OR = 0.42, 95%CI: 0.10-1.87, P = 0.257) and in the case-control sub-sample (OR = 0.37, 95%CI: 0.08-1.67, P = 0.197). No participant without anti-hypertensive medication had a systolic BP greater than 140 mmHg.
To shed further light on the associations between smoking and stroke, additional analyses assessing lifetime tobacco use and current smoking were conducted. In the whole sample every additional pack of cigarettes smoked in a year (pack/year) was associated with a 1% increased risk of stroke (OR = 1.01, 95%CI: 1.01-1.02, P = 0.007). In analyses restricted to “ever” smokers (i.e., those reporting being either current smokers or having smoked in the past) only a trend suggested that every pack/year was associated with increased risk (OR = 1.01, 95%CI: 0.99-1.02, P = 0.118). Being a current smoker (n = 152) did not present significant additional risk (OR = 1.21, 95%CI: 0.36-4.07, P = 0.762). The interaction between smoking and systolic blood pressure was also tested but did not reach significance.
Four main findings emerged from this study. First, high systolic BP and smoking, two of the strongest and most consistent stroke risk factors identified in the literature were also associated with increased risk in this prospective investigation. Second, a number of established risk factors including obesity, low physical activity, alcohol and depression were not found to be associated with an increased risk of future stroke. Third, while most of the cognitive measures investigated were not significant predictors of stroke, poorer dexterous bimanual hand function was associated with an increased risk of stroke. Finally, personality traits were not associated with an increased risk of stroke in this a large epidemiological sample of individuals in their early 60’s.
The fact that systolic BP was associated with an increased risk of stroke is not surprising but it is interesting that no such effect was detected for diastolic BP since some previous studies have found that higher diastolic BP is also associated with an increased risk. However, our findings are consistent with those of a large (n = 19698) population-based study which found that any risk associated with diastolic BP disappeared once controlling for systolic BP[26]. Of greater interest is the finding that every 1 mmHg increase in systolic BP over 140 is associated with a 4% increased risk of stroke in the following 8 years. Thus, an individual with a systolic reading greater than 165 mmHg (10% of this cohort) in this age group has more than a two-fold increased risk of stroke than participants with an average BP.
The demonstrated association between smoking and stroke risk is also well-documented and uncontroversial. In this sample smoking was associated with approximately a two-fold increased risk of stroke. This is higher than estimates from a recent meta-analysis which covered a broader age-range (20-107 years) and may reflect the narrow age-range of the present sample and the follow-up period which spans a period of life which is associated with the highest levels of stroke incidence. What is particularly notable is that the risk associated with smoking is present in this population despite a generally high level of education (mean 14 years) and further supports the view that the increased risk is due to intrinsic qualities of tobacco smoking and not to correlates of low education such as poorer health or sub-optimal lifestyle behaviours.
The novel and possibly most significant finding of this study is the demonstration of an association between poorer sensorimotor skill and increased risk of stroke. Despite extensive search, we are not aware of any previous study reporting such a finding. The pegboard task is timed, visually guided and involves dexterous coordinated movements of both hands. Performance on this task is therefore likely mediated by circuits involving the basal ganglia[27] and lesions in these brain regions would be expected to be associated with deteriorating performance. Consistent with this hypothesis are findings from previous research indicating that lacunar and non-lacunar strokes occur in 7% to 18% of cases in the basal ganglia[28]. Moreover, microbleeds in the basal ganglia and other deep brain regions have been found to be specifically associated with hypertensive arteriopathy and more generally greater prevalence of microbleeds is associated with poorer motor performance[29]. Taken together this evidence suggests that cerebro-vascular disease and its risk factors, particularly hypertension, may lead in some instances to the development of striatal microbleeds thus impairing fine motor control. Consequently, a possible interpretation of our findings is that impaired sensorimotor performance is indicative of the presence or development of silent lacunar strokes which predict and foreshadow the future occurrence of a major stroke.
This finding emphasises the benefits of prospective observational research and acknowledges the limitations of clinical and retrospective investigations which cannot test such associations. Our finding is particularly interesting because it may provide a link between research showing that higher blood pressure is associated with poorer sensorimotor skills[30,31] and investigations showing that silent strokes - which have high prevalence in the hypertensive population (approximately 10%) and are a known risk factor for future stroke - occur particularly in the striatum[32]. They also suggest that dexterous sensorimotor hand function may be a particularly sensitive biomarker for the progression of cerebral ischemic disease and stroke.
Finally, in this cohort personality measures were not associated with stroke risk. A trend suggesting that stronger neurotic traits may be associated with a somewhat increased risk of stroke was present; however, further investigation of this question is needed to determine whether this is reflective of a robust effect. Some converging evidence showing an association between hypertension and neuroticism exists[33] but a link between neuroticism and BP has not been supported by other investigations[34,35]. Consequently, these results should be treated with caution.
This study had a number of limitations. While participants were randomly selected from the population, this was done in a narrow age range and therefore this cohort may not be fully representative of the older population. The large sample size and 8-year follow-up may in part mitigate this issue. Conversely, the narrow age range may be considered a strength of the study as it provides a window into the factors that may specifically predict stroke in healthy individuals at 60-64 years of age; just prior to the age when the incidence of stroke increases[4]. The incidence of 197 per 100000 individuals in this study is consistent with that projected for stroke in previous reports[4]. Participants were generally well-educated and in good-health which may have led to an under-estimation of the effects of certain risk factors. On the other hand this is also a strength as it provides evidence that hypertension and smoking, even in a relatively affluent population where risk associated with low education is minimized, remain the major and salient risk factors for incident stroke. It should be noted, however, that a number of emerging vascular risk factors such as sleep-related breathing disorders, drug abuse, chronic inflammation and others were not available for investigation in this dataset and should be the focus of future research in this field[36]. Finally, the number of participants lost to follow-up was relatively high and this may have somewhat biased the reported estimates. Furthermore, the number of participants developing a stroke over the follow-up period was relatively small which may have limited our capacity to detect risk factors. Nevertheless, the combination of analyses and the concordance of results in the whole cohort and in the case-control sub-sample - which was effective in achieving very close matches - suggest that the detected effects are real and reliable.
In conclusion, sustained efforts should be made to keep healthy blood pressure levels in the population and across the lifespan and to decrease smoking rates. Focused investigations of the links between sensorimotor function, blood pressure and stroke should also be conducted to establish whether assessment of this functional domain may be useful as a biomarker of stroke risk.
The authors are grateful to Patricia Jacomb, Karen Maxwell, Tony Jorm, Helen Christensen, Bryan Rodgers, Simon Easteal, Peter Butterworth, and the PATH interviewers.
Stroke is a major population health concern and while a number of risk factors for stroke have been identified this has been done mostly retrospectively. In order to better assess risk at the population level there is a need to determine the extent to which the presence of specific risk factors across multiple domains is predictive of incident stroke in community-living individuals.
Given the availability of strong evidence linking cardio-vascular risk factors and stroke, current research hotspots include the estimation of risk associated with single and combination of risk factors across a broader range of domains including cognitive, lifestyle, personality and mental health with the view of early identification of those at risk at the population level and at specific ages.
This study used a large representative sample of individuals in their 60’s living in the community to assess a broad range of risk factors for incident stroke over an 8-year follow-up. While the confirmation that some established factors including blood pressure and smoking were associated with an increased risk of incident stroke was unsurprising the produced estimates characterising the risk associated with unitary increases in blood pressure contribute valuable new information that can be used to better model population risk. Moreover, the finding that poorer fine motor skill performance is a significant predictor of incident stroke provides a novel target to identifying those at risk.
In addition to established stroke risk factors, fine motor skill performance should be considered as a new marker to detect those at risk against population norms.
Sensorimotor skills refer to the fine motor control of hands and fingers. They involve precise movements and the coordination of actions through perceptual and visual feedback. In the present study sensorimotor skills were assessed with the Purdue Pegboard task using two hands. This task requires placing small metal pins in holes on a board as quickly as possible for a specified length of time.
The authors present a well conducted and scientifically interesting study on predictors of future acute stroke.
P- Reviewer: Arboix A, Juan DS S- Editor: Gong XM L- Editor: A E- Editor: Jiao XK
| 1. | O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2026] [Cited by in RCA: 2179] [Article Influence: 145.3] [Reference Citation Analysis (0)] |
| 2. | Allen CL, Bayraktutan U. Risk factors for ischaemic stroke. Int J Stroke. 2008;3:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Marrugat J, Arboix A, García-Eroles L, Salas T, Vila J, Castell C, Tresserras R, Elosua R. [The estimated incidence and case fatality rate of ischemic and hemorrhagic cerebrovascular disease in 2002 in Catalonia]. Rev Esp Cardiol. 2007;60:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Australian Institute of Health and Welfare. Stroke and its management in Australia: An update. Canberra: Australian Institute of Health and Welfare, 2013. . |
| 5. | Appelros P, Stegmayr B, Terént A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 694] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 6. | Brunner EJ, Shipley MJ, Britton AR, Stansfeld SA, Heuschmann PU, Rudd AG, Wolfe CD, Singh-Manoux A, Kivimaki M. Depressive disorder, coronary heart disease, and stroke: dose-response and reverse causation effects in the Whitehall II cohort study. Eur J Prev Cardiol. 2014;21:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Lambiase MJ, Kubzansky LD, Thurston RC. Prospective study of anxiety and incident stroke. Stroke. 2014;45:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Peters SA, Huxley RR, Woodward M. Smoking as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 81 cohorts, including 3,980,359 individuals and 42,401 strokes. Stroke. 2013;44:2821-2828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Reimers CD, Knapp G, Reimers AK. Exercise as stroke prophylaxis. Dtsch Arztebl Int. 2009;106:715-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1216] [Cited by in RCA: 1104] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 11. | Rexrode KM, Hennekens CH, Willett WC, Colditz GA, Stampfer MJ, Rich-Edwards JW, Speizer FE, Manson JE. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA. 1997;277:1539-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 322] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Strazzullo P, D’Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke. 2010;41:e418-e426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 359] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 13. | Rostamian S, Mahinrad S, Stijnen T, Sabayan B, de Craen AJ. Cognitive impairment and risk of stroke: a systematic review and meta-analysis of prospective cohort studies. Stroke. 2014;45:1342-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Anstey KJ, Christensen H, Butterworth P, Easteal S, Mackinnon A, Jacomb T, Maxwell K, Rodgers B, Windsor T, Cherbuin N. Cohort profile: the PATH through life project. Int J Epidemiol. 2012;41:951-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7971] [Cited by in RCA: 8960] [Article Influence: 280.0] [Reference Citation Analysis (0)] |
| 16. | Lamont AJ, Mortby ME, Anstey KJ, Sachdev PS, Cherbuin N. Using sulcal and gyral measures of brain structure to investigate benefits of an active lifestyle. Neuroimage. 2014;91:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Goldberg D, Bridges K, Duncan-Jones P, Grayson D. Detecting anxiety and depression in general medical settings. BMJ. 1988;297:897-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 783] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 18. | Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737-1744. [PubMed] |
| 19. | Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary. 3rd ed. New York: Oxford University Press, 2006. . |
| 20. | Pisoni DB, Kronenberger WG, Roman AS, Geers AE. Measures of digit span and verbal rehearsal speed in deaf children after more than 10 years of cochlear implantation. Ear Hear. 2011;32:60S-74S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 21. | Rannikko I, Paavola L, Haapea M, Huhtaniska S, Miettunen J, Veijola J, Murray GK, Barnes A, Wahlberg KE, Isohanni M. Verbal learning and memory and their associations with brain morphology and illness course in schizophrenia spectrum psychoses. J Clin Exp Neuropsychol. 2012;34:698-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Mackinnon A, Christensen H. An investigation of the measurement properties of the Spot-the-Word test in a community sample. Psychol Assess. 2007;19:459-468. [PubMed] |
| 23. | Tiffin J, Asher EJ. The Purdue pegboard; norms and studies of reliability and validity. J Appl Psychol. 1948;32:234-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 808] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 24. | Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21058] [Cited by in RCA: 18839] [Article Influence: 509.2] [Reference Citation Analysis (0)] |
| 25. | Eysenck SBG, Eysenck HJ, Barnett P. A revised version of the psychoticism scale. Pers Indiv Differ. 1985;6:21-29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1910] [Cited by in RCA: 1608] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 26. | Lindenstrøm E, Boysen G, Nyboe J. Influence of systolic and diastolic blood pressure on stroke risk: a prospective observational study. Am J Epidemiol. 1995;142:1279-1290. [PubMed] |
| 27. | Martino D, Leckman JF. Tourette syndrome. New York: Oxford University Press, 2013. . |
| 28. | Arboix A, Bell Y, García-Eroles L, Massons J, Comes E, Balcells M, Targa C. Clinical study of 35 patients with dysarthria-clumsy hand syndrome. J Neurol Neurosurg Psychiatry. 2004;75:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Charidimou A, Werring DJ. Cerebral microbleeds and cognition in cerebrovascular disease: an update. J Neurol Sci. 2012;322:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Telles S, Yadav A, Kumar N, Sharma S, Visweshwaraiah NK, Balkrishna A. Blood pressure and Purdue pegboard scores in individuals with hypertension after alternate nostril breathing, breath awareness, and no intervention. Med Sci Monit. 2013;19:61-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Jarmuzewska EA, Ghidoni A, Mangoni AA. Hypertension and sensorimotor peripheral neuropathy in type 2 diabetes. Eur Neurol. 2007;57:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Delgado P, Riba-Llena I, Tovar JL, Jarca CI, Mundet X, López-Rueda A, Orfila F, Llussà J, Manresa JM, Alvarez-Sabín J. Prevalence and associated factors of silent brain infarcts in a Mediterranean cohort of hypertensives. Hypertension. 2014;64:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Coelho R, Hughes AM, da Fonseca AF, Bond MR. Essential hypertension: the relationship of psychological factors to the severity of hypertension. J Psychosom Res. 1989;33:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Hozawa A, Ohkubo T, Tsuji I, Kikuya M, Matsubara M, Suzuki T, Nagai K, Kitaoka H, Arai Y, Hosokawa T. Relationship between personality and self-measured blood pressure value at home: the Ohasama study. Clin Exp Hypertens. 2002;24:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Santonastaso P, Canton G, Ambrosio GB, Zamboni S. Hypertension and neuroticism. Psychother Psychosom. 1984;41:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Arboix A. Cardiovascular risk factors for acute stroke: Risk profiles in the different subtypes of ischemic stroke. World J Clin Cases. 2015;3:418-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |