Peer-review started: October 2, 2014
First decision: December 12, 2014
Revised: July 7, 2015
Accepted: July 21, 2015
Article in press: July 23, 2015
Published online: September 28, 2015
Processing time: 366 Days and 5.2 Hours
Glioblastoma multiforme (GBM), the literal apogee on the hierarchy of malignant brain tumors, remains one of the greatest therapeutic challenges in oncology and medicine. Historically this may be contextualized in the fact that the medical and scientific communities have had a very elementary understanding of its intricate and complex pathophysiology. The last 10-15 years have yielded a number of studies that have elucidated much of the molecular and genetic complexities of GBM that underlie its pathogenesis. Excitingly, some of these discovered genetic mutations and molecular profiles in GBM have demonstrated value in prognostication and utility in predicting response to treatment. Despite this, however, treatment options for patients have remained somewhat limited. These treatment options are expected to expand with the availability of new data and with the transition of novel treatment modalities from animal to human studies. This paper will have a threefold objective: provide an overview of the traditional paradigm in understanding and treating GBM, describe recent discoveries in the molecular pathogenesis of GBM against this historical backdrop, and acquaint the reader with new treatment modalities that hold significant therapeutic potential for patients.
Core tip: This paper provides the reader with an overview some of the primary molecular markers that are implicated in the pathogenesis glioblastoma multiforme (GBM). It provides a robust review of the evidence that supports the use of these molecular markers for both prognostication and prediction for response to treatment. It gives the reader context for understanding the hypoxia model and how it informs treatment resistance in GBM. It provides an overview of cancer stem cells and their role in GBM biology. And it acquaints the reader with a few of the new, promising treatment modalities that are emerging.
- Citation: Joshi SK, Lucic N, Zuniga R. Molecular pathogenesis of glioblastoma multiforme: Nuances, obstacles, and implications for treatment. World J Neurol 2015; 5(3): 88-101
- URL: https://www.wjgnet.com/2218-6212/full/v5/i3/88.htm
- DOI: https://dx.doi.org/10.5316/wjn.v5.i3.88
Glioblastoma multiforme (GBM) belongs to a class of brain tumors known as gliomas, so named because they arise from glial cells (astrocytes, oligodendrocytes, ependymal and schwann cells). Glial cells have traditionally been understood as the workhorse cells of the central nervous system (CNS), providing the needful nutrients, oxygen and stromal support for neural cells. Recent studies have shown glial cells to have a more central and independent role in the CNS than historically thought, acting alongside neural cells in neurotransmission[1].
Since 1979, gliomas have been classified by the World Health Organization into 4 classes based upon histopathology, each successive class exemplifying features more consistent with malignancy[2] (Table 1). Indeed, of the 4 classes, only grades III and IV are considered malignant gliomas due to possessing telltale histological features (increased cellularity, abnormally increased mitotic activity, nuclear atypia). On this hierarchy, GBM is classified as grade IV due to the typifying unique characteristics of ubiquitous neovascularization and dramatic necrosis of neoplastic tissue (due to the extent of cell turnover).
| Localized astrocytoma |
| WHO grade I |
| Pilocytic astrocytoma |
| Pleomorphic xanthoastrocytoma |
| Subependymal giant cell astrocytoma |
| Diffuse astrocytomas/oligodendrogliomas |
| WHO grade II (Astrocytoma) |
| Fibrillary |
| Protoplasmic |
| Gemistocytic |
| WHO grade II (Oligodendroglioma) |
| WHO grade III (Anaplastic astrocytoma) |
| WHO grade III (Anaplastic oligodendroglioma) |
| WHO grade IV (Glioblastoma multiforme) |
| Giant cell glioblastoma |
| Gliosarcoma |
GBM is the most common malignant brain tumor, and histologically is second in incidence only to meningiomas when considering all intracranial neoplasms, both malignant and benign. Based upon data compiled by the Central Brain Tumor Registry of the United States Statistical Report, GBM makes up 15.6% of all brain tumors and 45.2% of primary malignant brain tumors[3]. The incidence of GBM increases with age; highest rates are observed in 75-84 years old and, conversely, comprise only about 3% of brain and CNS tumors in 0-19 year olds. For reasons unclear, GBM is slightly more common in males.
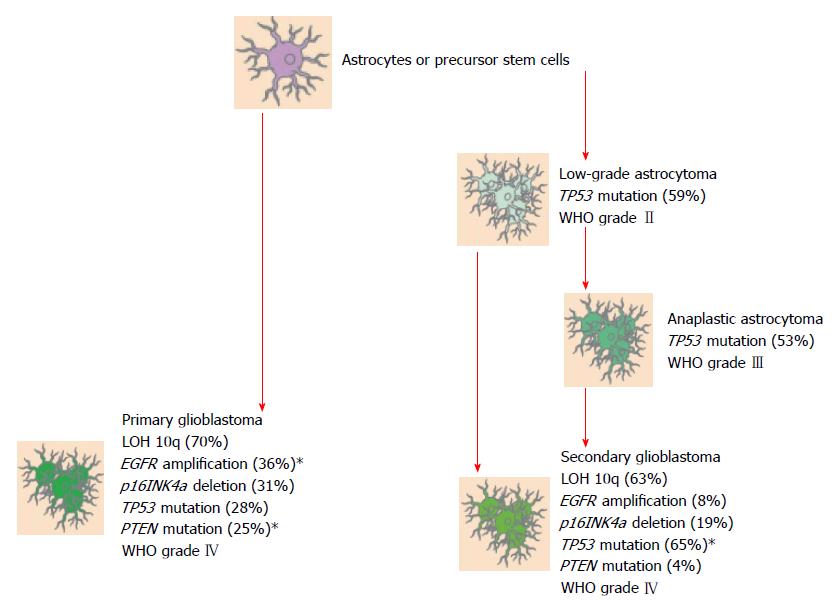
GBM may be classified as either primary or secondary. As connoted by the names, primary GBM comes from native, wild-type glial tissue whereas secondary GBM comes about through malignant changes in lower grade gliomas (Grades I and II). When a case of GBM is diagnosed, determining whether it is primary or secondary is germane to the clinician for it allows him/her to make initial informed impressions on the biological and clinical behavior of the tumor, provides utility in prognostication and, increasingly, is guiding clinicians in predicting responses to molecular/targeted therapies. Before proceeding to characterize key genotypic differences between primary and secondary GBM, it is of interest to briefly delineate defining epidemiological and clinical features of these respective categories. Upwards of 90% of GBM cases are primary. These tumors are afflictions of the elderly, the mean age at diagnosis being 62. And they carry with them a uniformly poor prognosis at the present time, with roughly two-thirds of patients dying less than 3 mo from the time of diagnosis[4].
Secondary GBMs, by contrast, are predominantly cancers of a younger population, the mean age at diagnosis being 45. This tumor is characterized by a more indolent time course than Primary GBM, progressing from lower grade gliomas over the course of years as opposed to months[4]. Indeed, a population-based study from 2005 reported a mean time of 5.3 years to be the amount of time it took for low-grade astrocytoma to develop into GBM. In the case of anaplastic astrocytoma the mean time reported was 1.4 years[5]. Secondary GBMs represent a small minority of cases, accounting for less than 10% of total GBMs.
Primary GBMs have trademark molecular abnormalities that distinguish them from secondary GBMs, and it is these unique genetic aberrations that give each class the distinct characteristics discussed above (Figure 1). These are: mutations in the gene encoding the epidermal growth factor receptor (EGFR) protein that result in its amplification, loss of heterozygosity (LOH) of Chromosome 10q, phosphatase and tensin homolog (PTEN) deletion on Chromosome 10, and p16 deletion. Conversely, in Secondary GBMs, mutations of the ubiquitous p53 oncogene and of the gene encoding the platelet-derived growth factor receptor (PDGFR) protein are culpable for malignant transformation of lower grade gliomas[6]. A few of these molecular anomalies will be treated in detail in the paragraphs to follow (Tables 2 and 3).
| Primary GBM | Secondary GBM | |
| Mean age at diagnosis | Approximately 62 yr of age | Approximately 45 yr of age |
| Percentage of cases | > 90% | < 10% |
| Clinical course | Rapid | Smoldering |
| Origin | De novo | Grade II/III astrocytomas |
| Primary GBM | Secondary GBM |
| EGFR overexpression/amplification | PDGFR overexpression |
| Loss of heterozygosity of Ch. 10q | Loss of heterozygosity of Ch. 10q |
| PTEN deletion on Ch. 10 | p53 mutations |
| p16 deletion | p16/Rb pathway aberrancies |
Many of the gene products inextricably involved in the development of GBM are growth factor signal transduction proteins that transduce an extracellular signal via ligand binding into a cellular response. The cellular response regulated by these proteins is proliferation and growth. A very carefully orchestrated combination of positive and negative regulatory ligands in the extracellular milieu ensures that in the normal homeostatic state, growth and proliferation of glial cells is kept in check. A common recurring theme in malignant transformation is mutations that cause amplification or overexpression of the signal transduction protein products.
One of the best characterized signal transduction proteins that brings about malignancy in more than 40% of cases of primary GBMs is EGFR[4]. Among tyrosine receptor kinases, EGFR belongs to the ErbB receptor family, bearing significant genetic homology to three others-HER2/c-neu (ErbB-2), Her3 (ErbB-3) and Her4 (ErbB-4). The wild type function of EGFR is contingent upon binding a specific extracellular ligand at its extracellular domain, ensuring that it remains coordinated with physiological needs. On binding the ligand, inactive monomers of EGFR dimerize to an active form and provoke autophosphorylateion of the intracellular, C-terminal domain at multiple tyrosine residues. Certain intracellular signaling proteins bind EGFR and concomitantly activate signal transduction cascades. The end result is increased expression of genes that are involved in a pro-growth phenotype.
When mutated in GBM as well as other malignancies, the EGFR gene is typically amplified, in which case the protein is autophosphorylated constitutively and is thereby overactive.
However, in GBM there exists a unique mutation that generates a mutant protein Epidermal growth factor receptor variant III (EGFRvIII) which is overexpressed. The mutation in the gene - a deletion of exons 2-7 -causes a deletion in the extracellular domain of the EGFRvIII protein that makes it inaccessible to extracellular regulatory ligands. This in turn leaves the protein in a constitutively active state that begets a slew of malignancy-specific features: cellular proliferation, the ability to invade other tissues, angiogenesis, and abnegation of the normal process of apoptosis. An interesting feature from the treatment perspective is that the deletion that yields the EGFRvIII protein encodes a codon that is not found in wild-type DNA and is unique to GBM[7]. Thus, conceivably, this sequence can be pursued as a specific molecular target in next generation treatment. In fact, studies are underway seeking to target EGFRvIII as a target. The phase III ACT IV trial underway is investigating the cancer vaccine rindopepimut for this very purpose[8].
Whereupon magnetic resonance imaging all but cinches the diagnosis, the gold standard for confirmation remains tissue biopsy. Though this may be accomplished by stereotactic brain biopsy alone, tissue is more commonly procured with maximally safe surgical resection. As it stands, maximally safe surgical resection is by no means curative as by the time of diagnosis, the tumor has invariably insinuated itself deep into vital, surgically inaccessible tissue. This said, rote surgical resection does still provide the patient with relief from symptoms wrought by mass effect of the tumor. There is also demonstrable improvement in survival by resection of tumor burden, albeit marginal[6].
Adjuvant radiotherapy has been an established cornerstone in the treatment of GBM since 1979, when publication of the seminal study by Walker et al[9] showed that patients treated with radiation showed longer survival than those treated with best supportive care.
A major obstacle in the radiotherapy of GBM is the problem of radiation resistance, which is recurrence of the tumor within the high dose region[7]. The existence of this phenomenon implies that the amount of radiation administered (and as tolerated without excessive toxicity) is not enough to eradicate in entirety tumor existing in the radiation field. It is hypothesized that some of the hallmark genetic mutations characteristic of GBM contribute to the phenomenon of radiation resistance. Studies that have looked at increasing the dose of radiation to the limit tolerated-up to 90 Gy-have not demonstrated a discernible benefit of this strategy.
Having discussed the limitations of current radiotherapy, it begs discussing new modalities being investigated that intend to overcome these limitations which will be discussed later.
The chemotherapeutic agent temozolomide has been available since 1999. Pharmacokinetically, temozolomide is an oral agent with effective absorption and excellent bioavailability. Temozolomide is metabolized into 5-(3-methyltriazen-1-yl) imidazol-4-carboxamid. The therapeutic potential of temozolomide lies in the alkylation/methylation of the DNA of tumor cells, typically occurring at the N-7 or O-6 positions of guanine residues. Methylation causes irreversible DNA damage which in turn provokes tumor cell death[10,11].
The use of temozolomide as standard of care adjuvant chemotherapy for GBM patients is largely the result of a seminal trial by Stupp et al[12] in 2005. This randomized controlled trial compared the use of irradiation alone to the use of concurrent radiation and temozolomide chemotherapy followed by 6 cycles of adjuvant temozolomide. In the experimental group, concurrent temozolomide was administered at 75 mg/m2 daily during irradiation (both arms received 30 fractions with total dose of 5500 to 6000 cGy) followed by 6 cycles of temozolomide 150 mg/m2 (days 1 to 5 of cycle 1) then 200 mg/m2 on days 1 to 5 of cycles 2-6, repeated on day 29.
It was found that temozolomide improved median overall survival (OS) (14.6 mo vs 12.1 mo), 2 year OS (27.2% vs 10.9%), 3 year OS (16.0% vs 4.4%), and 5 year OS (9.8% vs 1.9%). These statistically significant results corroborated the superiority of temozolomide and that continuously improved over time.
It is of importance to reconcile the positive results of temozolomide use in GBM treatment with the fact that, as discussed at the beginning of this paper, the overall outcomes in patients with GBM still remain unequivocally poor. One important concept that helps to explain this in part is chemotherapy resistance and in the case of temozolomide, through damaged DNA repair.
These chemotherapy resistant cells express a protein, O6-alkylguanine DNA alkyltransferase (AGT), encoded in humans by the O-6-methylguanine-DNA methyltransferase (MGMT) gene. The AGT protein removes the alkylated moiety on the O6 position of guanine and renders the therapeutic modality of temozolomide obsolete. It has been found that there exists an epigenetic variant of tumor cells that are able to circumvent this mechanism of chemotherapy resistance. These tumor cells possess a protein that is responsible for methylation of the MGMT promoter; this methylation serves to silence the MGMT gene. As a result, such tumors are thought to be more sensitive to temozolomide. Based upon this, this molecular marker for MGMT methylation has been investigated as a means of predicting response to treatment with temozolomide. As early as 2005, the group of Stupp et al[13] recognized the implications of this gene in therapy and conducted a retrospective analysis on the tumors culled from subjects in their pivotal study establishing superiority of adjuvant chemotherapy and radiation to radiation alone. For 203 patients whose tumors were found to possess the MGMT methylation gene, a substantive difference was found, with the progression free survival (PFS) being substantially greater in the experimental arm receiving temozolomide with radiation therapy (RT) than the control group receiving RT alone. On the basis of these findings, the prevailing thought was that possession of the MGMT methylation gene predicted favorable response to treatment with temozolomide. This premise of using the MGMT methylation gene for prediction of response to treatment with temozolomide was challenged in 2011 by the RTOG 0525 study[14]. The purpose of this study was to look at a proposed strategy for overcoming acquired temozolomide resistance, specifically whether there was a survival difference between the use of standard schedule of temozolomide or an altered schedule in which the same total dose of temozolomide was delivered in higher fractions, allowing for a 3 wk on, 1 wk off dosing. When these patients were stratified based on MGMT status, there was an OS of 23.2 mo in patients with tumors possessing the MGMT methylation gene vs 16 mo in those harboring unmethylated MGMT status. Thusly, the current paradigm is that possession of the MGMT methylation gene prognostically bodes better for patients receiving standard adjuvant treatment than those that do not possess in the general population but does not necessarily predict response to treatment with temozolomide. An important demographic caveat exists, however. It was found on that basis of multiple studies that the MGMT methylation gene does predict favorable responses in terms of survival benefit in elderly patients (age greater than 70) with GBM who receive TMZ and radiation vs RT alone[15-17]. This is important in that uses of different modalities of treatment necessarily must be used more conservatively and sparingly in elderly patients who have more limited physiological reserve with which to contend with the ill effects of such treatments.
Interestingly, there is another more nuanced twist to the MGMT story. Recent studies have revealed that in some tumors, the MGMT gene-and resistance to TMZ--is effectively silenced even without possession of the MGMT methylation gene. What these studies have found is that MGMT expression is also post-transcriptionally regulated by micro-RNAs[18]. MicroRNAs (miRNAs) are non-encoding RNA molecules 20-23 nucleotides in length that inhibit the translation and stability of messenger RNA (mRNA). MicroRNAs have a potent presence in the regulation of post-transcriptional gene expression as they “flag” mRNAs which leads to their decay and influences essential cell functions, i.e., replication, proliferation, metabolism, programmed cell death, etc.[19]. Low MGMT expression in promoter unmethylated tumors was found to be due in part to the expression of miR-181d, a miRNA that suppresses MGMT expression. There have been additional micro-RNAs identified that bind directly to the MGMT 3’UTR and purportedly result in loss of MGMT protein expression both in pre-clinical and clinical studies.
There are a number of genes on chromosome 10 of which mutation, deletion or LOH has been established in the development of GBM malignancy. These will be considered in turn.
A well-described phenomenon engendering tumorigenesis is LOH. In somatic cells, many tumor suppressor genes bear heterozygosity by merit of having inherited unique single nucleotide polymorphisms in different regions in that gene. Thusly, one allele in the pair for that gene is different from the other. In the process of LOH, a portion of or a complete chromosome in a diploid pair is deleted. If this portion contains a tumor suppressor gene, then the cell containing that deletion exhibits LOH for that gene or chromosome. When the remaining copy of the tumor suppressor gene incurs a mutation, the cell is no longer protected by that tumor suppressor gene and the biology of malignancy is begotten.
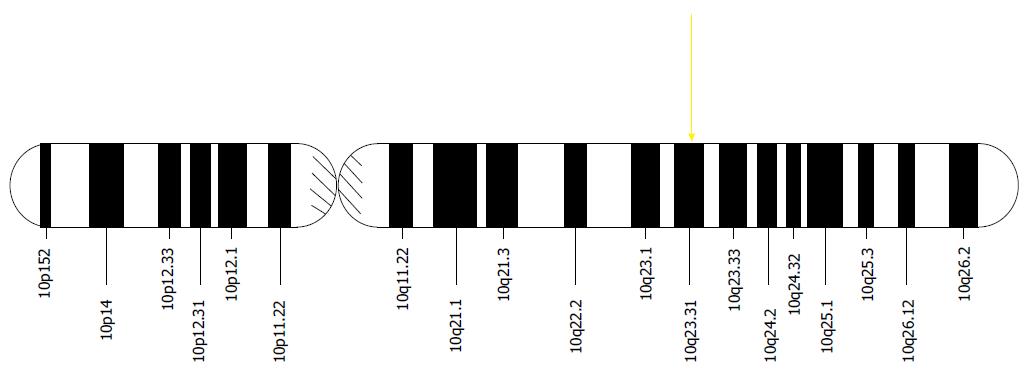
The LOH phenomenon specifically involving alleles of tumor suppressor genes in parts or all of chromosome 10q has reliably been demonstrated in the molecular pathogenesis of GBM[20]. A specific example is allelic deletion of the phosphatase and tensin homolog gene, or PTEN, located at locus 10q23[21] (Figure 2).
The wild type PTEN gene is a tumor suppressor. The product of this gene is involved in many different signaling pathways in its capacity as a phosphatase. The most important of these pathways is the PI3K/Akt pathway[21]. When an extracellular ligand binds to its correspondent receptor, e.g., EGFR, HER2, IGFR, the protein PI3K is activated and creates PIP13. PIP13 in turn recruits the Akt to the intracellular surface of the cell membrane and subsequently activates the PI3K/Akt pathway. Activity is positively regulated by the PIP3 gene product. This pathway promotes a number of progrowth phenotypes, including cell cycle progression, protein synthesis, inhibition of apoptosis and cell migration. When PIP3 is dephosphorylated by PTEN to PIP2-a, the PI3K/Akt pathway is downregulated and antagonizes the progrowth phenotype. Thusly, when PTEN activity is lost through mutation or LOH, PIP3 accumulates and begets malignant growth though constitutive activation of the PI3K/Akt pathway. Mutations in PTEN have been implicated in a variety of malignancies, including prostate, gyn malignancies, breast, pancreatic, melanoma and GBM[21].
PTEN LOH mediating malignant features in GBM has been found to occur in as much as 60%-80% of all cases[21]. Historically, studies concerning LOH or mutation in PTEN had proposed a value in prognostication, i.e., that loss of PTEN would make for a poorer prognosis[21]. This was particularly so prior to acceptance of TMZ as standard adjuvant treatment of GBM. However, a recent study out of Cedars-Sinai medical center appears to refute this understanding[22]. Indeed, the premise of the study was to update the understanding of the significance of this molecular marker in the current TMZ treatment era. In this study, the authors retrospectively looked at the presence or absence of PTEN in 155 tissue samples from patients who underwent craniotomy for resection of GBM between 2007 and 2010. The majority of these patients (80.7%) were treated with standard adjuvant radiation and TMZ chemotherapy after surgical tumor resection. What they found was that the loss of PTEN via LOH, mutation, or deletion was not associated independently with poorer prognosis as had been previously assumed. What they did find was that in their multivariate analysis, certain features assessed were significant predictors of worse prognosis; these included: older age (≥ 65), poorer functional level based on KPS score, partial resection of tumor, and not instituting standard adjuvant therapy. Interestingly, the authors also found evidence that appeared to corroborate the supposition that GBM cells that had PTEN loss were more susceptible to TMZ treatment. This was suggested in an in vitro study that found that glioma cell lines lacking PTEN were more sensitive to TMZ treatment than PTEN possessing glioma cell lines. The thought process is that the lack of PTEN makes those cells less capable of repairing double-stranded DNA breaks effected by TMZ, and thus makes TMZ more chemotherapeutically effectual. What this implies is that the reason the authors found no statistically significant difference in patient outcome based upon PTEN loss or presence alone is because the increased effectiveness of TMZ in PTEN loss would effectively even out the outcomes between the two groups. This would appear to explain the difference found between this study and prior studies that did not evaluate patients who had undergone adjuvant TMZ treatment. Thusly, it is not entirely clear that PTEN loss is not an independently poor prognostic factor. Moreover, this seems to suggest that PTEN loss would predict a more favorable response to TMZ though the outcome would appear not to be substantively different from patients with the presence of PTEN.
LOH has been found to occur in increased incidence in other genes located on chromosome 10 in GBM patients. Summarily, these, alongside LOH of PTEN on chromosome 10q23, indicate that this phenomenon alongside mutations on chromosome 10 may possess prognostic value when found in patients with newly-diagnosed GBM. However, as discussed above, there remains more to be elucidated in the context of the contemporary treatment paradigm before such mutations may be reliably used for such prognostication purposes.
The next molecular phenomenon in GBM pathogenesis to be discussed is the 1p/19q codeletion. This results from an unbalanced translocation between chromosomes 1p and 19q and leads to LOH. This molecular signature has been found to have tremendous significance and clinical utility in the evolving paradigm of molecular based prognostication and treatment of high grade glioma.
There have been three randomized clinical trials that have investigated the 1p/19q codeletion in GBM and found that it actually confers a survival benefit to patients whose tumors possess this codeletion and are receiving RT and/or alkylating chemotherapy. These trials will be discussed in turn.
The first trial to be considered here is the RTOG 9402 phase III randomized study, which included 289 patients with grade III anaplastic oligodendroglioma or anaplastic oligoastrocytoma treated with either adjuvant RT alone or four cycles of chemotherapy (Procarbazine/CCNU/Vincristine) followed by RT (PCV - > RT)[23]. The primary endpoints were assessing differences in PFS and OS between the two arms in the study. In this, they found at 3 years out that there was a benefit in PFS in the PCV - > RT arm (2.6 years) over the RT alone arm (1.7 years); however, there was no significant difference at that time in OS (4.7 years in the RT arm vs 4.9 years in the PCV - > RT arm). The researchers also assayed tissue samples for the 1p/19q codeletion and assessed whether this had any bearing upon either PFS or OS. Of 201 patients assayed with fluorescence in situ hybridization (FISH), 93 (46%) were positive for the codeletion. It was found that these patients had a survival benefit conferred by the codeletion over the wild type tumors. Irrespective of the treatment arm, those patients possessing the 1p/19q codeletion had a median OS of > 7 years whereas the median OS in patients without the codeletion was 2.8 years. Though treatment at this juncture did not appear to have any bearing on survival, extended follow up 2012 confirmed the better prognosis for the 1p/19q codeletion group and that PCV - > RT also appeared to improve survival over RT alone in those with the codeletion. The median OS in non-codeleted tumors was 2.6 years and 2.7 years in the PCV - > RT and RT alone group, respectively. However, patients with codeleted tumors had a median OS of 14.7 and 7.3 years in the PCV - > RT and RT alone groups respectively. Thusly, it appeared from this trial that the 1p/19q codeletion possessed both prognostic and predictive value.
Soon thereafter, the EORTC 26951 trial was conducted, compared RT alone to RT followed by six cycles of PCV (RT - > PCV) in 368 patients with anaplastic oligodendroglioma or anaplastic oligoastryocytoma randomized between the two arms[24]. Summarily, the outcome was analogous to the aforementioned trial on the primary endpoints of PFS and OS; PCV and sequential RT did increase PFS from 13.2 mo to 23 mo but had no bearing on OS (40.3 mo for PCV - > RT vs 30.6 mo for RT alone) at 60 mo out. The researchers in this study also used FISH to assay tissue for the 1p/19q codeletion; 78 patients (21%) were positive for the codeletion. As with the RTOG study, 1p/19q codeletion was prognostic and conferred a better outcome irrespective of therapeutic intervention. At 60 mo out, those patients possessing the 1p/19q codeletion did not reach a discrete median OS whereas those without the codeletion and treated with RT followed by PCV had a median OS of 25.2 and 21.4 mo for those treated with RT alone. Results after extended follow up of 12 years in 2012, again mirrored those of the RTOG trial at extended follow up, with RT - > PCV yielding a greater OS (no median OS reached in these patients) over RT alone (median OS of 9.3 years) in patients with the 1p/19q codeletion. This survival benefit was not seen in those patients without the codeletion; in this contingent, those receiving RT - > PCV had an OS of 25 mo and those receiving RT alone 21 mo.
A third trial known as NOA-04 conducted by the German Neuro-Oncology Group prospectively evaluated 318 patients with anaplastic astrocytoma, anaplastic oligodendroglioma, and mixed anaplastic oligoastrocytoma treated with RT, PCV, or TMZ-by the ratio of 2:1:1, respectively[25]. Patients who experienced excessive toxicity or progression after RT were then randomized to receive either PCV or TMZ, or patients with similar outcomes during or after primary treatment with chemotherapy were then administered RT. The primary endpoint was treatment failure, and 43% reached that endpoint at 54 mo out. On PFS and OS, the three groups were found to have similar results. In this study, FISH assays of tissue found 74 patients (23%) to possess the 1p/19q codeletion. When assessing these patients against wild type patients on the primary endpoint, there was, regardless of therapeutic intervention, an improvement of almost 50% in treatment failure. What should be understood, however, is that the benefits conferred by the codeletion had no bearing upon those patients with anaplastic astrocytoma. The aggregate of the studies described above show that the 1p/19q codeletion has both prognostic and predictive utility in malignant gliomas and may thereby represent a tool to guide clinicians in prognostication and treatment planning for patients with these malignancies.
The genes IDH1 and IDH2 are molecular markers that demonstrate prognostic value in patients with glioblastomas as well as lower grade gliomas. Isocitrate dehydrogenase (encoded by IDH1 in the cytoplasm and by IDH2 in the mitochondria) in its wild type form produces alpha-ketoglutarate[26]. Mutations in these genes encode an aberrant enzyme that turns alpha-ketoglutarate into an onco-metabolite, D-2 hydroxyglutarate. D-2 hydroxyglutarate controls the oncogenicity of IDH mutations. Based upon mutation status, gliomas may be classified as IDH-wild-type or IDH-mutant. IDH-wild-type gliomas include grade I pilocytic astrocytomas and primary GBMs. Tumorigenesis in this case is, therefore, independent of the IDH status and is mediated by other oncogenes. IDH-mutant gliomas include grade ii and grade III gliomas as well as some secondary GBMs. What is interesting is that within a given histological class, IDH mutants carry a better prognosis than IDH wild types. For example, in WHO class IV tumors, secondary GBMs (IDH mutants) carry a better prognosis than primary GBMs (IDH wild types). An analysis of 382 high grade gliomas in 2010 found that IDH status has greater prognostic value than histological grade[27]. Thusly, it is now being realized that grouping gliomas by IDH status is more useful for prognostication than grouping by histological grade and morphology.
It is well known, that protein p53, with a gene located on the short arm of chromosome 17 (17p13.1), is one of the main tumor suppressors. It is a transcription factor that activates the expression of genes that will induce the G1 cell cycle arrest in response to cell stress and DNA damage. Hence, the somatic and the germline mutations of p53 are associated with a variety of human cancers[28,29].
The Cancer Genome Atlas Network (TCGA) reported p53 mutations in 37.5% of the newly diagnosed, and in 58% of the previously treated GBM samples[30]. As far as the pathogenesis of malignant gliomas is concerned, the mutations in p53 and its regulatory pathways primarily play a role in development of secondary gliomas as opposed to the primary glioblastomas[31-33].
Other than the cell cycle progression, several cellular processes are thought to be affected by p53 such as: the response to DNA damage, apoptosis, and the cellular differentiation and neovascularization[34]. Based upon cellular homeostasis, the p53 gene product is typically low in normal cells and increased in cells affected by DNA damage, where it exhibits a relatively short half life, being degraded by the Murine Double Minute 2 (MDM2) protein in the cytoplasmic milieu[35].
Apart from the events that mutate the p53 itself, the mutation of genes encoding its functional regulation are found in approximately 70% of GBM samples, mainly ARF, 55%, MDM2, 11%, and MDM4, 4%[36]. The MDM2 and MDM4 proteins that function as E-3 ubiquitin ligase, degrade the p53 and repress its function. It has been confirmed, that the amplification of MDM2/MDM4 proteins inactivates the transcriptional activity of p53, resulting in abrogation of its antiproliferative and apoptotic effects[37,38].
The CDKN2A locus has been shown to present another frequent mutation in glioblastomas. In addition to encoding the p16INK4a, that is a specific inhibitor of CDK4/6, this locus encodes a second protein, the p14ARF, whose expression also induces a cell cycle arrest. The p14ARF acts by binding MDM2, thus promoting its rapid destruction, and leading to the stabilization and accumulation of p53. For INK4a/ARF locus mutation, the protective, antitumorogenic role of the p14ARF is lost, due to the suppression of p53[39]. The importance of this locus is also confirmed by observation, that in mice models the homozygous deletions of both p16 and p14, are correlated with the increased progression from lower to higher grade gliomas and poorer survival rate in patients older than 50 years[40].
In 2010, the group of Verhaak et al[41] published results of a study in which they utilized the genomic sequences of 91 GBM patient made available by TCGA Research Center to look at patterns of gene mutations and expression in different tumors that may allow for categorization of these tumors into distinct subclasses. They found they were able to find distinct genomic patterns that hewed to a classification system that would allow them to classify any given tumor into one of four subtypes: Proneural (PNL), Neural, Classical (CL) and Mesenchymal. In addition to allowing for the distinct biology for tumors of each of these classes to be contrasted with the others, it was posited that this may have utility for prognostication and/or predicting response to treatment.
As discussed, GBM by histology is characterized as one entity. This has been found to be a considerable oversimplification that does not account for the differences in biology between different GBM tumors. Genetics have revealed that there exist multiple subtypes of GBM.
What is clinically significant here is that the biology of each subtype confers upon it differences in prognosis and/or response to treatment from the other subtypes. As to the final consensus on how many genetics-based subtypes there actually are, this remains to be determined and studies are ongoing to this end.
One such study that provided a compelling insight into what is likely to be representative of the future of GBM classification came from a group out of Belgium in 2012[42]. The authors predicated their investigation upon the results of Verhaak et al[41] cited above, namely exploiting unique patterns of genetic mutations to classify GBM into distinct biological subtypes that each have unique clinical characteristics. The goal of the study was broadly twofold: to devise a relatively simple assay of mutations in tumor samples for classification into one of two subgroups; and to try to ascertain biological features of tumors from each subgroup that have demonstrable value in making clinical inferences. To this end, the authors did a retrospective analysis of 100 patients with new, treatment naive GBM. They utilized immunohistochemistry (IHC) to quantitatively assay tumor samples from these patients for the presence or absence of mutations in 3 well-characterized genes in GBM-EGFR, PDGFRA, and p53. Based upon the pattern of presence or absence of mutations in these genes, the investigators were able to discern two subtypes of GBM: the CL subtype and the PNL subtype. To be sure, these subtypes had been initially described by Verhaak et al[38] but the association with the IHC mutational analysis done here was entirely new. The CL subtype is characterized by positive immunostaining for EGFR and is negative for p53 and PDGFRA mutations. The PNL subtype, on the other hand, is EGFR negative and demonstrates positive immunostaining for p53 and/or PDGFRA. Of the initial cohort of 100 GBM specimens, 93 were able to be quantitatively assessed for these genetic signatures. Based upon the criteria set outlined, 35 specimens were found to belong to the CL subtype and 56 were found to belong to the PNL subtype; the other 2 specimens did not stain for any of the three markers. The endpoints assessed for the patients in this retrospective analysis were PFS and OS. The former was defined as the time elapsed from the date on which the tumor was resected to the date on which the tumor was found to have recurred or if the patient died from recurrence of tumor. The latter was defined as the time elapsed from the date on which the tumor was resected to the date the patient died due to tumor progression. Summarily, the study found the following of notable clinical significance. Firstly, patients with tumors of the PNL subtype had a statistically significantly higher median OS of 10.5 mo than the median OS of 5 mo for patients of the CL subtype (P = 0.047). Similarly, a mortality risk reduction of 52% was linked to the PNL subtype when compared to the CL subtype. Hence, it was suggested that the delineation of a given GBM patient to one of the two subtypes would possess value in prognostication. Furthermore and not insignificantly, the authors demonstrated that the information needed to make this categorization, i.e., PDGFRA, EGFR, and p53 status, is relatively easily obtainable through IHC staining. Secondly, the authors found that these two subtypes possess biological characteristics conferred by their respective genetic signatures that make their response to adjuvant adjuvant chemotherapy different from one another.
Specifically, they found that temozolomide chemotherapy with radiotherapy did dramatically improve survival of patients of the CL subtype. This was not the case in that contingent of CL patients receiving radiotherapy alone, who showed no significant improvement in OS compared to patients receiving no treatment or palliative management. Interestingly, treatment modalities had quite the opposite effect on patients of the PNL subytype. These patients who received radiotherapy alone saw a significant improvement in OS over those who received no treatment or palliative management. However, the addition of temozolomide to radiotherapy did not improve survival in this subset of patients as it did in the CL patients.
When considering the multimodal actions of TMZ as a chemotherapy agent and contextualizing this in the problem of chemotherapy resistance, a topic of recent research interest is autophagy.
Autophagy, known as type II programmed cell death, is a catabolic process during which cells self-digest intracellular organelles. When allowed to go to completion, autophagy results in cell death[43,44]. Biologically, it serves two functions: as an intracellular mechanism of disposing of damaged organelles and proteins, and for catabolism of substrates during cellular stress in order to generate energy needed for cell survival. As may be intuited, persistent autophagy does in many cases result in cancer cell death. However, there is also mounting evidence that autophagy may also drive the damage response that cancer cells use to avoid death when exposed to metabolic and therapeutic stresses.
Knizhnik et al[45] demonstrated in glioma cells that TMZ can induce cell death via a complex process between apoptosis, autophagy, and senescence. Senescence represents a state when viable cells stop synthesizing DNA with the unknown endpoint of either survival or death. They demonstrated that TMZ - induced cell death could be accomplished by two mutually exclusive pathways: by apoptosis alone (via the caspase-mediated pathway) or by autophagy followed by cellular senescence. It was found that the autophagy pathway inhibited the appositional apoptosis pathway, and the cells progressed to senescence. Thus, it is proposed that autophagy may be a survival mechanism whereby gliomas undergo senescence rather than immediate death via apoptosis when therapeutic doses of TMZ are used. Knizhnik et al[45] also found that autophagy, senescence, and apoptosis of glioma cells occurred at 72, 96, and 120 h after TMZ exposure, respectively.
As a result of TMZ-induced autophagy, it is possible that the high recurrence rate in glioblastoma patients and the unsatisfactory clinical survival rate might not only be due to the resistant mechanisms of tumors such as MGMT and deficiency of MMR but also due to autophagy allowing the tumor to survive where it should otherwise undergo apoptosis. As such, investigations are underway to see if adjunctive treatment with an autophagy inhibitor may enhance the beneficial therapeutic effects of TMZ for patients with GBM.
GBM is one of the most vascularized human tumors and, alongside high expression of various proangiogenic factors, vascular proliferation is one of its defining pathologic features[2]. GBM cells produce proangiogenic factors; one of, if not the, best characterized of these is vascular endothelial growth factor (VEGF).
VEGF consists of a family of 5 glycoproteins: VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placenta growth factor. These factors bind with their corresponding tyrosine kinase receptor (VEGFR-1, VEGFR-2, and VEGFR-3) and activate a signal transduction cascade that results in the development of angiogenesis, increased vascular permeability, and lymphangiogenesis. Of these, VEGF-A plays the greatest role in tumor angiogenesis along with tumor cell proliferation and migration. Thusly, elevated levels of VEGF-A in patients with cancer--specifically that of breast, lung, colon, uterus, and ovary--confers a graver prognosis[46].
Bevacizumab is a humanized monoclonal antibody to VEGF-A[47]. This antibody prevents the interaction of VEGF with target receptors VEGFR-1 and VEFGR-2 on the surface of endothelial cells. This in turn prevents downstream signaling that would normally induce tyrosine phosphorylation and the subsequent cascade of signal transduction events that would lead to endothelial cell survival, proliferation and vascular permeability. The composite effect of causing regression of existing microvasculature, inhibition of new vessel growth and normalization of the surviving vasculature (which leads to reduced vascular permeability and reverses peritumoral edema) bears a particularly germane pertinence to GBM. In the United States, bevacizumab has been approved for recurrent GBM based on studies that showed improvement in PFS but not OS[47].
In February 2014, Chinot et al[48] published randomized, double-blinded, placebo controlled trial on newly diagnosed GBM patients where they compared standard radiotherapy and TMZ for newly diagnosed GBM with or without bevacizumab. The study met the first primary endpoint of improved median PFS with statistical significance (P < 0.0001), finding a 4.4 mo improvement in median PFS of the experimental group (10.6 mo) over the control group (6.2 mo).
The OS at 1 year (P = 0.049) was 72.4% and 66.3% in the experimental and control groups, respectively. At 2 years (P = 0.24), the OS was found to be 33.9% and 30.1%, respectively, which was not statistically significant. The experimental group receiving Bevacuzimab maintained a longer quality of life and performance status and required less steroids. However, the study noted that there was a clearly greater number of clinically significant adverse events in the Bevacizumab group than the control group.
The RTOG 0825 study, published in the same month as the study by Olivier et al[49], came to a similar conclusion: namely that adding bevacizumab to standard of care RT/TMZ provided discernible benefits for PFS but not for OS. Notable adverse effects in the bevacizumab group were hypertension, thromboembolic events and intestinal perforation, consistent with previously reported side effects of this medication.
The BELOB trial by Taal et al[50] out of Europe investigated three lines of therapy for patients with recurrent GBM: single-agent bevacizumab, single-agent lomustine and combination therapy with bevacuzimab plus lomustine. Results demonstrated 9-mo OS to be 43% in the lomustine group, 38% in the bevacizumab group and 59% in the combination group. Extrapolating from these results, the authors strongly questioned the role for single agent bevacizumab in recurrent glioblastoma. However, it provided a compelling indication for further investigations of combination bevacuzimab with lomustine, particularly in a phase III trial.
The aggregate of data from clinical trials on bevacuzimab for newly-diagnosed and recurrent GBM reveals that the proposed mechanism of action of bevacuzimab in antagonizing the VEGF pathway is not enough on its own to explain the observed results. It has led investigators and the scientific community to realize that there are much more complex regulatory mechanisms in angiogenesis at work than previously recognized.
Recent evidence has indicated that prolonged anti-angiogenic treatment leads to development of progressive hypoxia in tumor tissues which in turn has led to the recognition of an entirely novel paradigm of treatment resistance. VEGF blockade of its own causes only a small reduction in tumor burden but does induce a strong depletion of large and intermediate-sized blood vessels with a subsequent reduction in vascular leakage and intratumoral blood flow.
This result in a hypoxic microenvironment within the tumor which is proposed to provoke significant tumor cell invasion.
Hypoxia-inducible factor-1 (HIF-1) is a transcriptional complex belonging to a family of transcriptional factors known as hypoxia inducible factors (HIFs) that is activated in response to hypoxia and growth factors. HIFs are heterodimers composed of an oxygen-sensitive HIF-alpha subunit and a HIF-beta subunit. Under normal homeostatic cellular conditions, HIF-alpha binds to the tumor suppressor protein von Hipel-Landau (vHL), which leads to degradation of HIF-alpha.
However, under conditions of hypoxia, there is an abrogation of the interaction between HIF-alpha and vHL as a result of which HIF-alpha gets stabilized. This leads to dimerization of HIF-alpha which then allows it to bind to hypoxia responsive elements on promoters of genes inolved in promoting cell survival, motility and metabolism. The activation of HIFα also plays a regulatory role in the expression of VEGF and inducible nitric oxide synthetase facilitating angiogenesis and the tumors cell’s access to the circulatory system. Two HIFα subunits, HIF-1α and HIF-2α are primarily responsible for regulating tumors adaptation to hypoxia. HIF-1α is widely expressed in several tissues, while HIF-2α has a more restricted expression pattern and is associated with cancer initiation or tumor progression. Thusly, HIF-1 plays a central role in tumor progression, invasion, and metastasis. Indeed, overexpression of the HIF-1α subunit has been observed in many human cancers and is associated with a poor prognostic outcome with conventional treatments[51].
Preclinical trials of recent have revealed some very intriguing characteristics of tumor vasculature. Three major mechanisms have been proposed for the development of new tumor vasculature: proliferation from preexisting vessels, colonization by circulating endothelial cells or colonization by proangiogenic bone marrow cells. This last phenomenon has come to be denoted as vasculogenesis[52].
In specific, vasculogenesis itself depend on three major pathways: (1) mobilization and recruitment of proangiogenic bone marrow derived cells (BMDCs) into tumor milieu; (2) retention of these BMDCs in hypoxic tumor tissues; and (3) vascularization dependent on CD11b+ myelomonocytic cells.
Hypoxia leads to induction of the transcription factor HIF-1 which has been shown to be a major recruiter of BMDCs to tumors including GBM. Retention of these cells is dependent on secretion of stromal cell derived factor-1 (SDF-1, CXCL12) which binds its receptor, CXCR4, on the BMDCs. Thus has been elucidated the link between hypoxia in GBM and vasculogenesis.
This in turn has led researchers to propose the means by which bevacuzimab engenders treatment resistance.
The proposed hypoxia model as discussed has been further supported by studies looking into inhibitors of the modulators of vasculogenesis. In xenograft models, the HIF-1 inhibitor NSC-134754 and AMD3100, an inhibitor of the SDF-1/CXCR4 interaction, compellingly found little to absent tumor regrowth following irradiation[53].
Hypoxia has been also been proposed as a means of activating autophagy, the lysosomal degradation pathway that, as discussed earlier, likely promotes tumor cell survival[54]. The mechanisms by which hypoxia induces autophagy need elucidation, but the finding that BNIP3, a downstream target of HIF-1α, is essential to hypoxia-induced autophagy suggests a likely mechanism.
An additional important developing point of interest with therapeutic potential is the identification of cancer cells with stem cell-like properties. It has been hypothesized that a subset of cells known as the cancer stem cells exist within a tumor with stem cell like properties and can initiate primary tumors as well as recurrences by way of their self-renewal capacity and inherent resistance to therapeutics.
Glioblastoma contains multipotent tumor stem cells (GSCs) that could be responsible for populating and repopulating tumors.
Specific criteria are required to define GSCs: (1) the ability to self renew; (2) the ability to differentiate into different lineages (multipotency); and (3) the ability to initiate tumors in animal models which recapitulate the original disease phenotype and heterogenicity[55,56].
Multipotent neural stem cells have the ability to differentiate into neurons and glia (astrocytes and oligodendrocytes). Physiologically, stem cells have a long life expectancy and divide frequently which makes them more susceptible for tumorigenesis.
The process of neurogenesis occurs in two major regions of the adult brain: the subventricular zone of the lateral ventricles (SVZ) and the subgranular layer of the hippocampal dentate gyrus[57].
Neuronal stem cells (NSCs) are regulated by the orchestration of intrinsic factors with extrinsic signals from surrounding microenvironment, defined as the neurogenic niche. A niche represents a specialized anatomic compartment formed by cellular and acellular components that integrates local and systemic factors, supports maintenance and survival and actively regulates the function and proliferation of NSCs.
It has been hypothesized that once neurogenic niches house NSCs (which have a relatively large chance of becoming cancerous cells) and support maintenance, survival and proliferation, they become vulnerable sites for growth and proliferation of transformed cells. It is believed that the SVZ gives rise to the highest number of glioblastomas and this has led to efforts looking at this cell population as a potential therapeutic target.
The sole process of neurogenesis depends on a complex cascade of molecular signaling pathways. These candidate pathways include Notch[58], bone morphogenic protein[59], Wnt[60] and sonic hedgehog (Shh)[61].
Blockage of Notch signaling with γ-secretase inhibition, inhibits self-renewal, and causes CD133+ cell depletion[62]. Transforming growth factor-β (TGF-β) signaling promotes GSC self-renewal[63]. Shh signaling (important during embryonic development) plays an important role in GSC maintenance by promoting self-renewal and expression of stem cell genes[64], whereas blockage leads to apoptosis, delay in tumorigenesis and inhibition of GSC self-renewal and migration[65].
Similarly to that of normal stem cells, GSCs are found in a microenvironment that provides ideal condition for tumor maintenance. The tumor perivascular niche is composed of heterogeneous cell groups, including astrocytes, endothelial cells, macrophages, microglia, non-tumor initiating cells, and, indeed, tumor stem-like cells[66].
Multiple mechanisms leading GSCs to chemo-resistance have been identified in pre-clinical studies. These include: increased activity of ABC-type transporters present on the cell surface that extrude chemotherapeutic agents to the extracellular space[67]. These chemo-resistant cells have been identified in GBM cells via flow cytometry with a specific pattern of expression of surface antigens (CD133+, CD117+, CD90+, CD71+, CD45+)[68]. Further corroborating the important role GSCs have in chemo-resistance, CD133 is highly expressed in recurrent tumors and transcriptional analysis of these cells demonstrates concurrent over-expression of anti-apoptotic genes[69]. Parada et al[70] applied these findings and showed that a restricted Nestin+ GSC population could regenerate tumors after being treated with temozolomide. Others have attempted selective ablation of this cell population and this only led to tumor growth arrest, supporting the hypothesis that GSCs resist current standard chemotherapy and have intrinsic properties of chemo-resistance. In addition to the above, GSCs have slow cell cycles, generally quiescent and are immune to exposure to chemotherapy because these traditionally target actively cycling cells. GSCs also have the ability to evade irradiation with the development of clones that over-express GSC markers as well as triggering over-activation of the Notch and TGF-β signaling pathways[71,72].
GBM has historically been and indeed remains a formidable challenge for clinicians and has maintained a grim prognosis not much changed from the very inception of conventional treatment. This is despite a profusion of significant recent discoveries regarding its unique biology and intricate molecular pathogenesis. However, with the elucidation of these recent and ongoing findings, there are a number of exciting studies underway investigating entirely novel treatment modalities that exploit these recent revelations. It is expected that with fruition of validated results in animal models and progression to phase III clinical trials, a veritable revolution will take place in both the diagnosis and treatment of this most malignant of primary brain cancers.
P- Reviewer: Castresana JS, Poltronieri P S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Smith SL, Smith IT, Branco T, Häusser M. Dendritic spikes enhance stimulus selectivity in cortical neurons in vivo. Nature. 2013;503:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 272] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 2. | Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7079] [Cited by in RCA: 8036] [Article Influence: 446.4] [Reference Citation Analysis (0)] |
| 3. | Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15 Suppl 2:ii1-i56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 1162] [Article Influence: 105.6] [Reference Citation Analysis (0)] |
| 4. | Ohgaki H, Kleihues P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 2009;100:2235-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 19.3] [Reference Citation Analysis (4)] |
| 5. | Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 6. | Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2955] [Cited by in RCA: 3158] [Article Influence: 185.8] [Reference Citation Analysis (0)] |
| 7. | Hopkins K. Phase III Study of Rindopepimut/GM-CSF in Patients With Newly Diagnosed Glioblastoma (ACT IV). gov. Bethesda (MD): National Library of Medicine (US), 2011- 2014; Available from: http://clinicaltrials.gov/show/NCT01480479. |
| 8. | Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1038] [Cited by in RCA: 1010] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 9. | Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 589] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Newlands ES, Stevens MF, Wedge SR, Wheelhouse RT, Brock C. Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev. 1997;23:35-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 557] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 11. | Stevens MF, Hickman JA, Langdon SP, Chubb D, Vickers L, Stone R, Baig G, Goddard C, Gibson NW, Slack JA. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & amp; B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res. 1987;47:5846-5852. [PubMed] |
| 12. | Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14033] [Cited by in RCA: 15789] [Article Influence: 789.5] [Reference Citation Analysis (0)] |
| 13. | Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5037] [Cited by in RCA: 5287] [Article Influence: 264.4] [Reference Citation Analysis (0)] |
| 14. | Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31:4085-4091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, Nikkhah G, Papsdorf K, Steinbach JP, Sabel M. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13:707-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 808] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 16. | Malmström A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, Abacioglu U, Tavelin B, Lhermitte B, Hegi ME. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 897] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 17. | Yin AA, Zhang LH, Cheng JX, Dong Y, Liu BL, Han N, Zhang X. The predictive but not prognostic value of MGMT promoter methylation status in elderly glioblastoma patients: a meta-analysis. PLoS One. 2014;9:e85102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Berthois Y, Delfino C, Metellus P, Fina F, Nanni-Metellus I, Al Aswy H, Pirisi V, Ouafik L, Boudouresque F. Differential expression of miR200a-3p and miR21 in grade II-III and grade IV gliomas: evidence that miR200a-3p is regulated by O6-methylguanine methyltransferase and promotes temozolomide responsiveness. Cancer Biol Ther. 2014;15:938-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Møller HG, Rasmussen AP, Andersen HH, Johnsen KB, Henriksen M, Duroux M. A systematic review of microRNA in glioblastoma multiforme: micro-modulators in the mesenchymal mode of migration and invasion. Mol Neurobiol. 2013;47:131-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 20. | McNamara MG, Sahebjam S, Mason WP. Emerging biomarkers in glioblastoma. Cancers (Basel). 2013;5:1103-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Bleeker FE, Molenaar RJ, Leenstra S. Recent advances in the molecular understanding of glioblastoma. J Neurooncol. 2012;108:11-27. [PubMed] |
| 22. | Carico C, Nuño M, Mukherjee D, Elramsisy A, Dantis J, Hu J, Rudnick J, Yu JS, Black KL, Bannykh SI. Loss of PTEN is not associated with poor survival in newly diagnosed glioblastoma patients of the temozolomide era. PLoS One. 2012;7:e33684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Cairncross JG, Wang M, Shaw EG, Jenkins RB, Scheithauer BW, Brachman D, Buckner JC, Fink KL, Souhami L, Laperriere N. Chemotherapy plus radiotherapy (CT-RT) versus RT alone for patients with anaplastic oligodendro-glioma: long-term results of the RTOG 9402 phase III study. J Clin Oncol. 2012;30:2008b. |
| 24. | van den Bent MJ, Carpentier AF, Brandes AA, Sanson M, Taphoorn MJ, Bernsen HJ, Frenay M, Tijssen CC, Grisold W, Sipos L. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24:2715-2722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 529] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 25. | Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, Stockhammer F, Sabel MC, Koeppen S, Ketter R, Meyermann R. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874-5880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 577] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 26. | Weller M, Pfister SM, Wick W, Hegi ME, Reifenberger G, Stupp R. Molecular neuro-oncology in clinical practice: a new horizon. Lancet Oncol. 2013;14:e370-e379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, Westphal M, Schackert G, Meyermann R, Pietsch T. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120:707-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 601] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 28. | Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5532] [Cited by in RCA: 5487] [Article Influence: 161.4] [Reference Citation Analysis (0)] |
| 29. | Hainaut P, Hollstein M. p53 and human cancer: the first ten thousand mutations. Adv Cancer Res. 2000;77:81-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 670] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 30. | Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6404] [Cited by in RCA: 5992] [Article Influence: 352.5] [Reference Citation Analysis (0)] |
| 31. | Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, Burkhard C, Schüler D, Probst-Hensch NM, Maiorka PC. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892-6899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 925] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 32. | Watanabe K, Tachibana O, Sata K, Yonekawa Y, Kleihues P, Ohgaki H. Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol. 1996;6:217-23; discussion 23-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 486] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 33. | Watanabe K, Sato K, Biernat W, Tachibana O, von Ammon K, Ogata N, Yonekawa Y, Kleihues P, Ohgaki H. Incidence and timing of p53 mutations during astrocytoma progression in patients with multiple biopsies. Clin Cancer Res. 1997;3:523-530. [PubMed] |
| 34. | Bögler O, Huang HJ, Kleihues P, Cavenee WK. The p53 gene and its role in human brain tumors. Glia. 1995;15:308-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Stott FJ, Bates S, James MC, McConnell BB, Starborg M, Brookes S, Palmero I, Ryan K, Hara E, Vousden KH. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 1998;17:5001-5014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 877] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 36. | Reifenberger G, Liu L, Ichimura K, Schmidt EE, Collins VP. Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res. 1993;53:2736-2739. [PubMed] |
| 37. | Riemenschneider MJ, Büschges R, Wolter M, Reifenberger J, Boström J, Kraus JA, Schlegel U, Reifenberger G. Amplification and overexpression of the MDM4 (MDMX) gene from 1q32 in a subset of malignant gliomas without TP53 mutation or MDM2 amplification. Cancer Res. 1999;59:6091-6096. [PubMed] |
| 38. | Ruas M, Peters G. The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim Biophys Acta. 1998;1378:F115-F177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 341] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 39. | Schmidt EE, Ichimura K, Reifenberger G, Collins VP. CDKN2 (p16/MTS1) gene deletion or CDK4 amplification occurs in the majority of glioblastomas. Cancer Res. 1994;54:6321-6324. [PubMed] |
| 40. | Labuhn M, Jones G, Speel EJ, Maier D, Zweifel C, Gratzl O, Van Meir EG, Hegi ME, Merlo A. Quantitative real-time PCR does not show selective targeting of p14(ARF) but concomitant inactivation of both p16(INK4A) and p14(ARF) in 105 human primary gliomas. Oncogene. 2001;20:1103-1109. [PubMed] |
| 41. | Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5060] [Cited by in RCA: 5588] [Article Influence: 372.5] [Reference Citation Analysis (0)] |
| 42. | Le Mercier M, Hastir D, Moles Lopez X, De Nève N, Maris C, Trepant AL, Rorive S, Decaestecker C, Salmon I. A simplified approach for the molecular classification of glioblastomas. PLoS One. 2012;7:e45475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 967] [Cited by in RCA: 981] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 44. | Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell. 2005;120:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 356] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 45. | Knizhnik AV, Roos WP, Nikolova T, Quiros S, Tomaszowski KH, Christmann M, Kaina B. Survival and death strategies in glioma cells: autophagy, senescence and apoptosis triggered by a single type of temozolomide-induced DNA damage. PLoS One. 2013;8:e55665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 213] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 46. | Los M, Roodhart JM, Voest EE. Target practice: lessons from phase III trials with bevacizumab and vatalanib in the treatment of advanced colorectal cancer. Oncologist. 2007;12:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 47. | Burkhardt JK, Riina H, Shin BJ, Christos P, Kesavabhotla K, Hofstetter CP, Tsiouris AJ, Boockvar JA. Intra-arterial delivery of bevacizumab after blood-brain barrier disruption for the treatment of recurrent glioblastoma: progression-free survival and overall survival. World Neurosurg. 2012;77:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 48. | Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1748] [Cited by in RCA: 1867] [Article Influence: 169.7] [Reference Citation Analysis (0)] |
| 49. | Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1903] [Cited by in RCA: 2076] [Article Influence: 188.7] [Reference Citation Analysis (0)] |
| 50. | Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D, de Vos FY. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15:943-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 563] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 51. | Flynn JR, Wang L, Gillespie DL, Stoddard GJ, Reid JK, Owens J, Ellsworth GB, Salzman KL, Kinney AY, Jensen RL. Hypoxia-regulated protein expression, patient characteristics, and preoperative imaging as predictors of survival in adults with glioblastoma multiforme. Cancer. 2008;113:1032-1042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 52. | Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 626] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 53. | Hu YL, DeLay M, Jahangiri A, Molinaro AM, Rose SD, Carbonell WS, Aghi MK. Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Res. 2012;72:1773-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 758] [Reference Citation Analysis (0)] |
| 54. | Louis DN. Molecular pathology of malignant gliomas. Annu Rev Pathol. 2006;1:97-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 488] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 55. | Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821-5828. [PubMed] |
| 56. | Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5422] [Cited by in RCA: 5561] [Article Influence: 264.8] [Reference Citation Analysis (0)] |
| 57. | Sawada M, Sawamoto K. Mechanisms of neurogenesis in the normal and injured adult brain. Keio J Med. 2013;62:13-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 58. | Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010;30:3489-3498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 532] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 59. | Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 842] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 60. | Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1313] [Cited by in RCA: 1418] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 61. | Palma V, Lim DA, Dahmane N, Sánchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 447] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 62. | Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, Koh C, Zhang J, Li YM, Maciaczyk J. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28:5-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 464] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 63. | Ikushima H, Todo T, Ino Y, Takahashi M, Miyazawa K, Miyazono K. Autocrine TGF-beta signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell. 2009;5:504-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 443] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 64. | Cayuso J, Ulloa F, Cox B, Briscoe J, Martí E. The Sonic hedgehog pathway independently controls the patterning, proliferation and survival of neuroepithelial cells by regulating Gli activity. Development. 2006;133:517-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 65. | Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524-2533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 478] [Cited by in RCA: 450] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 66. | Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2012;60:502-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 405] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 67. | Bleau AM, Huse JT, Holland EC. The ABCG2 resistance network of glioblastoma. Cell Cycle. 2009;8:2936-2944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 68. | Kang MK, Kang SK. Tumorigenesis of chemotherapeutic drug-resistant cancer stem-like cells in brain glioma. Stem Cells Dev. 2007;16:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 69. | Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1292] [Cited by in RCA: 1366] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 70. | Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1518] [Cited by in RCA: 1754] [Article Influence: 134.9] [Reference Citation Analysis (0)] |
| 71. | Hardee ME, Marciscano AE, Medina-Ramirez CM, Zagzag D, Narayana A, Lonning SM, Barcellos-Hoff MH. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-β. Cancer Res. 2012;72:4119-4129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 72. | Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 981] [Article Influence: 54.5] [Reference Citation Analysis (0)] |