Revised: May 28, 2013
Accepted: June 18, 2013
Published online: September 28, 2013
Processing time: 128 Days and 15.3 Hours
AIM: To explore the existence of potential correlations of cortical thickness between different functional brain areas.
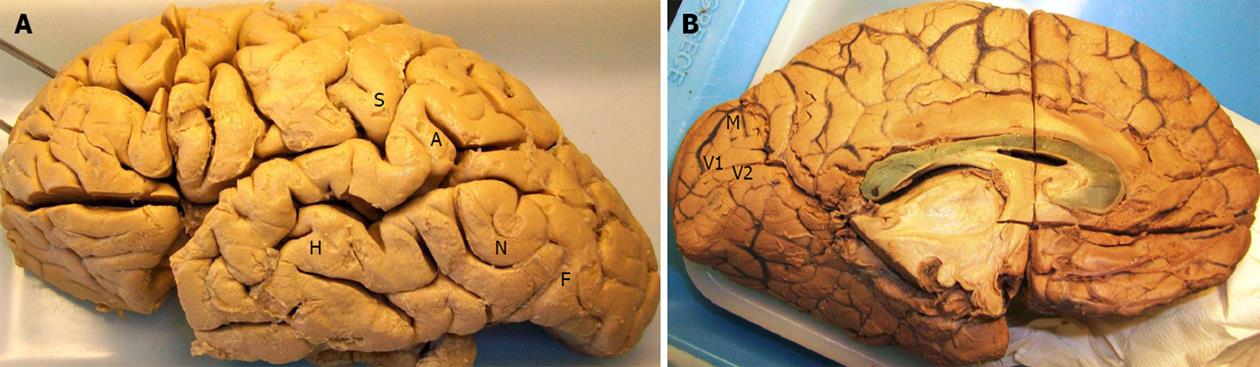
METHODS: Our material consisted of 38 formalin-fixated human cerebral hemispheres from twenty males and three females, cadaver donors for students’ education. We selected the following cortical areas at each hemisphere to examine: supramarginal gyrus (S), angular gyrus (A), area of colors recognition (F), area of names recognition (N), area of auditory attention (H), area of place memory (M), cortex of the superior wall of the calcarine sulcus (V1) and cortex of the inferior wall of the calcarine sulcus (V2). We measured the thickness of each cortical area and statistically analyzed our data.
RESULTS: We found a significant difference of the mean value of the V1 (P < 0.05) between right and left hemispheres, as well as very significant correlations (P < 0.001) between the following cortical areas: N and F, A and F, S and F, A and S, A and N, S and N. We also found significant correlations (P < 0.01) between the following areas: S and M, S and H, N and H, as well as between the following areas (P < 0.05): V1 and V2, M and F, M and N, A and H.
CONCLUSION: Our results suggest that there could be a potentially stronger impact for objects placed in the left inferior quarter of the visual field. Our study revealed several thickness-based correlations among different functional cortical areas. Most of them seem to have a more or less rational explanation.
Core tip: Our primary purpose was to explore the existence of potential correlations of cortical thickness between different functional areas of the human brain. Our material consisted of 38 formalin-fixated cerebral hemispheres. We examined eight specific cortical areas at each hemisphere. We found several statistically significant correlations. Our results suggest that there could be a potentially stronger impact for objects placed in the left inferior quarter of the visual field. Our study also revealed several thickness-based correlations among different functional cortical areas. Most of them seem to have a more or less rational explanation.
- Citation: Mavridis I, Lontos K, Anagnostopoulou S. Thickness-based correlations of cortical areas involved in senses, speech and cognitive processes. World J Neurol 2013; 3(3): 67-74
- URL: https://www.wjgnet.com/2218-6212/full/v3/i3/67.htm
- DOI: https://dx.doi.org/10.5316/wjn.v3.i3.67
The human brain is the most mysterious organ of our body. Despite the significant research progress on neurosciences during the last century, there is still a lot to learn. Although functional neuroimaging methods, mainly the functional magnetic resonance imaging (fMRI), offered a window with a magnificent view to brain functions, there are still well kept secrets regarding these complex functions. The knowledge that specific cortical areas of the brain are correlated with specific functions, known at least since the 19th century, resulted in several functional maps of the cerebral cortex as well as the famous motor and sensory homunculi. Modern functional neuroimaging techniques allowed this knowledge to evolve, revealing, however, the complexity of such correlations.
In human anatomy, information regarding structure can indirectly provide us with information regarding function. Neuroanatomical studies are traditionally the ‘gold standard’ method of exploring the brain structure and they offer information indirectly about its function. But could anatomical studies, focused on the cerebral cortex, offer indirect information regarding cortical function? That is what we aimed to do with the present study. Our primary purpose was specifically to explore the existence of potential correlations of cortical thickness between different functional cortical areas of the human brain, as well as to compare such thickness between sides.
Our material consisted of 38 formalin-fixated normal human cerebral hemispheres (23 left, 15 right) in our department. They came from twenty males (32 hemispheres) and three females (6 hemispheres), predominately middle-aged cadaver donors (with cause of death irrelevant to brain pathology) for students’ education. We selected the following cortical areas[1] at each hemisphere to examine (Figure 1).
S = supramarginal gyrus (auditory center of speech at the dominant hemisphere[2]) (Brodmann area 40); A = angular gyrus (visual center of speech at the dominant hemisphere[2]) (Brodmann area 39); F = area of colors recognition (Brodmann area 19); N = area of names understanding (Brodmann area 37); H = area of auditory attention (Brodmann area 21).
M = area of place memory (Brodmann area 19); V1 = cortex of the superior wall of the calcarine sulcus (primary visual cortex, Brodmann area 17); V2 = cortex of the inferior wall of the calcarine sulcus (primary visual cortex, Brodmann area 17).
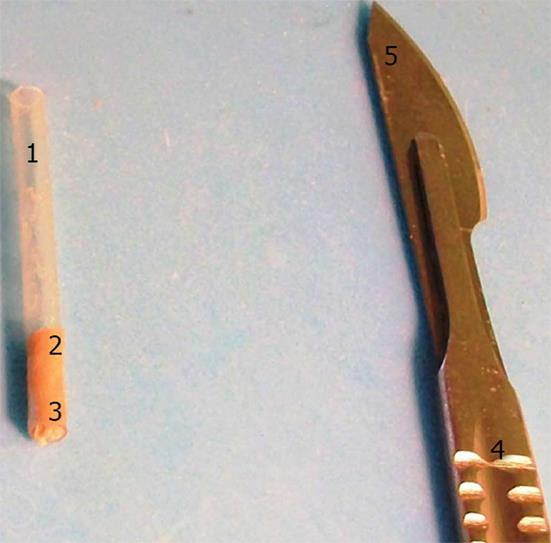
After removing the choroid meninge, we measured the thickness of each of these eight cortical areas. Methodologically, we used a transparent plastic tube approximately 25 mm long and 2 mm wide which was inserted perpendicularly to the cerebral surface in order to remove a cylindrical piece of brain tissue from each selected area. The transparency of the tube allowed the identification of the cortex-white matter limit and hence the measuring of the cortical thickness without even removing the sample from the tube. A scalpel was occasionally used to help the removal of the brain tissue piece by cutting at its bottom (Figure 2).
Our results were statistically analyzed by calculating each thickness’ mean value (MV) and standard deviation (SD), as well as by applying student’s t-test among each side’s MVs. We also applied Pearson’s correlation coefficient (r) among different areas’ thicknesses.
Table 1 shows the measured data with their MVs and SDs and the measured data separately for right and left hemispheres (hemisphere column numbers are for specimen identifying purposes). As it can be seen on these tables: (1) The S varied from 0.9 mm to 4.0 mm and its MV ± SD was S = 2.20 ± 0.76 mm (n = 34). For right hemispheres we found S = 2.15 ± 0.82 mm (n = 13) and for left S = 2.23 ± 0.74 mm (n = 21); (2) The A varied from 1.0 mm to 4.5 mm and its MV ± SD was A = 2.23 ± 0.76 mm (n = 34). For right hemispheres we found A = 2.14 ± 0.90 mm (n = 13) and for left A = 2.29 ± 0.68 mm (n = 21); (3) The F varied from 1.0 mm to 4.0 mm and its MV ± SD was F = 2.03 ± 0.62 mm (n = 37). For right hemispheres we found F = 2.12 ± 0.73 mm (n = 15) and for left F = 1.97 ± 0.54 mm (n = 22); (4) The N varied from 0.9 mm to 4.2 mm and its MV ± SD was N = 2.14 ± 0.71 mm (n = 37). For right hemispheres we found N = 2.13 ± 0.79 mm (n = 15) and for left N = 2.14 ± 0.68 mm (n = 22); (5) The H varied from 0.9 mm to 3.9 mm and its MV ± SD was H = 2.11 ± 0.70 mm (n = 34). For right hemispheres we found H = 2.00 ± 0.66 mm (n = 15) and for left H = 2.20 ± 0.74 mm (n = 19); (6) The M varied from 1.4 mm to 4.0 mm and its MV ± SD was M = 2.16 ± 0.65 mm (n = 30). For right hemispheres we found M = 2.37 ± 0.65 mm (n = 11) and for left M = 2.04 ± 0.63 mm (n = 19); (7) The V1 varied from 1.2 mm to 4.2 mm and its MV ± SD was V1 = 2.07 ± 0.64 mm (n = 30). For right hemispheres we found V1 = 2.42 ± 0.80 mm (n = 11) and for left V1 = 1.87 ± 0.43 mm (n = 19); and (8) The V2 varied from 1.3 mm to 4.0 mm and its MV ± SD was V2 = 2.13 ± 0.65 mm (n = 30). For right hemispheres we found V2 = 2.28 ± 0.65 mm (n = 11) and for left V2 = 2.04 ± 0.65 mm (n = 19).
| Hemisphere | S (mm) | A (mm) | F (mm) | N (mm) | H (mm) | M (mm) | V1 (mm) | V2 (mm) | |
| Human cerebral cortex | |||||||||
| 1 | R3 | 2.5 | 2.8 | 1.7 | 2.6 | 3.5 | 2.9 | 2.2 | 2.9 |
| 2 | R7 | 4 | 4.5 | 4 | 4.2 | 2.5 | 3.4 | 2.6 | 3.1 |
| 3 | L8 | 2.7 | 3.3 | 2 | 2.9 | 2.9 | 1.9 | 2.3 | 4 |
| 4 | L11 | - | - | - | - | - | 2 | 1.9 | 2.1 |
| 5 | L9 | 2 | 1.9 | 2.3 | 2.3 | 2.6 | 2.2 | 1.5 | 1.6 |
| 6 | L12 | 3.5 | 3.9 | 2.3 | 1.7 | 2.9 | 1.9 | 1.8 | 1.7 |
| 7 | L10 | 3.9 | 3 | 2 | 2.9 | 2 | 2.5 | 2 | 1.8 |
| 8 | L2 | 2.5 | 2 | 2 | 2.5 | 3.9 | 3 | 1.5 | 2.5 |
| 9 | R2 | 2.5 | 3.1 | 3 | 2.4 | 2.5 | 2.4 | 3 | 1.9 |
| 10 | L3 | 3.1 | 2.7 | 3.1 | 2.8 | 3.1 | 2.1 | 3 | 3.1 |
| 11 | R5 | 3.3 | 1.5 | 2 | 2.3 | 2.4 | 2.8 | 2.5 | 3 |
| 12 | R1 | 2 | 2.4 | 2.8 | 2.5 | 2 | 2.9 | 4.2 | 2.2 |
| 13 | L6 | 1.5 | 2 | 1.9 | 2.1 | 1.8 | 1.5 | 1.8 | 1.6 |
| 14 | R6 | 1.2 | 1.6 | 2.4 | 2.2 | 1.3 | 1.9 | 1.4 | 1.8 |
| 15 | R4 | 1.6 | 1.5 | 1.5 | 2 | 2.3 | 1.6 | 1.5 | 1.6 |
| 16 | L7 | 1.5 | 2 | 1.2 | 1 | 2.2 | 1.9 | 2 | 2 |
| 17 | L1 | 2 | 2.1 | 2.5 | 2.6 | 2.1 | 1.8 | 1.5 | 2.7 |
| 18 | L4 | 2.9 | 2.1 | 2.5 | 2.9 | 2.5 | 4 | 1.2 | 1.3 |
| 19 | L5 | 2 | 2.3 | 2 | 2 | - | 2.1 | 2.2 | 2 |
| 20 | L15 | 2 | 2.1 | 2.1 | 2 | 2.2 | 1.7 | 1.7 | 1.5 |
| 21 | L16 | 3 | 2.9 | 2.2 | 3 | 2.5 | 2.5 | 2 | 1.4 |
| 22 | L13 | 2.4 | 2.6 | 2 | 2.5 | 2.4 | 1.5 | 1.7 | 1.9 |
| 23 | L14 | 1.9 | 1.2 | 1.2 | 0.9 | - | 1.5 | 2.3 | 1.9 |
| 24 | R8 | 2 | 1.7 | 2.2 | 2 | 2.2 | 2.5 | 1.9 | 1.6 |
| 25 | R9 | 1.9 | 2 | 2.3 | 3 | 2.3 | 1.4 | 2.4 | 2.6 |
| 26 | L18 | 1.6 | 1.9 | 1.6 | 1.4 | 1.6 | 1.5 | 1.5 | 2 |
| 27 | R17 | - | - | 1.9 | 2 | 1.9 | - | - | - |
| 28 | L25 | - | - | 1.6 | 1.8 | - | - | - | - |
| 29 | R10 | - | - | 1.9 | 1.6 | 1.5 | 1.6 | 1.9 | 1.4 |
| 30 | L17 | 1.6 | 2.1 | 2 | 3.2 | 2 | 1.6 | 2.3 | 1.9 |
| 31 | R13 | 2.1 | 1.4 | 1.5 | 1 | 1.8 | - | - | - |
| 32 | L21 | 2.5 | 3 | 3 | 2 | 0.9 | - | - | - |
| 33 | R15 | 2.2 | 2.3 | 2.1 | 1.4 | 1.9 | - | - | - |
| 34 | L23 | 1.1 | 2.3 | 1.6 | 1.2 | 1 | - | - | - |
| 35 | R14 | 0.9 | 1.5 | 1.2 | 1.4 | 1 | - | - | - |
| 36 | L22 | 1.8 | 1 | 1 | 1.5 | 1.2 | - | - | - |
| 37 | R16 | 1.8 | 1.5 | 1.3 | 1.4 | 0.9 | 2.7 | 3 | 3 |
| 38 | L24 | 1.4 | 1.7 | 1.3 | 1.9 | 2 | 1.5 | 1.3 | 1.7 |
| MV | 2.2 | 2.23 | 2.03 | 2.14 | 2.11 | 2.16 | 2.07 | 2.13 | |
| SD | 0.76 | 0.76 | 0.62 | 0.71 | 0.7 | 0.65 | 0.64 | 0.65 | |
| Human cerebral cortex, right hemispheres | |||||||||
| 1 | R3 | 2.5 | 2.8 | 1.7 | 2.6 | 3.5 | 2.9 | 2.2 | 2.9 |
| 2 | R7 | 4 | 4.5 | 4 | 4.2 | 2.5 | 3.4 | 2.6 | 3.1 |
| 3 | R2 | 2.5 | 3.1 | 3 | 2.4 | 2.5 | 2.4 | 3 | 1.9 |
| 4 | R5 | 3.3 | 1.5 | 2 | 2.3 | 2.4 | 2.8 | 2.5 | 3 |
| 5 | R1 | 2 | 2.4 | 2.8 | 2.5 | 2 | 2.9 | 4.2 | 2.2 |
| 6 | R6 | 1.2 | 1.6 | 2.4 | 2.2 | 1.3 | 1.9 | 1.4 | 1.8 |
| 7 | R4 | 1.6 | 1.5 | 1.5 | 2 | 2.3 | 1.6 | 1.5 | 1.6 |
| 8 | R8 | 2 | 1.7 | 2.2 | 2 | 2.2 | 2.5 | 1.9 | 1.6 |
| 9 | R9 | 1.9 | 2 | 2.3 | 3 | 2.3 | 1.4 | 2.4 | 2.6 |
| 10 | R17 | - | - | 1.9 | 2 | 1.9 | - | - | - |
| 11 | R10 | - | - | 1.9 | 1.6 | 1.5 | 1.6 | 1.9 | 1.4 |
| 12 | R13 | 2.1 | 1.4 | 1.5 | 1 | 1.8 | - | - | - |
| 13 | R15 | 2.2 | 2.3 | 2.1 | 1.4 | 1.9 | - | - | - |
| 14 | R14 | 0.9 | 1.5 | 1.2 | 1.4 | 1 | - | - | - |
| 15 | R16 | 1.8 | 1.5 | 1.3 | 1.4 | 0.9 | 2.7 | 3 | 3 |
| MV | 2.15 | 2.14 | 2.12 | 2.13 | 2 | 2.37 | 2.42 | 2.28 | |
| SD | 0.82 | 0.9 | 0.73 | 0.79 | 0.66 | 0.65 | 0.8 | 0.65 | |
| Human cerebral cortex, left hemispheres | |||||||||
| 1 | L8 | 2.7 | 3.3 | 2 | 2.9 | 2.9 | 1.9 | 2.3 | 4 |
| 2 | L11 | - | - | - | - | - | 2 | 1.9 | 2.1 |
| 3 | L9 | 2 | 1.9 | 2.3 | 2.3 | 2.6 | 2.2 | 1.5 | 1.6 |
| 4 | L12 | 3.5 | 3.9 | 2.3 | 1.7 | 2.9 | 1.9 | 1.8 | 1.7 |
| 5 | L10 | 3.9 | 3 | 2 | 2.9 | 2 | 2.5 | 2 | 1.8 |
| 6 | L2 | 2.5 | 2 | 2 | 2.5 | 3.9 | 3 | 1.5 | 2.5 |
| L3 | 3.1 | 2.7 | 3.1 | 2.8 | 3.1 | 2.1 | 3 | 3.1 | |
| 8 | L6 | 1.5 | 2 | 1.9 | 2.1 | 1.8 | 1.5 | 1.8 | 1.6 |
| 9 | L7 | 1.5 | 2 | 1.2 | 1 | 2.2 | 1.9 | 2 | 2 |
| 10 | L1 | 2 | 2.1 | 2.5 | 2.6 | 2.1 | 1.8 | 1.5 | 2.7 |
| 11 | L4 | 2.9 | 2.1 | 2.5 | 2.9 | 2.5 | 4 | 1.2 | 1.3 |
| 12 | L5 | 2 | 2.3 | 2 | 2 | - | 2.1 | 2.2 | 2 |
| 13 | L15 | 2 | 2.1 | 2.1 | 2 | 2.2 | 1.7 | 1.7 | 1.5 |
| 14 | L16 | 3 | 2.9 | 2.2 | 3 | 2.5 | 2.5 | 2 | 1.4 |
| 15 | L13 | 2.4 | 2.6 | 2 | 2.5 | 2.4 | 1.5 | 1.7 | 1.9 |
| 16 | L14 | 1.9 | 1.2 | 1.2 | 0.9 | - | 1.5 | 2.3 | 1.9 |
| 17 | L18 | 1.6 | 1.9 | 1.6 | 1.4 | 1.6 | 1.5 | 1.5 | 2 |
| 18 | L25 | - | - | 1.6 | 1.8 | - | - | - | - |
| 19 | L17 | 1.6 | 2.1 | 2 | 3.2 | 2 | 1.6 | 2.3 | 1.9 |
| 20 | L21 | 2.5 | 3 | 3 | 2 | 0.9 | - | - | - |
| 21 | L23 | 1.1 | 2.3 | 1.6 | 1.2 | 1 | - | - | - |
| 22 | L22 | 1.8 | 1 | 1 | 1.5 | 1.2 | - | - | - |
| 23 | L24 | 1.4 | 1.7 | 1.3 | 1.9 | 2 | 1.5 | 1.3 | 1.7 |
| MV | 2.23 | 2.29 | 1.97 | 2.14 | 2.2 | 2.04 | 1.87 | 2.04 | |
| SD | 0.74 | 0.68 | 0.54 | 0.68 | 0.74 | 0.63 | 0.43 | 0.65 | |
The statistical analysis of our measurements revealed no statistically significant difference between sides regarding the S, A, F, N, H, M and V2. It also revealed a statistically significant difference of the V1 MV between right (2.42 ± 0.80 mm) and left (1.87 ± 0.43 mm) hemispheres (t = 2.11, degrees of freedom = 28, P < 0.05), as well as the following correlations (beginning from the most powerful): (1) Statistically significant correlation between the N and F (r = 0.711, degrees of freedom = 35, P < 0.001); (2) Statistically significant correlation between the Α and F (r = 0.698, degrees of freedom = 32, P < 0.001); (3) Statistically significant correlation between the Α and S (r = 0.680, degrees of freedom = 32, P < 0.001); (4) Statistically significant correlation between the Α and N (r = 0.586, degrees of freedom = 32, P < 0.001); (5) Statistically significant correlation between the S and F (r = 0.579, degrees of freedom = 32, P < 0.001); (6) Statistically significant correlation between the S and N (r = 0.579, degrees of freedom = 32, P < 0.001); (7) Statistically significant correlation between the S and M (r = 0.558, degrees of freedom = 26, P < 0.01); (8) Statistically significant correlation between the S and H (r = 0.530, degrees of freedom = 30, P < 0.01); (9) Statistically significant correlation between the N and H (r = 0.514, degrees of freedom = 32, P < 0.01); (10) Statistically significant correlation between the V2 and V1 (r = 0.447, degrees of freedom = 28, P < 0.05); (11) Statistically significant correlation between the M and F (r = 0.441, degrees of freedom = 27, P < 0.05); (12) Statistically significant correlation between the M and N (r = 0.431, degrees of freedom = 27, P < 0.05); (13) Statistically significant correlation between the Α and H (r = 0.394, degrees of freedom = 30, P < 0.05); (14) Statistically ambiguous correlation between the V1 and F (r = 0.340, degrees of freedom = 27, P < 0.1); (15) Statistically ambiguous correlation between the N and V2 (r = 0.326, degrees of freedom = 27, P < 0.1); and (16) Statistically ambiguous correlation between the H and F (r = 0.303, degrees of freedom = 32, P < 0.1).
As shown on Table 1, from the eight areas studied, area A was the thickest one in left hemispheres and area V1 in right hemispheres. The latter area was in contrast the thinnest one in left hemispheres, whereas the thinnest in right hemispheres was area H. The absence of significant difference between sides regarding areas S and A is somehow surprising, given the location of Wernicke’s area at the dominant hemisphere.
The significantly thicker V1 for right hemispheres is an interesting finding. This cortical area receives visual pathway information from the left inferior quarter of the visual field, while the significantly thinner left V1 area receives visual pathway information from the right inferior quarter of the visual field. Could this finding mean that there is a potentially stronger impact for objects placed in the left inferior quarter of our visual field? Further research is needed to determine whether this difference reflects real functional differences between the right and left V1 cortical fields. It is also interesting that there was no such difference observed regarding the V2 areas (right and left) of the visual cortex which receive visual pathway information from the superior half of the visual field.
Considering the correlations (very significant, significant) we found, it seems quite difficult to find a probable explanation. However, we suggest the following potential explanations and interpretations:
N and F: Our mind possibly correlates names with color images (of the respective people or objects etc.), mainly based on our previous experience.
A and F, S and F: When we perceive (hear or see) the name of the color, the image of the color comes up into our mind.
A and S: It seems expected as they constitute the Wernicke’s area.
A and N, S and N: The functions of the auditory and visual centers of speech are obviously crucial for a name understanding process. When we read or hear the name of a person (or object, place etc.) we know, this particular person usually comes to our mind. Moreover, we can sometimes recognize somebody from the style they write or the way they speak.
S and M: The auditory perception of particular phrases regarding places we know can bring relative images from the past to our mind.
S and H, A and H: When we perceive somebody’s speech, they usually attract our attention, at least initially. We might even move towards them to listen better.
N and H: A simple example of the usefulness of such a correlation is that, when we hear our name, we focus our attention on the particular person talking to us.
V2 and V1: It seems expected as they both consist the primary visual cortex.
M and F: The images of places usually include several types of color.
M and N: Many places have specific names or are subconsciously connected with specific people.
Falk et al[3] described a method for obtaining clear 3D MRIs of the cortical surface of the brain in living human subjects. By combining volume composite and depth encoded images, they obtained surface coordinate data that resulted in highly repeatable measurements of sulcal lengths and cortical surface areas in eight normal adult volunteers. Sulcal lengths were determined for specific parts of the Sylvian fissure, central sulcus and frontal operculum. They observed previously unrecognized directional asymmetries in the length of the anterior limb of the pars triangularis, length of the ascending limb of the posterior Sylvian fissure, and position of the lateral end of the central sulcus. They attributed the finding of three new directional asymmetries for the human cortex, as well as the high repeatability of their measurements, to the sensitivity and accuracy of the 3D MRI technology[3].
The analysis of the human cerebral cortex and the measurement of its thickness based on MRI data can provide insight into normal brain development and neurodegenerative disorders. Accurate and reproducible results of the cortical thickness measurement are desired for sensitive detection[4]. Lüsebrink et al[4] compared ultra-high resolution data acquired at 7 Tesla (T) with 3T data for determination of the cortical thickness of the human brain. At identical resolution, the cortical thickness determination yielded consistent results between 3T and 7T, confirming the robustness of the acquisition and processing against potential field strength related effects. However, the ultra-high resolution 7T data resulted in significantly reduced values for the cortical thickness estimation compared to the lower resolution data. The reduction in thickness amounts to approximately one sixth to one third, depending on the processing algorithm and software used. This suggests a bias in the gray matter segmentation due to partial volume effects and indicates that true cortical thickness is overestimated by most current MR studies using both a voxel-based or surface-based method and can be more accurately determined with high resolution imaging at 7T[4].
Westlye et al[5] reported that cortical thickness decreases from childhood throughout life, as estimated by MRI. This monotone trajectory does not reflect the fundamentally different neurobiological processes underlying morphometric changes in development versus aging. The spatial pattern of intracortical neurodevelopment followed a posterior-anterior gradient, with earliest maturation of occipital visual cortices and most protracted in superior frontal regions[5].
Lyoo et al[6] reported that subjects with bipolar disorder exhibited significantly decreased cortical thickness in the left cingulate cortex, left middle frontal cortex, left middle occipital cortex, right medial frontal cortex, right angular cortex, right fusiform cortex and bilateral postcentral cortices, compared to healthy subjects (all P < 0.001). Duration of illness in bipolar subjects was inversely correlated with the cortical thickness of the left middle frontal cortex. Cortical thinning was present in multiple prefrontal cortices in bipolar disorder. There was also cortical thinning in sensory and sensory association cortices, which has not been reported in previous studies using region-of-interest or voxel-based morphometry methods. They proposed that cortical thinning may be related to impairment of emotional, cognitive and sensory processing in bipolar disorder but longitudinal studies will be necessary to test this hypothesis[6].
Developmental and psychiatric disorders, including schizophrenia, may be associated with altered cortical thickness and folding. Reduced cell density has been reported in the fundi of some sulci in the temporal lobe in schizophrenia[7]. Moreover, functional imaging studies suggested changes in primary visual cortex activity in subjects with schizophrenia. Interestingly, postmortem studies of subjects with schizophrenia reported an increased density of neurons in the primary visual cortex (Brodmann’s area 17)[8]. Dorph-Petersen et al[8] estimated the total volume and neuron number of Brodmann’s area 17 in postmortem brains from 10 subjects with schizophrenia and 10 matched normal comparison subjects. In addition, they assessed cortical thickness. They found a marked and significant reduction in total neuron number (25%) and volume (22%) of Brodmann’s area 17 in the schizophrenia group compared to the normal subjects. Subjects with schizophrenia therefore have a smaller cortical area allocated to primary visual perception. This finding suggests the existence of a schizophrenia-related change in cortical parcellation[8].
Brain atrophy is common in subcortical ischemic vascular disease but the underlying mechanisms are poorly understood[9]. Duering et al[9] provided in vivo evidence for secondary cortical neurodegeneration after subcortical ischemia as a mechanism for brain atrophy in cerebrovascular disease.
The use of computational approaches in the analysis of high resolution MRI of the human brain provides a powerful tool for in vivo studies of brain anatomy[10]. Watkins et al[10] reported results obtained with a voxel-wise statistical analysis of hemispheric asymmetries in regional ‘amounts’ of gray matter, based on MRI scans obtained in 142 healthy young adults. The voxel-wise analysis detected the well-known frontal (right > left) and occipital (left > right) petalias. Their analysis confirmed the presence of left-greater-than-right asymmetries in several posterior language areas, including the planum temporale and the angular gyrus; no significant asymmetry was detected in the anterior language regions. They also confirmed previously described asymmetries in the cingulate sulcus (right > left) and the caudate nucleus (right > left). In some brain regions they observed highly significant asymmetries that were not reported before, such as in the anterior insular cortex (right > left)[10].
Brain asymmetry has been observed in animals and humans in terms of structure, function and behavior. This lateralization is thought to reflect evolutionary, hereditary, developmental, experiential and pathological factors[11]. Toga and Thompson[11] reviewed the diverse literature describing brain asymmetries, focusing primarily on anatomical differences between the hemispheres and the methods that have been used to detect them. Brain-mapping approaches in particular can identify and visualize patterns of asymmetry in whole populations, including subtle alterations that occur in disease, with age and during development. These and other tools show great promise for assessing factors that modulate cognitive specialization in the brain, including the ontogeny, phylogeny and genetic determinants of brain asymmetry[11].
Numerous studies measuring field potentials or functional imaging signals have reported auditory cortex activations to either visual or somatosensory stimuli and together with neuroanatomical studies provide compelling evidence for the presence of synaptic inputs from other sensory modalities to the auditory cortex. Visual and somatosensory stimuli can modulate low frequency oscillations in the auditory cortex and by doing so, shape the excitability of local networks and mediate stimulus selection. Multisensory influences also interact with attentional selection, suggesting the existence of a shared substrate for both mechanisms of selective stimulus enhancement[12].
The study of Lyttelton et al[13] revealed several new findings, including a striking leftward increase in surface area of the supramarginal gyrus (peak effect 18%) compared with a smaller areal increase in the left Heschl’s gyrus and planum temporale region (peak effect 8%). A second finding was a rightward increase in surface area (peak effect 10%) in a band around the medial junction between the occipital lobe and parietal and temporal lobes[13].
According to the results of Rosenthal et al[14], the right angular gyrus had a critical role in perceptual sequence learning, whereas the primary motor cortex had a causal role in developing experience-dependent functional attributes relevant to conscious knowledge on manual but not perceptual sequence learning[14].
Sakurai et al[15] aimed to determine the features of alexia or agraphia with a left angular or supramarginal gyrus lesion. They assessed the reading and writing abilities of three patients using kanji (Japanese morphograms) and kana (Japanese syllabograms). They found that alexia occurs as “angular” alexia only when the lesion involves the adjacent lateral occipital gyri. Transposition errors suggest disrupted sequential phonological processing from the angular and lateral occipital gyri to the supramarginal gyrus. Substitution errors suggest impaired allographic conversion between hiragana (one form of kana) and katakana (another form of kana) attributable to a dysfunction in the angular/lateral occipital gyri[15].
As limitations of our study we should mention the lack of functional data (it is a purely anatomical study), the absence of gender balance, the formalin effect on the specimens and the potential cerebral atrophy. However, the last two limitations could potentially affect the absolute cortical thickness values but not the correlations we found. Furthermore, potentially useful details concerning the manual dominance or potential neurological history of the donors were unfortunately not available. On the other hand, thickness overestimation (observed in MRI studies[4]) was not an issue in our study due to the anatomical method of cortical thickness measuring used.
Our results could be parallelized with a glimpse of our brain’s mysteries. Their interpretation is anything but easy. However, our results suggest that there could be a potentially stronger impact for objects placed in the left inferior quarter of the visual field. Our study also revealed several thickness-based correlations among different functional cortical areas. Most of them seem to have a more or less rational explanation. Furthermore, we believe that functional imaging of the cerebral cortex could be able to provide us with a more documented approach to the interpretation of such findings, especially when combined with clinical data.
The knowledge that specific cortical areas of the brain are correlated with specific functions, known at least since the 19th century, resulted in several functional maps of the cerebral cortex as well as the famous motor and sensory homunculi. Modern functional neuroimaging techniques allowed this knowledge to evolve, revealing, however, the complexity of such correlations.
In human anatomy, information regarding structure can indirectly provide us with information regarding function.
Despite the significant research progress on neurosciences during the last century, there is still a lot to learn. Although functional neuroimaging methods, mainly the functional magnetic resonance imaging, offered a window with a magnificent view to brain functions, there are still well kept secrets regarding these complex functions.
The authors found a significant difference of the mean value of the cortical thickness of the superior wall of the calcarine sulcus between right and left hemispheres, as well as very significant correlations among several functional cortical areas.
The authors’ results suggest that there could be a potentially stronger impact for objects placed in the left inferior quarter of the visual field. The study also revealed several thickness-based correlations among different functional cortical areas.
The question of the study is interesting. The authors reveal several thickness-based correlations among different functional cortical areas. This is important research to help us enhance our understanding of how functional brain areas are associated with their structural substrates.
P- Reviewers Arboix A, Ardila A, Bereczki D, Bravo ERC S- Editor Wen LL L- Editor Roemmele A E- Editor Yan JL
| 1. | Bähr M, Frotscher M, Duus P. Duus’ Neurologisch-topische Diagnostik, Anatomie-Funktion-Klinik, 8 Auflage. Stuttgart: Georg Thieme Verlag 2003; . |
| 2. | Snell RS. Clinical Neuroanatomy for Medical Students. 3rd ed. Philadelphia: Lippincott Williams and Wilkins 1992; . |
| 3. | Falk D, Hildebolt C, Cheverud J, Kohn LA, Figiel G, Vannier M. Human cortical asymmetries determined with 3D MR technology. J Neurosci Methods. 1991;39:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Lüsebrink F, Wollrab A, Speck O. Cortical thickness determination of the human brain using high resolution 3T and 7T MRI data. Neuroimage. 2013;70:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A, Grydeland H, Tamnes CK, Østby Y, Fjell AM. Differentiating maturational and aging-related changes of the cerebral cortex by use of thickness and signal intensity. Neuroimage. 2010;52:172-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Lyoo IK, Sung YH, Dager SR, Friedman SD, Lee JY, Kim SJ, Kim N, Dunner DL, Renshaw PF. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006;8:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 225] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 7. | Chance SA, Tzotzoli PM, Vitelli A, Esiri MM, Crow TJ. The cytoarchitecture of sulcal folding in Heschl’s sulcus and the temporal cortex in the normal brain and schizophrenia: lamina thickness and cell density. Neurosci Lett. 2004;367:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Dorph-Petersen KA, Pierri JN, Wu Q, Sampson AR, Lewis DA. Primary visual cortex volume and total neuron number are reduced in schizophrenia. J Comp Neurol. 2007;501:290-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Duering M, Righart R, Csanadi E, Jouvent E, Hervé D, Chabriat H, Dichgans M. Incident subcortical infarcts induce focal thinning in connected cortical regions. Neurology. 2012;79:2025-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 10. | Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, Taylor J, Worsley KJ, Evans AC. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cereb Cortex. 2001;11:868-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 342] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 11. | Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1049] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 12. | Kayser C. The multisensory nature of unisensory cortices: a puzzle continued. Neuron. 2010;67:178-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Lyttelton OC, Karama S, Ad-Dab’bagh Y, Zatorre RJ, Carbonell F, Worsley K, Evans AC. Positional and surface area asymmetry of the human cerebral cortex. Neuroimage. 2009;46:895-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Rosenthal CR, Roche-Kelly EE, Husain M, Kennard C. Response-dependent contributions of human primary motor cortex and angular gyrus to manual and perceptual sequence learning. J Neurosci. 2009;29:15115-15125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Sakurai Y, Asami M, Mannen T. Alexia and agraphia with lesions of the angular and supramarginal gyri: evidence for the disruption of sequential processing. J Neurol Sci. 2010;288:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |