Revised: April 17, 2013
Accepted: May 7, 2013
Published online: September 28, 2013
Severe tetraparesis resulting from cervical cord compression due to osteochondroma of the Atlas is a rare condition, especially in young children. In this report, the author discusses the clinical presentation, and outcome of surgical excision of a large C1 osteochondroma presenting with severe myelopathic tetraparesis, in a 10-year-old girl. Computed tomography and magnetic resonance images revealed a large bony lump arising from the posterior arch of atlas, filling most of the spinal canal, and compressing the cervical spinal cord. Another histologically proven exostosis was incidentally found at the spinous process of T1. There was no history of exostosis in the family, and the patient improved dramatically after removal of the C1 osteochondroma.
Core tip: Solitary cervical spine osteochondroma affects C1, whereas multiple exostoses involve C2 more. Osteochondroma usually originates from the posterior element, and continues to grow slowly until puberty. It appears in computed tomography as an extradural mass, and spinal cord changes are better seen in magnetic resonance imaging. The author highlighted this case of a child with multiple exostoses arising from the posterior arch of C1 and a smaller one at the spinous process of C7, because the associated severe neurological symptoms are more pronounced than reported previously, and adequate decompression by total excision was associated with significant neurological recovery.
- Citation: Elgamal EA. Complete recovery of severe tetraparesis after excision of large C1-osteochondroma. World J Neurol 2013; 3(3): 79-82
- URL: https://www.wjgnet.com/2218-6212/full/v3/i3/79.htm
- DOI: https://dx.doi.org/10.5316/wjn.v3.i3.79
Spinal exostosis causing severe spinal cord compression is a rare form of bone dysplasia representing 4% to 7% of all primary benign spinal tumors, which generally becomes symptomatic during the second and third decades of life[1-3]. Most spinal exostoses grow from the external parts of the lamina and occasionally arise from the posterior arch of the atlas, causing cord compression and subsequently quadriparesis[4].
Spinal exostoses are better visualized by computed tomography (CT) than by magnetic resonance imaging (MRI). However, spinal cord compression is best studied by MRI, as is the size of the cartilaginous cap, which assists in differentiating exostoses from chondrosarcoma[1-5].
Neurological recovery after surgical excision of intraspinal exostoses and decompression of the spinal cord is excellent, and the recurrence rate is low[2-5]. The author reports complete recovery of severe tetraparesis after excision of a large intraspinal C1 osteochondroma in a 10 year old girl who also had an T1 spinous process exostosis, found incidentally.
A 10 years old girl, the product of uneventful pregnancy for a non-consanguine couple was born at term by caesarian section. She presented with a 7 d history of left leg pain, and frequent falls at school. She further complained of moderate neck pain and swelling at the back of the neck which was thought to be related to the fall. She admitted that she has been troubled by unsteadiness and had noticed weakness and stiffness of both upper and lower limbs, particularly on the left side, over the previous year. She had a history of iron-deficiency anaemia in early childhood, and there was no family history of neurologic or musculoskeletal disorder.
On examination, the patient’s neck was tilted to the right side and a hard swelling was felt just below the vertebra prominens at the T1 spinous process. She was able to move her neck in all directions, with some limitations due to stiffness. She was found to have G2/5 power in the left arm and leg and G3/5 power in the right upper and lower limbs, along with myelopathic manifestations affecting all 4 limbs but mainly the left side, with hypertonia, brisk reflexes, unsustained left ankle clonus, upgoing planters, and positive Hoffman’s sign bilaterally. Pin prick sensation was impaired with a sensory level at the root of the neck, but deep sensation was preserved.
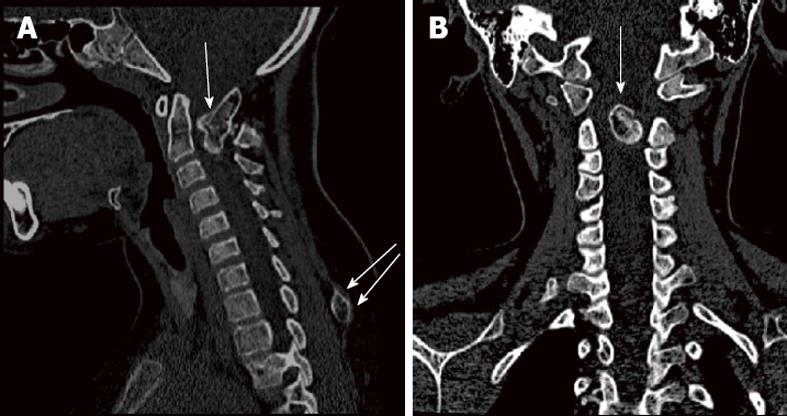
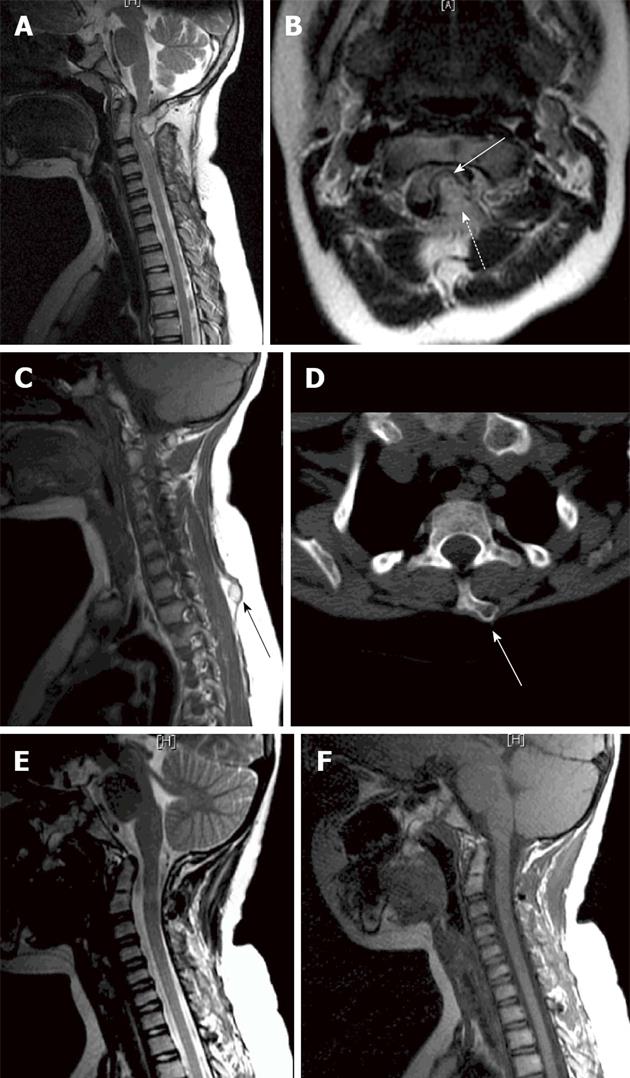
Radiological investigations, including CT and MRI scans, revealed an irregular bony outgrowth arising from the posterior arch of the atlas extending into the spinal canal, the posterior part forming a pseudo joint with the posterior arch of the axis. Findings were suggestive of a congenital anomalous bony outgrowth from C1 (Figure 1). This bony overgrowth was mainly at the left side, extending anteriorly into the spinal canal and causing significant compression, flattening and displacement of the cord to the right side. There was no evidence of alteration of cord signal intensity (Figure 2). Prominence of the T1 spinous process with a rounded bony outgrowth was found incidentally on screening the rest of the patient’s spine (Figure 2). Skeletal survey excluded similar bony lesions.
The patient underwent excision of the masses through a posterior approach. The bony lump was composed mainly of cancellous bone lined by a thin cortical layer. Drilling of the posterior arch of C1 and piecemeal removal of the cancellous content, extended deep into the spinal canal and a cap which was found adhering to the underlying dura was removed. The mass arising at the spinous process of T1 was removed through a separate incision.
The patient tolerated the procedure well and started to recover immediately following surgery. Postoperative CT and MRI scans showed widening of the spinal canal, relief of the neural compression, and improvement of the spinal cord deformation (Figure 2). Histological examination of both specimens proved them to be osteochondromas.
The patient continued to improve with the help of physiotherapy until she was back to her normal self at 6 mo follow-up after surgery. Physical examination showed no muscle weakness, normal sensation, improved hyperreflexia, and no lower limb clonus.
Osteochondromas are considered the most common benign skeletal tumor. It has been reported that about 3% occur in the spine. However, spinal exostoses are probably under-reported in relation to their true incidence because most are asymptomatic[5,6]. Most solitary and hereditary osteochondromas tend to occur in the cervical spine, while the most common site for solitary lesions is C1. In hereditary multiple exostosis (HME), it the C2 vertebra seems to be the most commonly affected[4-7].
In a review of 165 cases of spinal exostoses reported in the literature, 89 of them (53%) were affected by solitary lesions, with the cervical spine the most affected site (54%), and C1 the most common level (10%). On the other hand, lesions associated with HME also occurred most commonly in the cervical spine (57%), most commonly at C2 level (19%)[8]. The results of Bess et al[8] also indicated that the majority of cervical spine exostoses were solitary.
Spinal osteochondromas usually arise from the posterior elements (the secondary centers of ossification), most commonly near the tips of the spinous processes[6]. Of the 121 exostoses whose origins were reported, 106 (88%) emanated from the posterior elements. The remaining 15 exostoses (12%) originated from the vertebral body. Solitary and HME exostoses had similar distributions of vertebral origin[8].
Neurological damage is usually caused by the progressive encroachment of the slowly expanding lesion. However, the reported incidence of spinal cord or nerve root compression is 0.5% to 1.0%, and a higher incidence of symptomatic exostoses was found with lesions associated with HME, especially in young patients[9,10].
Osteochondromas cease to grow after skeletal maturity and do not develop in postpubertal individuals. However, in the majority of children, the tumor remains asymptomatic until skeletal maturity. The clinical presentation in the present case started at the age of nine and progressed relentlessly over the course of a year, resulting in myelopathy affecting all extremities by one week before presentation[11].
In osteochondroma a CT scan is the diagnostic imaging modality of choice. CT shows the extent of the cartilaginous and osseous components, and their relationship to the vertebral and neural elements of the spine[8]. MRI is more useful than CT in defining the extradural component and is the preferred method for examining the spinal canal and the effect of pressure on the spinal cord[1-5].
The incidence of malignant transformation of osteochondroma into sarcoma is between 5% and 11%, and is associated with sudden pain or neurological deterioration[5,12]. In this case, rapid neurological deterioration was probably due to sudden decompensation of the compressed spinal cord and not to malignant transformation, as confirmed by histological examination.
The management of this case was aimed at relieving neurological symptoms, by excision of the lump and decompressing the spinal cord. Improvement and satisfactory recovery of function after resection of the lesion are to be expected in most cases[13]. This patient’s favorable prognostic factors included her young age, short period of rapid symptom progression, and absence of signal cord changes in the MRI. Although spinal cord compression was severe, the patient showed such rapid and satisfactory recovery that she was back to almost her normal self, within 6 mo after surgery.
Albrecht et al[14] emphasized that neurological recovery after surgical treatment of intraspinal exostoses causing spinal cord compression is excellent, and the recurrence rate is low. They found that 89% of symptomatic patients treated operatively reported improvement of symptoms. All patients except two in the Bess et al[8] series surgically treated for intraspinal exostoses, had eventual resolution of presenting symptoms after surgery.
This patient had no family history of similar condition, and no family screening was performed. However, having multiple spinal exostosis affecting C1 and C7, this case could be the first in the family to develop HME. It is an autosomal dominant disorder, with full penetrance and has an equal gender prevalence[15].
In conclusion, Spinal exostosis occurs most commonly in the cervical spine, and the associated neurological symptoms are more common than reported previously. Multiple exostoses tend to affect younger patients and have a higher incidence of neurological symptoms than solitary lesions.
Although MRI is the preferred method for examining the spinal canal and the effect of pressure on the spinal cord, CT scan can be more specific and is the imaging modality of choice. Total excision of the exostosis is associated with a high probability of neurological recovery.
P- Reviewer Kahveci R S- Editor Gou SX L- Editor Hughes D E- Editor Yan JL
| 1. | Aldea S, Bonneville F, Poirier J, Chiras J, George B, Carpentier A. Acute spinal cord compression in hereditary multiple exostoses. Acta Neurochir (Wien). 2006;148:195-198; discussion 198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Er U, Simşek S, Yiğitkanlı K, Adabağ A, Kars HZ. Myelopathy and Quadriparesis due to Spinal Cord Compression of C1 Laminar Osteochondroma. Asian Spine J. 2012;6:66-70. [PubMed] [DOI] [Full Text] |
| 3. | Ozturk C, Tezer M, Hamzaoglu A. Solitary osteochondroma of the cervical spine causing spinal cord compression. Acta Orthop Belg. 2007;73:133-136. [PubMed] |
| 4. | Chooi YS, Siow YS, Chong CS. Cervical myelopathy caused by an exostosis of the posterior arch of C1. J Bone Joint Surg Br. 2005;87:257-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Oga M, Nakatani F, Ikuta K, Tamaru T, Arima J, Tomishige M. Treatment of cervical cord compression, caused by hereditary multiple exostosis, with laminoplasty: a case report. Spine (Phila Pa 1976). 2000;25:1290-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Giudicissi-Filho M, de Holanda CV, Borba LA, Rassi-Neto A, Ribeiro CA, de Oliveira JG. Cervical spinal cord compression due to an osteochondroma in hereditary multiple exostosis: case report and review of the literature. Surg Neurol. 2006;66 Suppl 3:S7-S11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Yoshida T, Matsuda H, Horiuchi C, Taguchi T, Nagao J, Aota Y, Honda A, Tsukuda M. A case of osteochondroma of the atlas causing obstructive sleep apnea syndrome. Acta Otolaryngol. 2006;126:445-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Bess RS, Robbin MR, Bohlman HH, Thompson GH. Spinal exostoses: analysis of twelve cases and review of the literature. Spine (Phila Pa 1976). 2005;30:774-780. [PubMed] |
| 9. | Gille O, Pointillart V, Vital JM. Course of spinal solitary osteochondromas. Spine (Phila Pa 1976). 2005;30:E13-E19. [PubMed] |
| 10. | Roach JW, Klatt JW, Faulkner ND. Involvement of the spine in patients with multiple hereditary exostoses. J Bone Joint Surg Am. 2009;91:1942-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Schomacher M, Suess O, Kombos T. Osteochondromas of the cervical spine in atypical location. Acta Neurochir (Wien). 2009;151:629-633; discussion 633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Ratliff J, Voorhies R. Osteochondroma of the C5 lamina with cord compression: case report and review of the literature. Spine (Phila Pa 1976). 2000;25:1293-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Rahman A, Bhandari PB, Hoque SU, Ansari A, Hossain AT. Solitary osteochondroma of the atlas causing spinal cord compression: a case report and literature review. BMJ Case Rep. 2012;2012:pii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Albrecht S, Crutchfield JS, SeGall GK. On spinal osteochondromas. J Neurosurg. 1992;77:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 147] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Chatzidakis E, Lypiridis S, Kazdaglis G, Chatzikonstadinou K, Papatheodorou G. A rare case of solitary osteochondroma of the dens of the C2 vertebra. Acta Neurochir (Wien). 2007;149:637-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |