Published online Dec 28, 2013. doi: 10.5316/wjn.v3.i4.148
Revised: September 16, 2013
Accepted: October 11, 2013
Published online: December 28, 2013
Processing time: 208 Days and 23.2 Hours
More than 40 CGG expansions in the 5’ noncoding region of the fragile X mental retardation 1 (FMR1) gene of the X chromosome give rise to several distinct clinical phenotypes, depending on the size of the expansion. First, more than 200 CGG expansions (full mutation) cause an inherited mental retardation called fragile X syndrome. Second, CGG expansions between 55 and 199 (premutation) cause a disorder called fragile X-associated tremor/ataxia syndrome (FXTAS) which typically includes intention tremor, ataxia and specific magnetic resonance imaging (MRI) findings. Indeed, it could develop parkinsonism although it usually shows features of postsynaptic parkinsonism. Finally, CGG expansions between 41 and 54 CGG (gray zone) are not consider normal but rarely develops abnormal neurological conditions. In this sense, the aim of this study is to report two atypical cases associated with CGG expansions of the FMR1 gene. First, a FMR1 premutation alleles carrier with an unusual phenotype, such as a presynaptic parkinsonism indistinguishable from Parkinson disease (PD) and a FMR1 gray zone alleles carrier presented with neurological features, namely hand tremor, parkinsonism and ataxia, usually described in FXTAS, as well as orthostatic tremor. We conclude that, on the one hand, FMR1 premutation alleles might cause two phenotypes of parkinsonism, such as a presynaptic phenotype, indistinguishable from PD, and a postsynaptic phenotype, associated with clinical features of FXTAS. On the other hand, although FMR1 gray zone alleles carriers were believed to have no abnormal neurological conditions, our study supports that they could develop FXTAS and other neurological disorders such as orthostatic tremor which has not been reported before associated with the FMR1 gene.
Core tip: In this study we report two atypical cases associated with CGG expansions of the fragile X mental retardation 1 (FMR1) gene. First, a FMR1 premutation alleles carrier presented with a parkinsonism indistinguishable from Parkinson disease. Second, a FMR1 gray zone alleles carrier presented with orthostatic tremor and neurological features associated with the fragile X-associated tremor/ataxia syndrome, such as hand tremor, parkinsonism and ataxia.
-
Citation: Peña E, Llanero M. Atypical neurological symptoms associated with CGG expansions of the
FMR1 gene. World J Neurol 2013; 3(4): 148-151 - URL: https://www.wjgnet.com/2218-6212/full/v3/i4/148.htm
- DOI: https://dx.doi.org/10.5316/wjn.v3.i4.148
More than 40 CGG expansions in the 5’ noncoding region of the fragile X mental retardation 1 (FMR1) gene of the X chromosome give rise to several distinct clinical phenotypes, depending on the size of the expansion. First, fragile X syndrome is an inherited mental retardation caused by more than 200 CGG expansions in the FMR1 gene (full mutation). Second, fragile X-associated tremor/ataxia syndrome (FXTAS) is a disorder described in premutation alleles carriers (55-199 CGG expansions) of the FMR1 gene. Typically, it includes intention tremor, ataxia and specific magnetic resonance imaging (MRI) findings, although the spectrum of neurological disorders associated with the FXTAS is increasing[1-3]. Finally, an interval of CGG expansions between 41 and 54 is known as gray zone which rarely develops abnormal neurological conditions. In this sense, the aim of this study is to report two atypical cases associated with CGG expansions of the FMR1 gene. First, a FMR1 premutation alleles carrier with an unusual phenotype, such as a parkinsonism indistinguishable from Parkinson disease (PD) and a FMR1 gray zone alleles carrier presented with neurological features, namely hand tremor, parkinsonism, ataxia and orthostatic tremor.
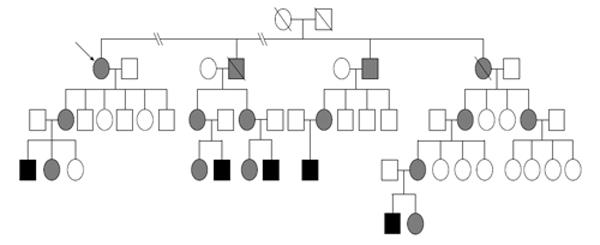
A 71-year-old woman presented with a 4-year history of progressive neurological disorder with tremor, bradykinesia and gait disorder. She was FMR1 alleles carrier (expansion of 82 CGG) with family history of fragile X syndrome (Figure 1). On examination, she had severe rest and postural tremor in her right hand and slight postural tremor in her left hand, severe bradykinesia and rigidity more marked on her right side, mild lost of postural stability and freezing. There was neither intention tremor nor ataxia. Indeed, patient did not show either autonomic dysfunction or Babinski sign or gaze palsy. Finally, She had significant improvement with 520 mg of levodopa (Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale I 8 II 17 III ON 42 OFF 60 IV 0). These clinical findings fulfilled the United Kingdom PD Society Brain Bank clinical diagnostic criteria for idiopathic PD[4].
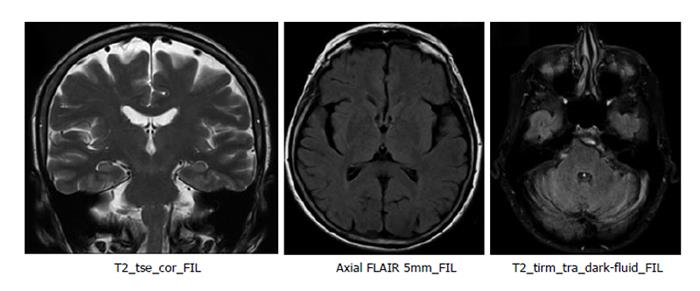
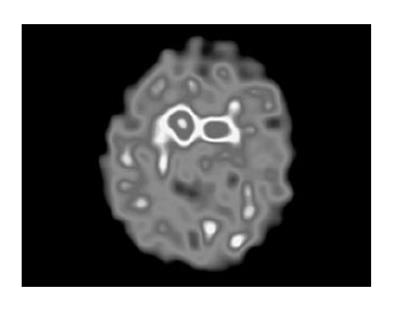
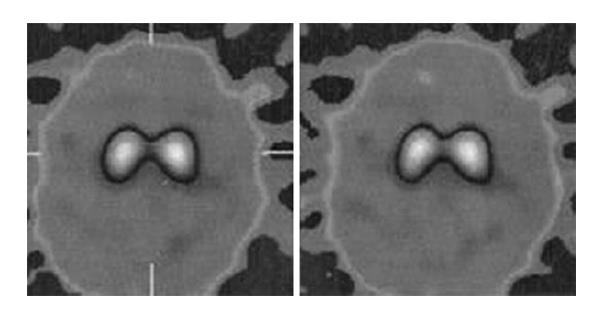
Blood tests and cranial MRI were normal (Figure 2). DAT SCAN showed an asymmetric decrease of the striatum tracer uptake more marked on the left side (Figure 3). Neuropsychological assessment did not show cognitive decline. However, it showed slight subcortical deficits (Table 1).
| Test | Subtest | Percentile |
| Orientation | Normal | |
| Attention and executive functions | ||
| D2 test of attention | Processing elements | p45 |
| Omissions | p25 | |
| Concentration | p40 | |
| Digit span (WMS-III) | Forward | p50 |
| Backward | p50 | |
| Verbal fluency | Semantic (animals) | p75 |
| Semantic (names) | p90 | |
| Phonological (FAS) | p30 | |
| Trail making test | A | p50 |
| B | p20 | |
| Stroop | p50 | |
| Hanoi tower | 3 disk | Normal |
| 4 disk | Not solved1 | |
| Similarities (WAIS) | p50 | |
| Wisconsin card sorting test | Categories completed | 4 (P > 16) |
| Failure to maintain set | p51 | |
| Errors | p30 | |
| Perseverative responses | p30 | |
| Perseverative errors | p30 | |
| Conceptual level responses | p20 | |
| BADS | Rule shift cards | p51 |
| DEX | Score 20 | |
| Episodic memory | ||
| Logical memory I (WMS-III) | Recall unit (Story A + B) | p20 |
| Recall thematic (Story A+ B) | p20 | |
| Logical memory II (WMS-III) | Recall unit (Story A + B) | p30 |
| Recall thematic (Story A + B) | p30 | |
| Word list I (WMS-III) | Recall total score | p50 |
| Short-delay recall | p85 | |
| Word list II (WMS-III) | Recall | p70 |
| Recognition | p85 | |
| Rey-osterrieth complex figure test | Recall | p50 |
| Naming | ||
| Boston naming test | p50 | |
| Praxis and visuo-constructive ability | ||
| Rey-osterrieth complex figure test | Copy | p75 |
| Gesture imitation | Normal | |
| Complex sequencing | Normal | |
A 76-year-old man, FMR1 gray-zone alleles carrier (expansion of 51 CGG) presented with a 3-year history of hand tremor and severe gait disorder. On examination he had mild postural tremor in both hands, mild rigidity more marked in the left side, lost of postural stability, severe tremor in a standing position, ataxia and freezing. He improved neither with 609 mg of levodopa nor with 1.5 mg of clonazepam. There were not cognitive decline.
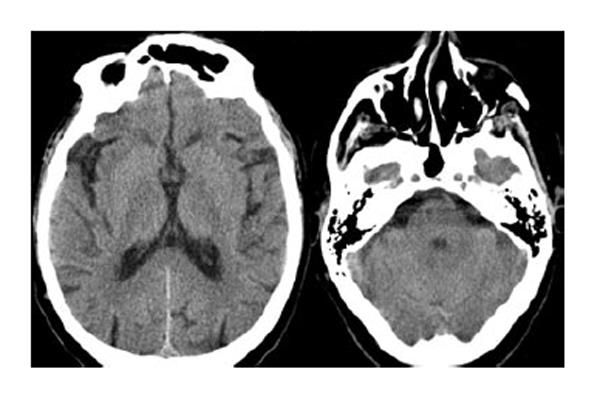
Blood tests were normal. Cranial computed tomography and DAT SCAN were also normal (Figures 4 and 5). Electromyography corroborated high-frequency (14 Hz) tremor in a standing position in both legs which confirmed the diagnosis of orthostatic tremor.
Parkinsonism is often related to FXTAS, although most patients do not meet clinical criteria for PD[1,2]. Indeed, in parkinsonism related to FXTAS, response to levodopa is often poor[5,6], DAT SCAN is usually normal[5] and Single Photon Emission Computed Tomography with iodobenzamide has shown abnormal tracer uptake[6]. It leads to conclude that parkinsonism in FXTAS is developed by dysfunction beyond the presynaptic nigroestriatal level[5]. However, our first case, a parkinsonism with clinical features indistinguishable from PD, as similar cases previously reported[2], without intention tremor or ataxia and with both good response to levodopa and abnormal DAT SCAN suggests damage restricted to the presynaptic level. Thus, although the relationship between these “presynaptic parkinsonisms” and the FMR1 premutation could be casual, it is necessary to consider that the FMR1 premutation might cause two phenotypes of parkinsonism, such as a presynaptic phenotype, indistinguishable from PD, and a postsynaptic phenotype, associated with clinical features of FXTAS. In fact, neuropathological features related to the FMR1 gene without Lewy bodies were found in a FMR1 premutation alleles carrier with clinical features of presynaptic parkinsonism[7] which supported the relationship between that presynaptic parkinsonism and the FMR1 gene as well as ruled out a concomitant PD. How to explain it remains unclear, it could reflect either different ranges of severity or different stages of the same process or the coexistence of the FMR1 premutation with others genes associated with PD[8]. Thus, we suggest that FMR1 premutation should be considered in the differential diagnosis of PD, almost in those patients with family history of disorders related to the FMR1 gene.
FMR1 gray zone alleles carriers were believed to have no abnormal neurological conditions. However, a recent report has suggested a relationship between FMR1 gray zone alleles carriers and FXTAS[3]. In this sense, our second case, presented with usual clinical features of FXTAS, such as parkinsonism, hand tremor and ataxia, supports the fact that FMR1 gray zone alleles carriers could develop FXTAS. Indeed, orthostatic tremor has not been described before in association with the FMR1 gene. Thus, the presence of orthostatic tremor in our second patient expands the clinical spectrum of neurological disorders associated with the FMR1 gene. Future research should validate findings on a larger group of patients.
This work was partially presented at the Movement Disorders Society’s 15th International Congress of Parkinson’s Disease and Movement Disorders, Toronto, June 2011.
P- Reviewers: Guo JF, Livshits L S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, Greco C, Des Portes V, Jardini T, Levine R. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 533] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 2. | Hall DA, Howard K, Hagerman R, Leehey MA. Parkinsonism in FMR1 premutation carriers may be indistinguishable from Parkinson disease. Parkinsonism Relat Disord. 2009;15:156-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Hall D, Tassone F, Klepitskaya O, Leehey M. Fragile X-associated tremor ataxia syndrome in FMR1 gray zone allele carriers. Mov Disord. 2012;27:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7288] [Cited by in RCA: 8000] [Article Influence: 242.4] [Reference Citation Analysis (0)] |
| 5. | Ceravolo R, Antonini A, Volterrani D, Rossi C, Goldwurm S, Di Maria E, Kiferle L, Bonuccelli U, Murri L. Dopamine transporter imaging study in parkinsonism occurring in fragile X premutation carriers. Neurology. 2005;65:1971-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Scaglione C, Ginestroni A, Vella A, Dotti MT, Nave RD, Rizzo G, De Cristofaro MT, De Stefano N, Piacentini S, Martinelli P. MRI and SPECT of midbrain and striatal degeneration in fragile X-associated tremor/ataxia syndrome. J Neurol. 2008;255:144-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Louis E, Moskowitz C, Friez M, Amaya M, Vonsattel JP. Parkinsonism, dysautonomia, and intranuclear inclusions in a fragile X carrier: a clinical-pathological study. Mov Disord. 2006;21:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Hedrich K, Pramstaller PP, Stübke K, Hiller A, Kabakci K, Purmann S, Kasten M, Scaglione C, Schwinger E, Volkmann J. Premutations in the FMR1 gene as a modifying factor in Parkin-associated Parkinson’s disease. Mov Disord. 2005;20:1060-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |