Peer-review started: July 10, 2014
First decision: August 14, 2014
Revised: June 11, 2015
Accepted: July 16, 2015
Article in press: July 17, 2015
Published online: September 28, 2015
Processing time: 447 Days and 3.2 Hours
Mechanical transduction by ion channels occurs in all cells. The physiological functions of these channels have just begun to be elaborated, but if we focus on the upper animal kingdom, these channels serve the common sensory services such as hearing and touch, provide the central nervous system with information on the force and position of muscles and joints, and they provide the autonomic system with information about the filling of hollow organs such as blood vessels. However, all cells of the body have mechanosensitive channels (MSCs), including red cells. Most of these channels are cation selective and are activated by bilayer tension. There are also K+ selective MSCs found commonly in neurons where they may be responsible for both general anesthesia and knockout punches in the boxing ring by hyperpolarizing neurons to reduce excitability. The cationic MSCs are typically inactive under normal mechanical stress, but open under pathologic stress. The channels are normally inactive because they are shielded from stress by the cytoskeleton. The cationic MSCs are specifically blocked by the externally applied peptide GsMtx4 (aka, AT-300). This is the first drug of its class and provides a new approach to many pathologies since it is nontoxic, non-immunogenic, stable in a biological environment and has a long pharmacokinetic lifetime. Pathologies involving excessive stress are common. They produce cardiac arrhythmias, contraction in stretched dystrophic muscle, xerocytotic and sickled red cells, etc. The channels seem to function primarily as “fire alarms”, providing feedback to the cytoskeleton that a region of the bilayer is under excessive tension and needs reinforcing. The eukaryotic forms of MSCs have only been cloned in recent years and few people have experience working with them. “Newbies” need to become aware of the technology, potential artifacts, and the fundamentals of mechanics. The most difficult problem in studying MSCs is that the actual stimulus, the force applied to the channel, is not known. We don’t have direct access to the channels themselves but only to larger regions of the membrane as seen in patches. Cortical forces are shared by the bilayer, the cytoskeleton and the extracellular matrix. How much of an applied stimulus reaches the channel is unknown. Furthermore, many of these channels exist in spatial domains where the forces within a domain are different from forces outside the domain, although we often hope they are proportional. This review is intended to be a guide for new investigators who want to study mechanosensitive ion channels.
Core tip: Mechanosensitive ion channels are found in all cells and their physiological function in most cells has yet to be defined inviting new researchers to the field. This review provides some guidelines to help newcomers understand key issues and potential artifacts.
- Citation: Sachs F. Mechanical transduction by ion channels: A cautionary tale. World J Neurol 2015; 5(3): 74-87
- URL: https://www.wjgnet.com/2218-6212/full/v5/i3/74.htm
- DOI: https://dx.doi.org/10.5316/wjn.v5.i3.74
We are all familiar with many forms of mechanical transduction[1] including hearing[2], touch[3] and mechanical pain[4-7] that feed the central nervous system. In addition, there are the unconscious motor pathways bearing information about muscle stress and joint position[8,9]. Afferents in the autonomic nervous system service blood pressure regulation[8,10-12] and the distension of hollow organs[13,14]. While these reminders may be familiar to the readers of this journal, what may be less familiar is that all cells in our body, including red cells[15,16], are mechanically sensitive[17]. This cell sensitivity probably reflects our evolutionary origins[18]. Single cell prokaryotes like Paramecia have differentiated touch senses; if they bump into walls they back up, and if you bite their tails they run away[19-24]. We have the same heritage, but what are these sensors?
Mechanical sensitivity requires that forces do work on the sensor. This means that the sensors must be deformable, but that only narrows the field to all molecules since they can bend and stretch; it’s a quantitative issue. Computational biology of molecular dynamics shows molecules moving under thermal and externally applied forces. How does one design a mechanical sensor? It’s a matter of numbers. Sensors have a big output energy compared to the input energy. I will define mechanical sensors as those molecules or organelles in which the output energy, whatever that may be, is significantly larger than the input energy. Ion channels can do this because they are enzymes that dissipate lots of stored energy by catalyzing ion transport and their output energy is a function of the turnover number which can be 107.
All sensors have a background noise due to the random shaking of molecules (thermal fluctuations). In this review I will focus on ion channels as sensors (since that is my background), but all structural molecules can be viewed as mechanical sensors since they are compliant to applied force. The channels serve to couple mechanical stress to electrophysiology and biochemistry, typically by changes in calcium levels. This coupling of biochemistry to mechanics is familiar in muscle contraction, mechanically induced changes in gene expression, stem cell differentiation and changes in cell shape. We conjecture that all pathologies involve mechanics since they all involve a change in cell shape and that requires changes in force.
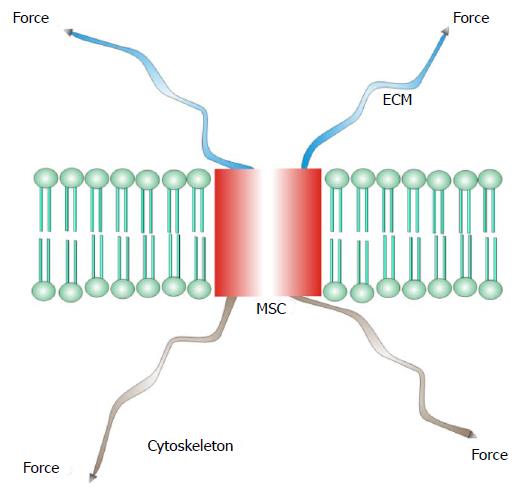
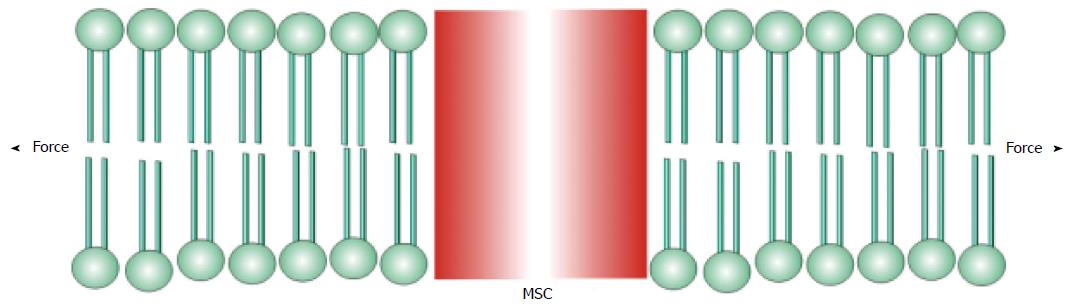
I will define mechanosensitive channels (MSCs), mechanically sensitive ion channels, as those channels whose dynamic range is fully accessible with physiologically relevant forces[25-37]. Many other ion channels, such as the voltage gated ion channels[38,39], are modulated by mechanical stress, but cannot span their dynamic range with mechanics alone. Many enzymes are also mechanically sensitive[40]. There are two basic types of MSCs: those gated by stress in attached structural proteins, and those gated by tension in the bilayer. MSCs in the differentiated sensory organs seem to be gated by forces in structural proteins[30,41-45] where the channel is in series with the extracellular matrix and the cytoskeleton (Figure 1). The other class of MSCs is gated by tension in the lipid bilayer (Figure 2)[24,42,46-50].
There is currently one drug known specific for MSCs of any kind, a peptide called GsMtx4 that is active on cation selective, bilayer-activated, MSCs[15,25,51-58]. Recently there has been a report of an organic molecule that tends to activate some of these channels[16].
This review is not intended to serve as a guide to the literature of MSCs, but addresses core issues that are required to understand how they work. For access to the general literature, there may be more review articles than research papers[18,24,42,46,47,59-63]! My primary goal is to familiarize newcomers to the field about what we have learned and to warn readers about some misleading dogmas to sensitize readers to critical interpretations of research papers. Many of the references in this paper come from the work in my lab, but that is not to say that they are the most important available, merely that I remember them better.
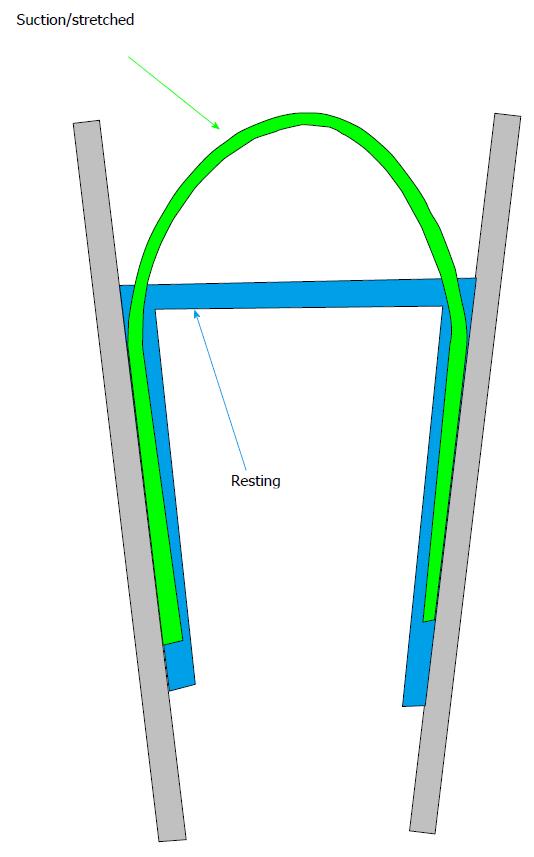
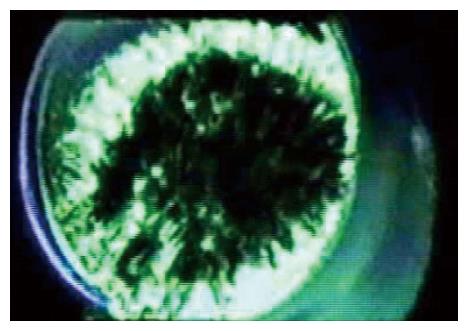
One of the critical limitations of work on MSCs is that the stimulus tends to be imprecise. We can pull, indent, swell or shrink a cell, but what does the channel feel? We don’t know, although but we can guess from the channel behavior. Even in plain lipid bilayer in a patch[46,47,61,64-66], we don’t know the stresses with precision. Let’s look at what might be considered the simplest of experimental systems: a patch of a liposome (Figure 3). Patches stick to the glass pipette. This adhesion produces a tension in the membrane that is a significant fraction of the lytic tension. This means that no one has ever recorded patch currents of any kind except under extreme, nonphysiological, tension (there is one exception to this statement; we were able to measure MSC kinetics under low stress for short periods of time[67]). Patch tension is far in excess of anything seen in resting cells[31,33,68-70]. We know that tension can modulate many ion channels[38,39,67,71], probably transporters[72] and other membrane bound enzymes. When you read a paper about patch clamp recording, remember that all data was obtained under extreme membrane tension[59] and the patch data may not apply to the same channels in situ.
Returning to our ideal lipid patch, without pressure applied to the pipette the patch is pulled flat by adhesion to the glass. When we apply pressure, the patch bends, and the average tension in the membrane can be calculated from Laplace’s law that states that the tension T=Pr/2 where P is the pressure across the membrane and r is the radius of curvature of the patch (not the radius of the pipette)[50,73-77]. From an image of a patch we should be able to calculate the membrane tension, and thus the stimulus that drives an embedded channel. Not so fast… notice that only the outer monolayer of the bilayer touches the glass. The inner monolayer is floating on the outer one[78-81], and as a fluid it cannot have any tension gradients at equilibrium[82,83].
Thus, even our simplest system has a gradient of tension normal to the membrane. You will notice that the papers on molecular dynamics simulations of MSCs apply a uniform tension across the bilayer in incorrectly compare those results to patch clamp data. While the mean tension in a membrane is related to the pressure across it by Laplace’s law, models of membrane patches ignore the high membrane curvature where the patch contacts the glass (Figure 3) at the upper end of a seal[44,68,84,85].
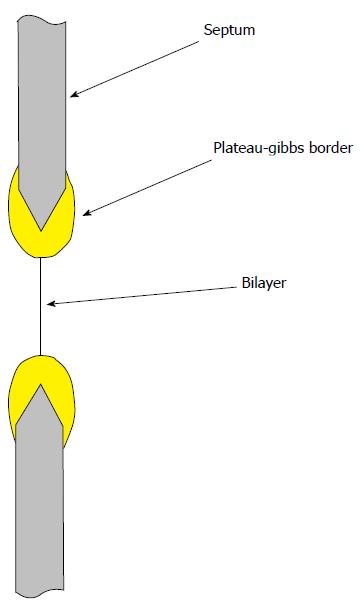
We might imagine that an even simpler experiment that gets rid of the glass, a planar bilayer where the membrane is floating in space[66,86-90]. However, these bilayer lipids are attached to a support structure such as a hole in a Teflon partition. Lipids wet the support, and this adherence creates significant tension (approximately 5 mN/m) in the bilayer (the bulk lipid lining the hole in the support is known as the Plateau-Gibbs border[91,92], Figure 4). No one has made a measurement of channel activity in an unstressed planar bilayer[66,93], and for MSCs, this is a serious bias. The bilayer experiments of Coste et al[66] on the PIEZO channels suffer from this bias. If one is working with channels that inactivate, the resting tension in membranes, either in planar bilayers or patches, can put channels in an inactivated state making them invisible to a patch recording, and making a reconstitution experiment appear to have failed[18,43,48,94,95].
There is one method, not currently in use, that might solve some of these problems. If the channels are reconstituted into large lipid vesicles (approximately 10 μm in diameter), and the vesicles are patched in “whole-cell-mode” then most of the membrane is not in contact with glass. By controlling the pressure in the vesicle which is large enough to measure accurately the radius of curvature, Laplace’s law will provide reliable estimates of the mean tension and there won’t be significant tension gradients.
While lipid membranes are simple model systems, they are not cells. Cells are not homogenous solutions of macro molecules, but heterogeneous, anisotropic, viscoelastic/plastic structures made of multiple proteins and lipids with an extracellular matrix[43] that can carry stress between the exterior and the interior of the cells. But let’s ignore these details for a moment and think of how we to experiment on cells.
The simplest stimulus is to poke a cell, usually with a fire polished pipette[93]. What does that do to MSCs? Imagine pressing a chopstick against a clump of Jello representing the cell interior. There is an indentation (more formally called a displacement) at the site of stimulation, but the amount of displacement decreases with distance from the site of stimulation. The stimulation is not uniform even at this macro level. This variation of stress/strain with distance occurs whether one stresses the cell with a pipette, a magnetic bead[96], a bead in a laser trap, an atomic force cantilever[97,98] or local perfusion[99]. It’s just a property of a deformable material[100]. When you record mechanically-induced currents from a cell, the response is represents a mean value from a distributed stress, and it decreases nonlinearly with distance but covers larger and larger areas[100]. The best you can hope for is that the response is proportional to the stimulus, and hopefully monotonic. Further complicating those assumptions is the fact that the cell is viscoelastic and plastic - local stresses change with time even with a constant stimulus. Modelling of channels in situ usually assume the channels are uniformly distributed, but this is a bad assumption since, in general, they aren’t (Figure 5). Remember to remain humble when interpreting your data since you really don’t know the details of the stimulus.
In case I haven’t yet scared you away from the field, let’s look more closely at real cells. We will stick with whole-cell recordings where the local effect of the pipette glass is not significant. The key problem is determining what the channel feels. The ability to reconstitute bacterial MSCs in lipids shows the channels respond to tension in the bilayer and don’t interact with a cytoskeleton, and that seems to apply to PIEZO MSCs as well[43]. What is the actual tension in the bilayer when MSCs are activated in cells? No one has measured it. The bilayer is supported by the cytoskeleton that shields the bilayer from excess stress (known as “mechanoprotection”[36,101-103]). One experiment dealt with the distribution of stresses between the cytoskeleton and the bilayer in patches of an human embryonic kidney (HEK) cell, it there it was about 50:50. The cytoskeleton can thus alter the stress in the bilayer. Defects in the cytoskeleton can lead to diseases like muscular dystrophy[104-112].
Laser trap measurements of bilayer tension in resting cells suggests that it is negligible[69,70,113-115]. That fits our common observation that MSCs are not active in resting cells (Figure 6)[53]. Why do cells make MSCs if they can’t be activated at normal stresses? Cortical stress is shared between the cytoskeleton and the bilayer, so bilayer stress reacts to cytoskeletal stress and vice versa, and these stresses are time dependent. The effective viscosity that makes responses time dependent arises from viscosity of the lipids and the dynamics of bonds in the cytoplasm[76,94,111,114,116-119]. The existence of connections between the bilayer and the cytoskeleton mean that any drugs that affect the cytoskeleton are likely to affect MSC activity, although drugs rarely are tested for these effects.
Adding to the complexity of defining the stimulus, lipids will flow under stress, and membrane lipids are not even homogeneous[46,76,116,120]. Spatial domains do exist[120-122] and physics tells us that the stress outside a domain is different from the stress inside a domain[73-75,123]. The energy gradient of stress at the edge of a domain is known as line tension. That affects the force inside the domain relative to that outside[75]. While we don’t know in detail the stimulus at an MSC, we are safe in assuming the stress is greater than zero and less than the lytic limit of the bilayer.
If the transmembrane domains of a channel are thicker or thinner than the surrounding bilayer, the bilayer will bend at the boundary and those stresses are likely to modify MSC activity[124]. This is termed a hydrophobic mismatch, but the local curvature does not extend more than a few lipids from the channel[90,125-127]. However, amphipaths can dissolve in the membrane[128-131] and interact locally with the channel modifying the local stress and affecting channel gating. For example, the general anesthetics at clinically relevant concentrations cause opening of two-pore domain K+ selective TREK-1 channels[132]. Opening these channels hyperpolarizes neurons possibly accounting for general anesthesia. The presence of these channels may explain why people can be knocked out by a blow to the head.
Suppose instead of these local mechanical stimuli we try for a more uniform stimulus like hypotonic stress? Cells swell with hypotonicity and we have been taught that swelling will stretch the membrane. If cells were spherical objects with a fluid cortex like red cells, that could work, but nucleated cells are filled with cross linked gels and the gels are what store most of the osmotic stress[133]. Consider the basic mechanics. Cells are not spherical so there are forces normal to the membrane. Secondly, with a given pressure across the membrane, the tension will depend upon the local radius of curvature (according to Laplace’s law), and cells do not have uniform curvature. But a more serious problem is that nucleated cells have a cytoskeleton that acts like a sponge, a three dimensional object that fills the cell volume. The mechanics of three dimensional objects are different[134] from those of two dimensional objects like membranes[98].
We found that osmotic swelling doesn’t make the membrane tense unless the cytoskeleton is disrupted[98], contrary to my intuition and years of textbook dogma. In fact, swelling tends to make cells softer[98]! How can that be? It turns out that everyone has done the experiment. When we pick up a dry kitchen sponge it is stiff. If we put it in the sink, it swells and soaks up water and it becomes softer. What is magic about a sponge? Nothing. It is just a set of cross-linked wettable polymers just like the cytoskeleton[26,135,136], and cells presumably can move water the same way without the need to move solutes. The cell membrane still remains the rate limiting step for water movement, but most of the energy from an osmotic gradient is in the cytoplasm and not in the membrane[133].
We visualized the distribution of osmotic stress in the cytoplasm using genetically coded optical stress sensors placed in structural proteins[26,27,29,30,119,137-139]. This three dimensional cross linked structure allows cells to withstand huge osmotic pressures[69]. (Ask yourself why sponges don’t lyse.) Many cells, like bovine endothelial cells (BAECs), can withstand distilled water for hours and remain viable. The predicted osmotic (hydrostatic) pressure due to exposure of cells to distilled water is about 7 Atm, twice the pressure in a car tire. The cell’s stability under this huge gradient arises because the cell interior is glued together like a sponge. In the case of BAECs, this bonding allows the cells to face severe viscous drag in arteries where blood flow tries to rip the apical cortex from the basal cortex[27,140,141]. Osmotic stress does not stress the cell membrane very much, and despite many citations in the literature, osmotic stress should not be considered a “mechanical stimulus” of the cell membrane. Do not accept the results of papers that claim it is. Instead, treat those papers literally as dealing with the effects of osmotic stress.
There are a vast number of papers on cell volume regulation[55,98,142-147] invoking various ion channels such as the BK channels[148] and other K+ channels[149], chloride channels[147,150-152] as well as neutral transporters[143,153,154] and water transporters[155-157] as well as the cytoskeleton[26-29,98,139,158] and host of calcium and other intracellular messengers[15,159-161]. Given the vast scale of modulators and potential effectors, it is unwise to think of cell volume as a specific stimulus.
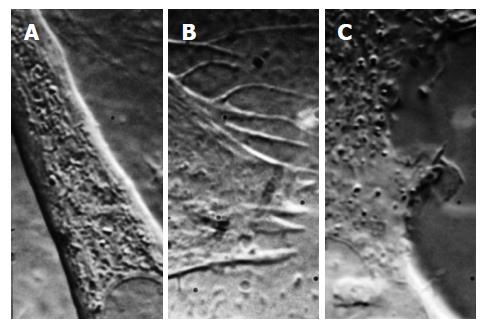
We all know about patch clamp recording and the revolution it created in our understanding of ion channels[162,163]. But what is a patch? The dogma says it is a bilayer containing channels[163] that spans the pipette. However, unless you are working with lipid vesicles, that is incorrect; patches are pieces of the cell cortex. Microscopy of patches (light microscopy[44,164] and electron microscopy[1,165-168]) show that patches are samples of the cell cortex, including the cytoskeleton (Figure 7) [1,165].
Whenever you make a patch, cell-attached or excised, the bilayer that contains your channels shares its stress with the cytoskeleton. How much does the bilayer feel in this composite structure? In the only published paper on the matter[169], we compared the amount of mechanical stress required to break a patch (pipette suction) with the voltage required to break the patch (typical of patch clamp “zap” voltages). The mechanical stress measured the lytic stress of the entire cortex. Voltage measured only the stress of the bilayer since that is where the voltage drop occurs. We measured the voltage required to lyse a patch as a function of the mechanical stress; the more mechanical stress, the less voltage. Since voltage only exerts force on the bilayer, we could separate the bilayer stress from the mean stress. It turns out that the bilayer lyses with a constant energy density, whether it comes from mechanics or voltage. For our particular cells, HEK-293, about half of the applied stress was in the bilayer and the rest in the cytoskeleton, but that result is from patches and we do not know how that applies to resting cells.
Regardless of the degree of stress sharing, no one has ever measured channel currents in a patch that emulates the tension characteristic of a resting cell[50,76]. The magnitude of the resting stress in a patch was emphasized to us when we tried to use used Triton-X100 to lyse patches. It doesn’t work. The patches are stable. The reason is that detergents work by forming micelles. If you want to form a micelle in a patch under tension, you need to increase the membrane area since a plane plus a sphere has more area than the plane. The energy required to change the area of a membrane under tension T is ΔG = TΔA where ΔA is the change in area (Hooke’s law in two dimensions). The energy available to the detergent is insufficient to form a micelle, but the same detergent works well in the resting cell the patch because its membrane is not under tension.
Is the cytoskeleton of a patch the same as that in a cell? We don’t know, but we do know that the chemical composition of a patch is different from that of the cell it was taken from[44]. We labelled different components of cell membranes and then patched them. We found that some elements made it to the pipette-spanning dome, and some didn’t, notoriously the extracellular matrix[44]. That never even made it into the seal region. Some ion channels made it, and some didn’t. You need to think of the pipette as a silica column. Biochemists know proteins stick to silica, so after dragging a membrane up a pipette, some things will stick to the glass and get filtered out, and some will make it to the dome.
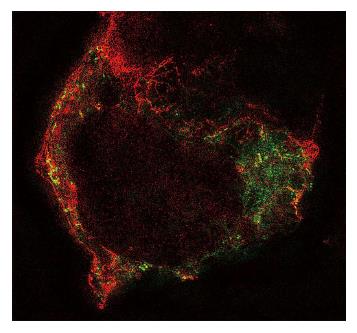
The heterogeneity of the patch emulates the heterogeneity of the cell membrane and we know that the cell membrane is not homogenous (Figure 8). Even pure bilayers may not be homogeneous. Like ice and water, there are phase separations[170]. The amount of each phase (the fraction of total membrane area) is modulated by internal and external conditions. Cell membranes are much more complicated. If you look at a time lapse movie of cells, you will be impressed by the motion of the cell surface. Imagine your patch pipette coming down on one of these cells and then try to figure out which piece of membrane you patched. The answer, of course, is that you have no idea. Furthermore, given the data showing changes in patch composition with patch formation[44], and visible domains in a patch, you are in fact recording from a new mixture of cellular components. We suspect that patches might contain membrane from the endoplasmic reticulum and other organelles as well as the plasmalemma. Someone needs to check on that. We think that patch clamp recordings are as reproducible as they are because the formation of the patch helps to homogenize the components. In any case, be cautious about assuming that the properties of currents you get from a patch are the same as you would observe in situ.
We know that most if not all types of cells have endogenous cationic MSCs[15,17,51,55,128,171-174]. You may not see them frequently as they are normally closed because bilayer stress is shielded by the cytoskeleton (“mechanoprotection”)[71,93]. You must know your background channel activity if you want to examine cells containing transfected channels. Treating the cells with cytochalasin or latrunculin to break up the cytoskeleton will reduce mechanoprotection and make background channels more visible[171]. Cell lines vary from lab to lab. According to the literature Coste et al[93] used N2A cells to clone PIEZO1, but Lee et al[71] used the same cell line and found no background PIEZO1 and 2 activity. Why? I expect that the cytoskeleton changes with passage number and with different batches of serum.
Because of the nearly universal presence of background channels, seeing a cationic MSC current after transfection does not mean you are seeing the channel coded by the DNA you used to transfect the cells. Furthermore, the expression of an MSC (or probably many other proteins) can cause massive structural changes in the cytoskeleton, even if the channel is nonconducting[175]. Thus, the process of transfection alone (not the effect of the transfection reagents, per se) can modify the forces that reach the channels.
We can now study cytoskeletal protein stress using genetically coded stress sensors[3,25,26,28-30,119,137-139,176]. The same issues apply to siRNA since suppression of one protein can affect others. For example, we showed that cytochalasin or colchicine affects the stress in actinin, spectrin and filamin and likely other structural proteins that are not judged to be the drug targets. When we modify any protein in a cell, we modify the stresses in the elements that are coupled to that protein.
Transfection can be a dangerous game. You can easily show modified RNA expression, we know that RNA expression is not cleanly related to the presence of functional channels. We cloned the human form of PIEZO1 and 2 from HEK cells[53], a human cell line of neural origin that usually exhibits little background MSC activity (Figure 6). The N2A cells that Coste et al[66] initially used to isolate PIEZO1, had no background MSC activity in other samples of the same cell line[71]. So how do we know what channels produced the currents we are looking at?
The best test would be to create a mutant channel with similar gating functions but with visibly different ionic selectivity than the endogenous channels. You cannot depend upon channel conductance alone[57] as a sound marker of expressed channels since it is easy to find situations in which the environment: cell-attached patch, inside-out or outside-out patch, or whole-cell, or planar bilayer have different conductances[66,177]. You would want a channel with big differences in selectivity, ideally a change from cation to anion selectivity!
You also cannot trust the channel kinetics as a marker since the kinetics of the channels depend upon their environment[177,178] and you have little control of that. Furthermore, you do not know if the channel you are trying to express might have a subunit that associates with an endogenous channel subunit or accessory protein, or induces the expression of previously unexpressed endogenous protein. It is well known that mechanical stress alters gene expression[179-182]. We know that expression of two different MSCs can create currents that do not belong to expression of either one alone[71]. There are no clean solutions in cells. We arbitrarily tend to look more closely at transfections that produce currents much larger than the background channels. We also don’t know that we are looking at homomers of the transfected proteins, but we often make the simplifying assumption they are (this also aids in grant funding). Expression of green fluorescent protein labelled channels on the cell surface[43,48] or in patch membranes[44] does not mean that the fluorescent object is a functional channel, simply that the protein is present. We have tested the mechanical sensitivity of labelled transient receptor potential-canonical channels in patches and found they are present but are not mechanically sensitive[183].
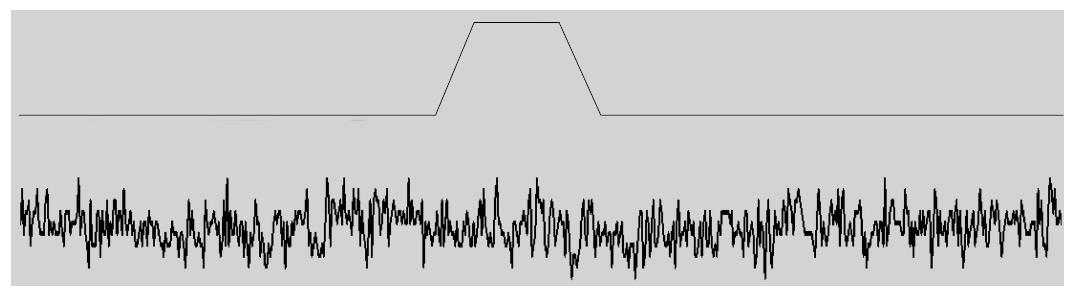
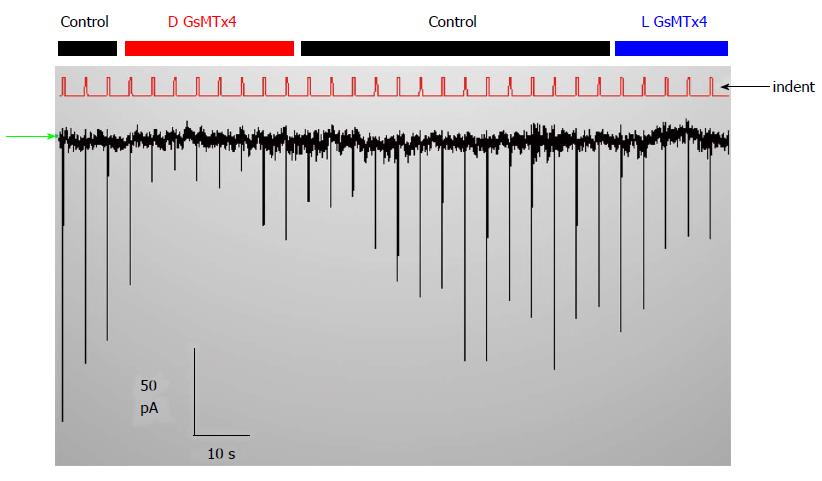
Cationic MSCs are normally protected from cell stress, so what do they do for a living? They do not seem to participate in behavior of normal hearts[184], but they do in stretched hearts where they seem to play a role in generating arrhythmias like atrial fibrillation[184]. They also play a role in muscular dystrophy where the channels produce a Ca2+ leak when the muscle is stretched[110,185]. We have come to believe that the typical cationic MSCs, like PIEZO1, serve primarily as sensors for potential bilayer failure. They would inform the cytoskeleton that the local bilayer is under excessive stress and likely to break, and the ion fluxes through the channel are signaling for mechanical reinforcement. The channels are functioning a bit like fire alarms whose function you unaware of until disaster looms. If the channels are closed in the resting cell you will not see the effect of inhibitory drugs like GsMtx4 on the currents (Figure 9). But if the same drug is active on open MSCs, you will see an effect, but to open the channels may require pathologic stress. Since channels like PIEZO inactivate, the information about the excess stress is transitory.
PIEZO1 mutations can cause anemias[43], and we have wondered why are these channels that inactivate quickly (< 30 ms) are present in red cells[186]. When does a red cell need such a short lived channel? We guess that the only time red cell stress becomes “pathological” is upon entry and exit from a capillary or perhaps a bifurcation. It might modify ion and water concentrations to reduce stress on the membrane as it is highly deformed upon entering the capillary. The same channels may be involved in sickle cell anemia where hemoglobin crystals push out on the membrane activating PIEZO1[15].
The universal presence of MSCs fits the common demand of all cells to avoid lysis and that occurs in disease. GsMtx4[187] and other agents that might act specifically on MSCs promise to be a new class of therapeutic agent with ideal selectivity they would only affect sick cells. We have found that GsMtx4 can be administered to mice daily for a month with no effect on behavior, and it can be injected into the CNS with no effect on behavior, but it does work to inhibit volume stimulated arrhythmias[188] and the phenotype of muscular dystrophy[109].
There is evidence that PIEZO2 channels may serve a sensory role in nociception[4]. Since PIEZO was only cloned a few years ago, we have a lot more work to do. A nagging problem is why is PIEZO so big[189] - it is the largest transmembrane protein (approximately 2500 amino acids) and even tends to form tetramers with a MW of about 106 with the N and C termini about 20-30 nm apart[190] making us suspicious that PIEZOs have other functions; a large size is not necessary for MSC function[43,67]. There are many kinds of MSCs[191-194], nearly a dozen in bacteria alone, so we have lots of interesting problems to keep us busy.
This review has two goals, nominally for investigators new to the field of mechanotransduction: (1) Be humble about your data because you generally don’t know your stimulus, and be explicit about your assumptions so people can read your paper properly. Quantitative models of the data have the intrinsic appeal of making the assumptions explicit; and (2) Create new preparations that can answer some of the pressing host of new questions.
This paper represents several decades of work and the contribution of dozens of people, too many to name here. Much of the data and the ideas in recent years come from Thomas Suchyna, Philip Gottlieb, Fanjie Meng, Chilman Bae, Radhakrishnan Gnanasambandam, Arthur Beyder, Chiara Spagnoli and Susan Hua. You can find many of the other collaborators in the cited papers.
P- Reviewer: Chen CC, Islas LD, Wu SN S- Editor: Gong XM L- Editor: A E- Editor: Jiao XK
| 1. | Delmas P, Hao J, Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat Rev Neurosci. 2011;12:139-153. [PubMed] |
| 2. | Gillespie PG, Müller U. Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell. 2009;139:33-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 328] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 3. | Krieg M, Dunn AR, Goodman MB. Mechanical control of the sense of touch by β-spectrin. Nat Cell Biol. 2014;16:224-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 4. | Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483:209-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 354] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 5. | Schmidt D, del Mármol J, MacKinnon R. Mechanistic basis for low threshold mechanosensitivity in voltage-dependent K+ channels. Proc Natl Acad Sci USA. 2012;109:10352-10357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Lolignier S, Eijkelkamp N, Wood JN. Mechanical allodynia. Pflugers Arch. 2015;467:133-139. [PubMed] |
| 7. | Hao J, Delmas P. Multiple desensitization mechanisms of mechanotransducer channels shape firing of mechanosensory neurons. J Neurosci. 2010;30:13384-13395. [PubMed] |
| 8. | Patel A, Sharif-Naeini R, Folgering JR, Bichet D, Duprat F, Honoré E. Canonical TRP channels and mechanotransduction: from physiology to disease states. Pflugers Arch. 2010;460:571-581. [PubMed] |
| 9. | Liu J, Schrank B, Waterston RH. Interaction between a putative mechanosensory membrane channel and a collagen. Science. 1996;273:361-364. [PubMed] |
| 10. | Stiber JA, Tang Y, Li T, Rosenberg PB. Cytoskeletal regulation of TRPC channels in the cardiorenal system. Curr Hypertens Rep. 2012;14:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Köhler R, Grundig A, Brakemeier S, Rothermund L, Distler A, Kreutz R, Hoyer J. Regulation of pressure-activated channel in intact vascular endothelium of stroke-prone spontaneously hypertensive rats. Am J Hypertens. 2001;14:716-721. [PubMed] |
| 12. | Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Delmas P, Patel A, Honoré E. Sensing pressure in the cardiovascular system: Gq-coupled mechanoreceptors and TRP channels. J Mol Cell Cardiol. 2010;48:83-89. [PubMed] |
| 13. | Gevaert T, Vandepitte J, Hutchings G, Vriens J, Nilius B, De Ridder D. TRPV1 is involved in stretch-evoked contractile changes in the rat autonomous bladder model: a study with piperine, a new TRPV1 agonist. Neurourol Urodyn. 2007;26:440-450; discussion 451-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Wang EC, Lee JM, Johnson JP, Kleyman TR, Bridges R, Apodaca G. Hydrostatic pressure-regulated ion transport in bladder uroepithelium. Am J Physiol Renal Physiol. 2003;285:F651-F663. [PubMed] |
| 15. | Vandorpe DH, Xu C, Shmukler BE, Otterbein LE, Trudel M, Sachs F, Gottlieb PA, Brugnara C, Alper SL. Hypoxia activates a Ca2+-permeable cation conductance sensitive to carbon monoxide and to GsMTx-4 in human and mouse sickle erythrocytes. PLoS One. 2010;5:e8732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Patel A, Demolombe S, Honoré E. An alternative to force. Elife. 2015;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Andolfo I, Alper SL, De Franceschi L, Auriemma C, Russo R, De Falco L, Vallefuoco F, Esposito MR, Vandorpe DH, Shmukler BE. Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood. 2013;121:3925-3935, S1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 244] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 18. | Sukharev S, Sachs F. Molecular force transduction by ion channels: diversity and unifying principles. J Cell Sci. 2012;125:3075-3083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Hennessey TM, Rucker WB, McDiarmid CG. Classical conditioning in paramecia. Animal Learning & Behavior. 1979;7:417-423. |
| 20. | Fujiu K, Nakayama Y, Iida H, Sokabe M, Yoshimura K. Mechanoreception in motile flagella of Chlamydomonas. Nat Cell Biol. 2011;13:630-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Tominaga T, Allen R. Electrophysiology of the in situ contractile vacuole complex of Paramecium reveals its membrane dynamics and electrogenic site during osmoregulatory activity. J Exp Biol. 1998;201:451-460. [PubMed] |
| 22. | Saimi Y, Martinac B, Gustin MC, Culbertson MR, Grinnell AD, Adler MR, Kung C. Ion channels of three microbes: Paramecium, Yeast and Escherichia Coli. Cold Spring Harbor Laboratory Press, 1988: 667-673. . |
| 23. | Schaefer WH, Hinrichsen RD, Burgess-Cassler A, Kung C, Blair IA, Watterson DM. A mutant Paramecium with a defective calcium-dependent potassium conductance has an altered calmodulin: a nonlethal selective alteration in calmodulin regulation. Proc Natl Acad Sci USA. 1987;84:3931-3935. [PubMed] |
| 24. | Martinac B. Bacterial mechanosensitive channels as a paradigm for mechanosensory transduction. Cell Physiol Biochem. 2011;28:1051-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Verma D, Ye N, Meng F, Sachs F, Rahimzadeh J, Hua SZ. Interplay between cytoskeletal stresses and cell adaptation under chronic flow. PLoS One. 2012;7:e44167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Meng F, Sachs F. Orientation-based FRET sensor for real-time imaging of cellular forces. J Cell Sci. 2012;125:743-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Meng F, Sachs F. Measuring strain of structural proteins in vivo in real time. Cardiac Mechano-Electric Coupling and Arrhythmia: From Pipette to Patient. Oxford: Oxford University Press 2011; 431-434. |
| 28. | Rahimzadeh J, Meng F, Sachs F, Wang J, Verma D, Hua SZ. Real-time observation of flow-induced cytoskeletal stress in living cells. Am J Physiol Cell Physiol. 2011;301:C646-C652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Wang Y, Meng F, Sachs F. Genetically encoded force sensors for measuring mechanical forces in proteins. Commun Integr Biol. 2011;4:385-390. [PubMed] |
| 30. | Meng F, Suchyna T, Sachs F. Real Time Detection of Mechanical Stress in Specific Cytoskeletal Proteins. Biophysical Journal. 2010;753A. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Gauthier NC, Fardin MA, Roca-Cusachs P, Sheetz MP. Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. Proc Natl Acad Sci USA. 2011;108:14467-14472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 275] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 32. | Rangamani P, Fardin MA, Xiong Y, Lipshtat A, Rossier O, Sheetz MP, Iyengar R. Signaling network triggers and membrane physical properties control the actin cytoskeleton-driven isotropic phase of cell spreading. Biophys J. 2011;100:845-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Xiong Y, Rangamani P, Fardin MA, Lipshtat A, Dubin-Thaler B, Rossier O, Sheetz MP, Iyengar R. Mechanisms controlling cell size and shape during isotropic cell spreading. Biophys J. 2010;98:2136-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Cai Y, Rossier O, Gauthier NC, Biais N, Fardin MA, Zhang X, Miller LW, Ladoux B, Cornish VW, Sheetz MP. Cytoskeletal coherence requires myosin-IIA contractility. J Cell Sci. 2010;123:413-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 35. | Moore SW, Roca-Cusachs P, Sheetz MP. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev Cell. 2010;19:194-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 317] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 36. | Glogauer M, Arora P, Chou D, Janmey PA, Downey GP, McCulloch CA. The role of actin-binding protein 280 in integrin-dependent mechanoprotection. J Biol Chem. 1998;273:1689-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 191] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 37. | Janmey PA, Hvidt S, Käs J, Lerche D, Maggs A, Sackmann E, Schliwa M, Stossel TP. The mechanical properties of actin gels. Elastic modulus and filament motions. J Biol Chem. 1994;269:32503-32513. [PubMed] |
| 38. | Morris CE. Why are so many ion channels mechanosensitive?. In: Sperelakis N, editor. Cell Physiology Source Book. Elsevier 2012; 493-505. |
| 39. | Morris CE. Voltage-gated channel mechanosensitivity: fact or friction? Front Physiol. 2011;2:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Johnson CP, Tang HY, Carag C, Speicher DW, Discher DE. Forced unfolding of proteins within cells. Science. 2007;317:663-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 276] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 41. | Suchyna T, Sachs F. The Membrane/Cytoskeleton Interface and Stretch-Activated Channels. Cardiac Mechano-Electric Coupling and Arrhythmia: From Pipette to Patient. Oxford, UK: Oxford University Press 2011; 57-65. |
| 42. | Martinac B. The ion channels to cytoskeleton connection as potential mechanism of mechanosensitivity. Biochim Biophys Acta. 2014;1838:682-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 43. | Huang H, Bae C, Sachs F, Suchyna TM. Caveolae regulation of mechanosensitive channel function in myotubes. PLoS One. 2013;8:e72894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Suchyna TM, Markin VS, Sachs F. Biophysics and structure of the patch and the gigaseal. Biophys J. 2009;97:738-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 45. | Suchyna TM, Sachs F. Mechanosensitive channel properties and membrane mechanics in mouse dystrophic myotubes. J Physiol. 2007;581:369-387. [PubMed] |
| 46. | Martinac B, Nomura T, Chi G, Petrov E, Rohde PR, Battle AR, Foo A, Constantine M, Rothnagel R, Carne S. Bacterial mechanosensitive channels: models for studying mechanosensory transduction. Antioxid Redox Signal. 2014;20:952-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Nomura T, Cranfield CG, Deplazes E, Owen DM, Macmillan A, Battle AR, Constantine M, Sokabe M, Martinac B. Differential effects of lipids and lyso-lipids on the mechanosensitivity of the mechanosensitive channels MscL and MscS. Proc Natl Acad Sci USA. 2012;109:8770-8775. [PubMed] |
| 48. | Bae C, Gottlieb PA, Sachs F. Human PIEZO1: removing inactivation. Biophys J. 2013;105:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 49. | Bae C, Gnanasambandam R, Nicolai C, Sachs F, Gottlieb PA. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc Natl Acad Sci USA. 2013;110:E1162-E1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 234] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 50. | Moe P, Blount P. Assessment of potential stimuli for mechano-dependent gating of MscL: effects of pressure, tension, and lipid headgroups. Biochemistry. 2005;44:12239-12244. [PubMed] |
| 51. | Ostrow LW, Suchyna TM, Sachs F. Stretch induced endothelin-1 secretion by adult rat astrocytes involves calcium influx via stretch-activated ion channels (SACs). Biochem Biophys Res Commun. 2011;410:81-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Kamaraju K, Gottlieb PA, Sachs F, Sukharev S. Effects of GsMTx4 on bacterial mechanosensitive channels in inside-out patches from giant spheroplasts. Biophys J. 2010;99:2870-2878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry. 2011;50:6295-6300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 427] [Cited by in RCA: 391] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 54. | Gottlieb PA, Barone T, Sachs F, Plunkett R. Neurite outgrowth from PC12 cells is enhanced by an inhibitor of mechanical channels. Neurosci Lett. 2010;481:115-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Hua SZ, Gottlieb PA, Heo J, Sachs F. A mechanosensitive ion channel regulating cell volume. Am J Physiol Cell Physiol. 2010;298:C1424-C1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Posokhov YO, Gottlieb PA, Morales MJ, Sachs F, Ladokhin AS. Is lipid bilayer binding a common property of inhibitor cysteine knot ion-channel blockers? Biophys J. 2007;93:L20-L22. [PubMed] |
| 57. | Gnanasambandam R, Bae C, Gottlieb PA, Sachs F. Ionic Selectivity and Permeation Properties of Human PIEZO1 Channels. PLoS One. 2015;10:e0125503. [PubMed] |
| 58. | Suffoletto K, Ye N, Meng F, Verma D, Hua SZ. Intracellular forces during guided cell growth on micropatterns using FRET measurement. J Biomech. 2015;48:627-635. [PubMed] |
| 59. | Slavchov RI, Sachs F, Martinac B, Sokabe M, Nomura T. Gigaseal Mechanics/Creep motion of gigaseal under the action of pressure gradient, adhesion and voltage. J Chem Phys. 2015;In press. |
| 60. | Friedrich O, Wagner S, Battle AR, Schürmann S, Martinac B. Mechano-regulation of the beating heart at the cellular level--mechanosensitive channels in normal and diseased heart. Prog Biophys Mol Biol. 2012;110:226-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | Zhou HX. Crowding effects of membrane proteins. J Phys Chem B. 2009;113:7995-8005. [PubMed] |
| 62. | Kumánovics A, Levin G, Blount P. Family ties of gated pores: evolution of the sensor module. FASEB J. 2002;16:1623-1629. [PubMed] |
| 63. | Okada K, Moe PC, Blount P. Functional design of bacterial mechanosensitive channels. Comparisons and contrasts illuminated by random mutagenesis. J Biol Chem. 2002;277:27682-27688. [PubMed] |
| 64. | Grage SL, Keleshian AM, Turdzeladze T, Battle AR, Tay WC, May RP, Holt SA, Contera SA, Haertlein M, Moulin M. Bilayer-mediated clustering and functional interaction of MscL channels. Biophys J. 2011;100:1252-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 65. | Martinac B, Rohde PR, Battle AR, Petrov E, Pal P, Foo AF, Vásquez V, Huynh T, Kloda A. Studying mechanosensitive ion channels using liposomes. Methods Mol Biol. 2010;606:31-53. [PubMed] |
| 66. | Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 635] [Cited by in RCA: 798] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 67. | Honoré E, Patel AJ, Chemin J, Suchyna T, Sachs F. Desensitization of mechano-gated K2P channels. Proc Natl Acad Sci USA. 2006;103:6859-6864. [PubMed] |
| 68. | Bae C, Markin V, Suchyna T, Sachs F. Modeling ion channels in the gigaseal. Biophys J. 2011;101:2645-2651. [PubMed] |
| 69. | Dai J, Sheetz MP, Wan X, Morris CE. Membrane tension in swelling and shrinking molluscan neurons. J Neurosci. 1998;18:6681-6692. [PubMed] |
| 70. | Sheetz MP, Dai J. Modulation of membrane dynamics and cell motility by membrane tension. Trends Cell Biol. 1996;6:85-89. [PubMed] |
| 71. | Lee W, Leddy HA, Chen Y, Lee SH, Zelenski NA, McNulty AL, Wu J, Beicker KN, Coles J, Zauscher S. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci USA. 2014;111:E5114-E5122. [PubMed] |
| 72. | Park EJ, Park J, Song HS, Kim SJ, Jung KC, Kim SM, Cho DG, Kim D, Park KS, Hong S. Nanovesicle-based platform for the electrophysiological monitoring of aquaporin-4 and the real-time detection of its antibody. Biosens Bioelectron. 2014;61:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 73. | Markin VS, Sachs F. Thermodynamics of mechanosensitivity. Mechanosensitive Ion Channels (Part A). 2007;58:87-119. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Wiggins P, Phillips R. Membrane-protein interactions in mechanosensitive channels. Biophys J. 2005;88:880-902. [PubMed] |
| 76. | Ursell T, Agrawal A, Phillips R. Lipid bilayer mechanics in a pipette with glass-bilayer adhesion. Biophys J. 2011;101:1913-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 77. | Wiggins P, Phillips R. Analytic models for mechanotransduction: gating a mechanosensitive channel. Proc Natl Acad Sci USA. 2004;101:4071-4076. [PubMed] |
| 78. | Evans E, Yeung A. Hidden dynamics in rapid changes of bilayer shape. Chem Phys Lipids. 1994;73:39-56. [RCA] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 237] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 81. | Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515:279-282. [PubMed] |
| 82. | Evans EA. Constitutive relation for red cell membrane. Correction. Biophys J. 1976;16:597-600. [PubMed] |
| 83. | Evans E, Needham D. Physical properties of surfactant bilayer membranes: thermal transitions, elasticity, rigidity, cohesion, and colloidal interactions. J Phys Chem. 1987;91:4219-4228. [RCA] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 500] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 84. | Suchyna TM, Besch SR, Sachs F. Dynamic regulation of mechanosensitive channels: capacitance used to monitor patch tension in real time. Phys Biol. 2004;1:1-18. [PubMed] |
| 85. | Sachs F. Stretch-activated ion channels: what are they? Physiology (Bethesda). 2010;25:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 86. | Poudel KR, Keller DJ, Brozik JA. Single particle tracking reveals corralling of a transmembrane protein in a double-cushioned lipid bilayer assembly. Langmuir. 2011;27:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 87. | Steltenkamp S, Müller MM, Deserno M, Hennesthal C, Steinem C, Janshoff A. Mechanical properties of pore-spanning lipid bilayers probed by atomic force microscopy. Biophys J. 2006;91:217-226. [PubMed] |
| 88. | Parthasarathy R, Groves JT. Protein patterns at lipid bilayer junctions. Proc Natl Acad Sci USA. 2004;101:12798-12803. [PubMed] |
| 89. | Borisenko V, Lougheed T, Hesse J, Füreder-Kitzmüller E, Fertig N, Behrends JC, Woolley GA, Schütz GJ. Simultaneous optical and electrical recording of single gramicidin channels. Biophys J. 2003;84:612-622. [PubMed] |
| 90. | Hwang TC, Koeppe RE, Andersen OS. Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry. 2003;42:13646-13658. [PubMed] |
| 91. | Needham D, Haydon DA. Tensions and free energies of formation of “solventless” lipid bilayers. Measurement of high contact angles. Biophys J. 1983;41:251-257. [PubMed] |
| 92. | Dimitrov DS. A hydrodynamic theory of bilayer membrane formation. Biophys J. 1981;36:21-25. [PubMed] |
| 93. | Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2492] [Cited by in RCA: 2140] [Article Influence: 142.7] [Reference Citation Analysis (0)] |
| 94. | Belyy V, Kamaraju K, Akitake B, Anishkin A, Sukharev S. Adaptive behavior of bacterial mechanosensitive channels is coupled to membrane mechanics. J Gen Physiol. 2010;135:641-652. [PubMed] |
| 95. | Belyy V, Anishkin A, Kamaraju K, Liu N, Sukharev S. The tension-transmitting ‘clutch’ in the mechanosensitive channel MscS. Nat Struct Mol Biol. 2010;17:451-458. [PubMed] |
| 96. | Fujiwara K. Platelet endothelial cell adhesion molecule-1 and mechanotransduction in vascular endothelial cells. J Intern Med. 2006;259:373-380. [PubMed] |
| 97. | Beyder A, Sachs F. Electromechanical coupling in the membranes of Shaker-transfected HEK cells. Proc Natl Acad Sci USA. 2009;106:6626-6631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 98. | Spagnoli C, Beyder A, Besch S, Sachs F. Atomic force microscopy analysis of cell volume regulation. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;78:031916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 99. | Sánchez D, Anand U, Gorelik J, Benham CD, Bountra C, Lab M, Klenerman D, Birch R, Anand P, Korchev Y. Localized and non-contact mechanical stimulation of dorsal root ganglion sensory neurons using scanning ion conductance microscopy. J Neurosci Methods. 2007;159:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 100. | Johnson KL. Contact Mechanics. 1999 th ed. Cambridge, UK: Cambridge University Press, 1985. . |
| 101. | Gu CX, Juranka PF, Morris CE. Stretch-activation and stretch-inactivation of Shaker-IR, a voltage-gated K+ channel. Biophys J. 2001;80:2678-2693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 102. | Morris CE. Mechanoprotection of the plasma membrane in neurons and other non-erythroid cells by the spectrin-based membrane skeleton. Cell Mol Biol Lett. 2001;6:703-720. [PubMed] |
| 103. | Peyronnet R, Sharif-Naeini R, Folgering JH, Arhatte M, Jodar M, El Boustany C, Gallian C, Tauc M, Duranton C, Rubera I. Mechanoprotection by polycystins against apoptosis is mediated through the opening of stretch-activated K(2P) channels. Cell Rep. 2012;1:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 104. | Allen DG, Whitehead NP. Duchenne muscular dystrophy--what causes the increased membrane permeability in skeletal muscle? Int J Biochem Cell Biol. 2011;43:290-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 105. | Allen DG, Zhang BT, Whitehead NP. Stretch-induced membrane damage in muscle: comparison of wild-type and mdx mice. Adv Exp Med Biol. 2010;682:297-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 106. | Mokri B, Engel AG. Duchenne dystrophy: electron microscopic findings pointing to a basic or early abnormality in the plasma membrane of the muscle fiber. 1975. Neurology. 1998;51:1 and 10 pages following. [PubMed] |
| 107. | Hoffman EP, Kunkel LM. Dystrophin abnormalities in Duchenne/Becker muscular dystrophy. Neuron. 1989;2:1019-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 243] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 108. | Hurko O, Johns DR. Dystrophin and Duchenne’s muscular dystrophy. N Engl J Med. 1989;321:398-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 109. | Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol. 2005;562:367-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 110. | Whitehead NP, Streamer M, Lusambili LI, Sachs F, Allen DG. Streptomycin reduces stretch-induced membrane permeability in muscles from mdx mice. Neuromuscul Disord. 2006;16:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 111. | Jiang L, Yang C, Zhao L, Zheng Q. Stress fiber response to mechanics: a free energy dependent statistical model. Soft Matter. 2014;10:4603-4608. [PubMed] |
| 112. | Khairallah RJ, Shi G, Sbrana F, Prosser BL, Borroto C, Mazaitis MJ, Hoffman EP, Mahurkar A, Sachs F, Sun Y. Microtubules underlie dysfunction in duchenne muscular dystrophy. Sci Signal. 2012;5:ra56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 113. | Khatibzadeh N, Spector AA, Brownell WE, Anvari B. Effects of plasma membrane cholesterol level and cytoskeleton F-actin on cell protrusion mechanics. PLoS One. 2013;8:e57147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 114. | Lau C, Brownell WE, Spector AA. Internal forces, tension and energy density in tethered cellular membranes. J Biomech. 2012;45:1328-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 115. | Khatibzadeh N, Gupta S, Farrell B, Brownell WE, Anvari B. Effects of cholesterol on nano-mechanical properties of the living cell plasma membrane. Soft Matter. 2012;8:8350-8360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 116. | Khatibzadeh N, Spector AA, Brownell WE, Anvari B. Rate-dependent dynamics of cellular membranes probed by laser tweezers and optical displacement sensing. In: Larin KV, Sampson DD, editors. Optical Elastography and Tissue Biomechanics 2014; . |
| 117. | Sachs F, Brownell WE, Petrov AG. Membrane Electromechanics in Biology, with a Focus on Hearing. MRS Bull. 2009;34:665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 118. | White CR, Frangos JA. The shear stress of it all: the cell membrane and mechanochemical transduction. Philos Trans R Soc Lond B Biol Sci. 2007;362:1459-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 119. | Guo J, Wang Y, Sachs F, Meng F. Actin stress in cell reprogramming. Proc Natl Acad Sci USA. 2014;111:E5252-E5261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 120. | Rawicz W, Smith BA, McIntosh TJ, Simon SA, Evans E. Elasticity, strength, and water permeability of bilayers that contain raft microdomain-forming lipids. Biophys J. 2008;94:4725-4736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 121. | Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1343] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 122. | Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 792] [Cited by in RCA: 769] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 123. | Markin VS, Sachs F. Thermodynamics of mechanosensitivity: lipid shape, membrane deformation and anesthesia. Biophys J. 2004;86:370A. [RCA] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 124. | Izumi C, Bird JE, Iwasa KH. Membrane thickness sensitivity of prestin orthologs: the evolution of a piezoelectric protein. Biophys J. 2011;100:2614-2622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 125. | Andersen OS, Nielsen C, Maer AM, Lundbaek JA, Goulian M, Koeppe RE. Ion channels as tools to monitor lipid bilayer-membrane protein interactions: gramicidin channels as molecular force transducers. Methods Enzymol. 1999;294:208-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 126. | Lundbaek JA, Andersen OS. Spring constants for channel-induced lipid bilayer deformations. Estimates using gramicidin channels. Biophys J. 1999;76:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 151] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 127. | Goforth RL, Chi AK, Greathouse DV, Providence LL, Koeppe RE, Andersen OS. Hydrophobic coupling of lipid bilayer energetics to channel function. J Gen Physiol. 2003;121:477-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 128. | Suchyna TM, Tape SE, Koeppe RE, Andersen OS, Sachs F, Gottlieb PA. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 2004;430:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 243] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 129. | Qi Z, Chi S, Su X, Naruse K, Sokabe M. Activation of a mechanosensitive BK channel by membrane stress created with amphipaths. Mol Membr Biol. 2005;22:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 130. | Sokabe M, Hasegawa N, Yamanori K. Blockers and activators for stretch-activated ion channels of chick skeletal muscle. Annals of the New York Academy of Sciences. 1993;707:417-420. |
| 131. | Martinac B, Adler J, Kung C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 1990;348:261-263. [PubMed] |
| 132. | Patel AJ, Honoré E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422-426. [PubMed] |
| 133. | Sachs F, Sivaselvan MV. Cell volume control in three dimensions: Water movement without solute movement. J Gen Physiol. 2015;145:373-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 134. | Wang N, Ingber DE. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys J. 1994;66:2181-2189. [PubMed] |
| 135. | Trichet L, Le Digabel J, Hawkins RJ, Vedula SR, Gupta M, Ribrault C, Hersen P, Voituriez R, Ladoux B. Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. Proc Natl Acad Sci USA. 2012;109:6933-6938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 364] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 136. | McCain ML, Parker KK. Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflugers Arch. 2011;462:89-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 137. | Meng F, Suchyna TM, Lazakovitch E, Gronostajski RM, Sachs F. Real Time FRET Based Detection of Mechanical Stress in Cytoskeletal and Extracellular Matrix Proteins. Cell Mol Bioeng. 2011;4:148-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 138. | Meng F, Sachs F. Visualizing dynamic cytoplasmic forces with a compliance-matched FRET sensor. J Cell Sci. 2011;124:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 139. | Meng F, Suchyna TM, Sachs F. A fluorescence energy transfer-based mechanical stress sensor for specific proteins in situ. FEBS J. 2008;275:3072-3087. [PubMed] |
| 140. | Niggel J, Sigurdson W, Sachs F. Mechanically induced calcium movements in astrocytes, bovine aortic endothelial cells and C6 glioma cells. J Membr Biol. 2000;174:121-134. [PubMed] |
| 141. | Girard PR, Nerem RM. Shear stress modulates endothelial cell morphology and F-actin organization through the regulation of focal adhesion-associated proteins. J Cell Physiol. 1995;163:179-193. [PubMed] |
| 142. | Kowalsky GB, Beam D, Oh MJ, Sachs F, Hua SZ, Levitan I. Cholesterol depletion facilitates recovery from hypotonic cell swelling in CHO cells. Cell Physiol Biochem. 2011;28:1247-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 143. | Kahle KT, Simard JM, Staley KJ, Nahed BV, Jones PS, Sun D. Molecular mechanisms of ischemic cerebral edema: role of electroneutral ion transport. Physiology (Bethesda). 2009;24:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 144. | Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 482] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 145. | Bortner CD, Cidlowski JA. The role of apoptotic volume decrease and ionic homeostasis in the activation and repression of apoptosis. Pflugers Arch. 2004;448:313-318. [PubMed] |
| 146. | Parkerson KA, Sontheimer H. Biophysical and pharmacological characterization of hypotonically activated chloride currents in cortical astrocytes. Glia. 2004;46:419-436. [PubMed] |
| 147. | Petrunkina AM, Harrison RA, Ekhlasi-Hundrieser M, Töpfer-Petersen E. Role of volume-stimulated osmolyte and anion channels in volume regulation by mammalian sperm. Mol Hum Reprod. 2004;10:815-823. [PubMed] |
| 148. | Sheu SJ, Wu SN, Hu DN, Chen JF. The influence of hypotonicity on large-conductance calcium-activated potassium channels in human retinal pigment epithelial cells. J Ocul Pharmacol Ther. 2004;20:563-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 149. | Hammami S, Willumsen NJ, Olsen HL, Morera FJ, Latorre R, Klaerke DA. Cell volume and membrane stretch independently control K+ channel activity. J Physiol. 2009;587:2225-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 150. | Klausen TK, Hougaard C, Hoffmann EK, Pedersen SF. Cholesterol modulates the volume-regulated anion current in Ehrlich-Lettre ascites cells via effects on Rho and F-actin. Am J Physiol Cell Physiol. 2006;291:C757-C771. [PubMed] |
| 151. | Parkerson KA, Sontheimer H. Contribution of chloride channels to volume regulation of cortical astrocytes. Am J Physiol Cell Physiol. 2003;284:C1460-C1467. [PubMed] |
| 152. | Sardini A, Amey JS, Weylandt KH, Nobles M, Valverde MA, Higgins CF. Cell volume regulation and swelling-activated chloride channels. Biochim Biophys Acta. 2003;1618:153-162. [PubMed] |
| 153. | Kirk K, Strange K. Functional properties and physiological roles of organic solute channels. Annu Rev Physiol. 1998;60:719-739. [PubMed] |
| 154. | Cala PM, Mandel LJ, Murphy E. Volume regulation by Amphiuma red blood cells: cytosolic free Ca and alkali metal-H exchange. Am J Physiol. 1986;250:C423-C429. [PubMed] |
| 155. | Heo J, Meng F, Hua SZ. Contribution of aquaporins to cellular water transport observed by a microfluidic cell volume sensor. Anal Chem. 2008;80:6974-6980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 156. | Verkman AS. Role of aquaporin water channels in eye function. Exp Eye Res. 2003;76:137-143. [PubMed] |
| 157. | Acher R. [Water homeostasis in the living: molecular organization, osmoregulatory reflexes and evolution]. Ann Endocrinol (Paris). 2002;63:197-218. [PubMed] |
| 158. | Zehnder SM, Suaris M, Bellaire MM, Angelini TE. Cell Volume Fluctuations in MDCK Monolayers. Biophys J. 2015;4:247-250. [RCA] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 159. | Hoffmann EK, Holm NB, Lambert IH. Functions of volume-sensitive and calcium-activated chloride channels. IUBMB Life. 2014;66:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 160. | Straub RH. Interaction of the endocrine system with inflammation: a function of energy and volume regulation. Arthritis Res Ther. 2014;16:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 161. | Aleu J, Martín-Satué M, Navarro P, Pérez de Lara I, Bahima L, Marsal J, Solsona C. Release of ATP induced by hypertonic solutions in Xenopus oocytes. J Physiol. 2003;547:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 162. | Sakmann B, Neher E. Single channel recording. New York: Plenum 1995; . |
| 163. | Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12857] [Cited by in RCA: 14047] [Article Influence: 319.3] [Reference Citation Analysis (0)] |
| 164. | Sokabe M, Sachs F, Jing ZQ. Quantitative video microscopy of patch clamped membranes stress, strain, capacitance, and stretch channel activation. Biophys J. 1991;59:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 182] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 165. | McEwen BF, Song MJ, Ruknudin A, Barnard DP, Frank J, Sachs F. Tomographic three dimensional reconstruction of patch clamped membranes imaged with the high voltage electron microscope. XII International Conference of Electron Microscopy; 1990: 522-523. . |
| 166. | Ruknudin A, Song M, Sachs F. The ultrastructure of patch-clamped membranes: a study using high voltage electron microscopy. J Cell Biol. 1991;112:125-134. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 167. | Jung F, Song MJ, Sachs F. Patch clamp anatomy: high voltage electron microscopy of in vivo patches. Biophys J. 1987;51:517A. |
| 168. | Sachs F, Song M. High-voltage electron microscopy of patch-clamped membranes. Proceeding in Electron Microscopic Society of America. 1987;45:582-583. |
| 169. | Akinlaja J, Sachs F. The breakdown of cell membranes by electrical and mechanical stress. Biophys J. 1998;75:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 170. | Baumgart T, Hess ST, Webb WW. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 2003;425:821-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1124] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 171. | Yang XC, Sachs F. Characterization of stretch-activated ion channels in Xenopus oocytes. J Physiol. 1990;431:103-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 173. | Ostrow LW, Sachs F. Mechanosensation and endothelin in astrocytes--hypothetical roles in CNS pathophysiology. Brain Res Brain Res Rev. 2005;48:488-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 174. | Ding JP, Salvi RJ, Sachs F. Stretch-activated ion channels in guinea pig outer hair cells. Hear Res. 1991;56:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 175. | Lauritzen I, Chemin J, Honoré E, Jodar M, Guy N, Lazdunski M, Jane Patel A. Cross-talk between the mechano-gated K2P channel TREK-1 and the actin cytoskeleton. EMBO Rep. 2005;6:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 176. | Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1328] [Cited by in RCA: 1134] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 177. | Gottlieb PA, Bae C, Sachs F. Gating the mechanical channel Piezo1: a comparison between whole-cell and patch recording. Channels (Austin). 2012;6:282-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 178. | Schmidt D, MacKinnon R. Voltage-dependent K+ channel gating and voltage sensor toxin sensitivity depend on the mechanical state of the lipid membrane. Proc Natl Acad Sci USA. 2008;105:19276-19281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 179. | Suzuki R, Nemoto E, Shimauchi H. Cyclic tensile force up-regulates BMP-2 expression through MAP kinase and COX-2/PGE2 signaling pathways in human periodontal ligament cells. Exp Cell Res. 2014;323:232-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 180. | Henstock JR, Rotherham M, Rose JB, El Haj AJ. Cyclic hydrostatic pressure stimulates enhanced bone development in the foetal chick femur in vitro. Bone. 2013;53:468-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 181. | Grenon SM, Jeanne M, Aguado-Zuniga J, Conte MS, Hughes-Fulford M. Effects of gravitational mechanical unloading in endothelial cells: association between caveolins, inflammation and adhesion molecules. Sci Rep. 2013;3:1494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 182. | Maeda E, Sugimoto M, Ohashi T. Cytoskeletal tension modulates MMP-1 gene expression from tenocytes on micropillar substrates. J Biomech. 2013;46:991-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 183. | Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflugers Arch. 2008;455:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 184. | Sachs F. Heart Mechanoelectric Transduction. Cardiac Electrophysiology; From Cell to Bedside. Philadelphia: Saunders (Elsevier) 2004; 96-102. |
| 185. | Bhatnagar S, Kumar A. Therapeutic targeting of signaling pathways in muscular dystrophy. J Mol Med (Berl). 2010;88:155-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 186. | Cahalan SM, Lukacs V, Ranade SS, Chien S, Bandell M, Patapoutian A. Piezo1 links mechanical forces to red blood cell volume. Elife. 2015;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 431] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 187. | Bowman CL, Gottlieb PA, Suchyna TM, Murphy YK, Sachs F. Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology. Toxicon. 2007;49:249-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 188. | Bode F, Sachs F, Franz MR. Tarantula peptide inhibits atrial fibrillation. Nature. 2001;409:35-36. [PubMed] |
| 189. | Volkers L, Mechioukhi Y, Coste B. Piezo channels: from structure to function. Pflugers Arch. 2015;467:95-99. [PubMed] |
| 190. | Wang Y, Cai E, Rosenkranz T, Ge P, Teng KW, Lim SJ, Smith AM, Chung HJ, Sachs F, Green WN. Small quantum dots conjugated to nanobodies as immunofluorescence probes for nanometric microscopy. Bioconjug Chem. 2014;25:2205-2211. [PubMed] |
| 191. | Anishkin A, Kung C. Stiffened lipid platforms at molecular force foci. Proc Natl Acad Sci USA. 2013;110:4886-4892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 192. | Kung C, Martinac B, Sukharev S. Mechanosensitive Channels in Microbes. Annual Review of Microbiology. 2010;64:313-329. [RCA] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 193. | Loukin SH, Su Z, Kung C. Hypotonic shocks activate rat TRPV4 in yeast in the absence of polyunsaturated fatty acids. FEBS Lett. 2009;583:754-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 194. | Su Z, Zhou X, Loukin SH, Saimi Y, Kung C. Mechanical force and cytoplasmic Ca(2+) activate yeast TRPY1 in parallel. J Membr Biol. 2009;227:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |