Peer-review started: August 30, 2021
First decision: December 2, 2021
Revised: December 13, 2021
Accepted: July 8, 2022
Article in press: July 8, 2022
Published online: July 29, 2022
Processing time: 331 Days and 11.9 Hours
Given the high prevalence of cardiovascular and pulmonary abnormalities associated with sickle cell anemia (SCA), the clinical impact caused in addition to compromising the quality of life of patients and the overcharge that it represents to the public health system, this study systematized and evaluated scientific publications on pulmonary complications and cardiovascular diseases in sickle cell patients from 1920 to 2020. This compilation aims to provide knowledge for health professionals and managers in order to draw attention to the importance of chronic diseases in SCA patients and in addition to providing elements that provide improvements in management of useful resources that contribute to improve the quality and increase the life expectancy of these patients.
To systematically compile information about cardiopulmonary changes in patients with SCA.
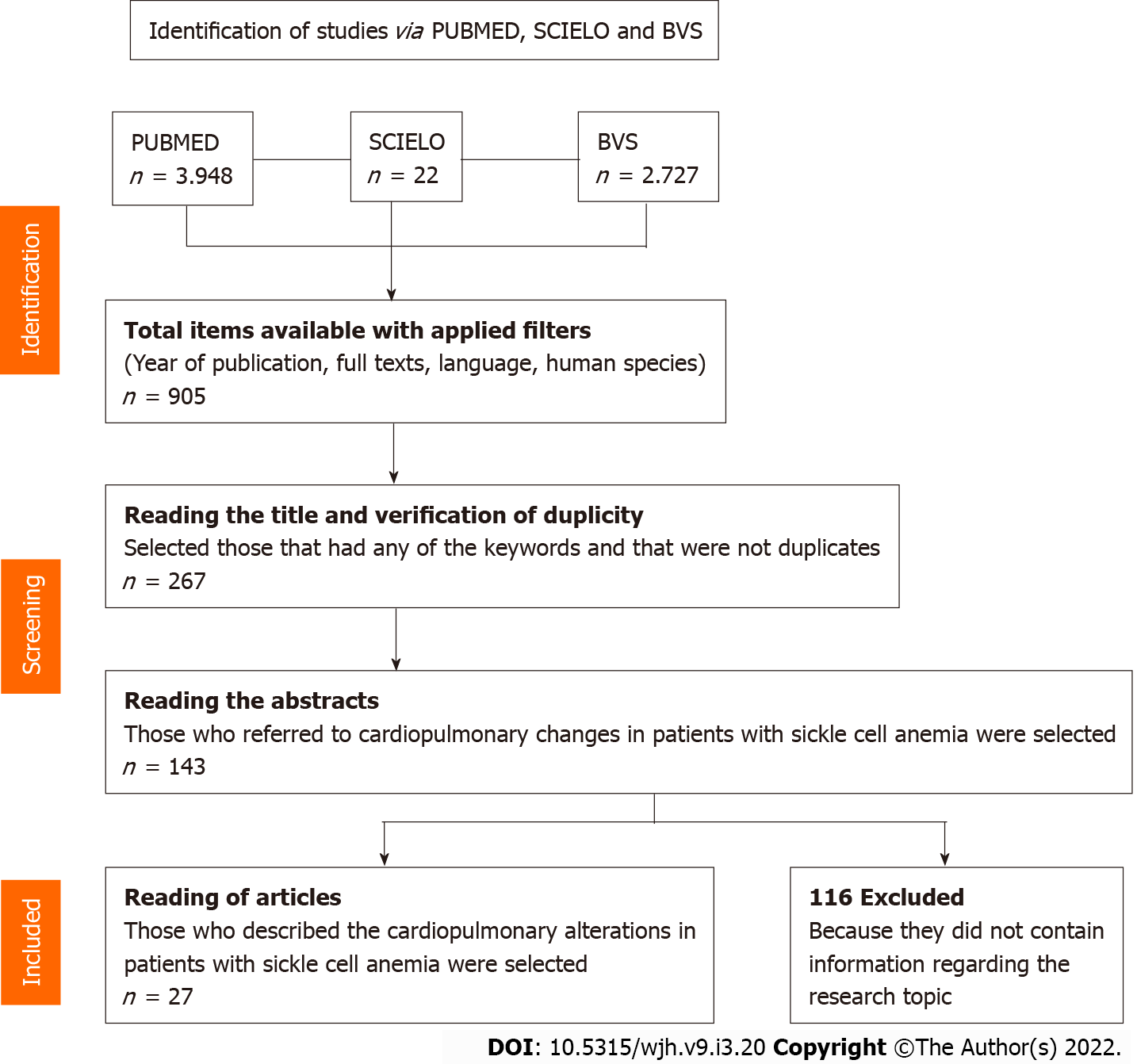
A systematic literature review was performed based on the PRISMA recom-mendation including scientific articles indexed in the Scientific Electronic Library Online databases of the United States National Library of Medicine and Biblioteca Virtual de Saúde. The search period was delimited between 1990 and 2020 and selected in Portuguese, English and Spanish. Three sets of descriptors were used for each database including research carried out with human beings. After reading the articles, those useful for this review were extracted using a collection instrument designed for this purpose.
The final selection included 27 studies. The year with the highest number of publications was 2016 with 5 studies (18.51%), followed by 2017 with 4 (14.81%). The type of study most carried out in the period was cohort 10 (37.03%) followed by cross-sectional and case-control with 8 studies in each (29.62%). Regarding the language of publication, the distribution was as follows: 25 (92.59%) in English, 1 (3.70%) in Spanish and 1 (3.70%) in Portuguese.
The findings of the present study suggest that cardiopulmonary alterations represent a serious clinical repercussion of SCA. Of the analyzed studies, the high occurrence of pulmonary hypertension, ventricular hypertrophy and diastolic dysfunction stands out as the main cardiopulmonary complications. In view of the increased survival in SCA, there is a need for surveillance and the development of strategies aimed at preserving the cardiopulmonary function and consequently improving the quality of life of these patients.
Core Tip: Sickle cell anemia (SCA) is the most common and severe form of sickle cell disease (SCD) accounting for approximately 70% of SCD cases worldwide. Illness related to SCA is an important public health problem as it is a serious chronic disease with limited possibility of cure and that causes suffering to its patients. With adult age and aging, cardiopulmonary changes are mainly observed. Given their high prevalence and the clinical impact caused to patients with SCA, this study compiled information about cardiopulmonary changes in patients with SCA.
- Citation: Silva Lopes J, Garcia Viana Í, Cordeiro Santos ML, Freire de Melo F, Oliveira MV, Souza CL. Cardiopulmonary changes in patients with sickle cell anemia: A systematic review. World J Hematol 2022; 9(3): 20-29
- URL: https://www.wjgnet.com/2218-6204/full/v9/i3/20.htm
- DOI: https://dx.doi.org/10.5315/wjh.v9.i3.20
Sickle cell anemia (SCA) is part of a group of hemoglobinopathies called sickle cell disease (SCD) in which individuals inherit hemoglobin variants derived from single-point mutations that result in morphological abnormalities in red blood cells. The SCA is the most common and severe form of the disease accounting for 70% of SCD cases in African ethnicity patients. Among the other forms of SCD, Sβ-thalassemia and heterozygous forms with hemoglobin C (HbC) and D (HbD) must be highlighted[1-3].
This hemoglobinopathy is characterized by an autosomal recessive mutation in the gene that produces HbA giving rise to HbS which forms red blood cells shaped like a crescent or sickle and makes blood oxygenation difficult causing various types of complications such as chronic hemolytic anemia, vaso-occlusive phenomena and consequent pain crises due to decreased blood perfusion. In addition, infarction and necrosis in various organs, such as bones, joints, spleen, lungs and kidneys may occur[4].
Illness related to SCD is an important public health problem worldwide as it has a great impact on morbidity and mortality in the affected population which in Brazil is estimated at 30000 patients with an annual increase of 3500 new cases. Furthermore, about 20% of children do not reach the first 5 years of life, especially when they do not have adequate medical care. It is a serious chronic disease with limited possibility of cure and that still causes significant suffering to its patients which requires special medical, genetic and psychosocial attention[5,6].
Advances in treatment and survival studies with patients with SCA demonstrates an improvement in life expectancy. A few years ago, this expectation was only 20 years which increased to an 85% chance of survival after 20 years and the implementation of neonatal diagnosis, education and comprehensive patient care programs. Although there is an increase in quality of life and longevity, clinical complications persist with adulthood and aging and others start to be observed. The chronic impact of hemolytic anemia and vaso-occlusive episodes lead to more evident progressive complications in target organs (lungs, heart, spleen, bones, brain, kidneys and skin). The development of cardiopulmonary manifestations associated with the disease include: Elevated pulmonary artery systolic pressure, pulmonary hypertension (PH), left ventricular diastolic heart disease, cardiac dysrhythmia and sudden death. In older patients, cardiopulmonary dysfunction is more intense and significantly contributes to morbidity and premature mortality[7,8].
Cardiomegaly is the main cardiac alteration seen in sickle cell patients. Myocardial dilatation and hypertrophy are other important manifestations. These changes result from hemodynamic dysfunction resulting from the reduced oxygen transport capacity imposing an increase in cardiac output (CO) which can reach up to 50% during rest in patients with SCA. This process occurs mainly due to a greater systolic volume because of the increase in preload: Product of cardiac dilation; and afterload: Due to lower peripheral vascular resistance (PVR). This hemodynamic overload also promotes the other clinical findings such as murmurs perceived on auscultation[9].
The overload in iron concentrations resulting from multiple blood transfusions is an additional factor in the pathogenesis of cardiac dysfunction. The main mechanism is related to the free iron ion that exceeds the body’s capacity to store and neutralize this element through the chelation process. Excess free iron is gradually deposited in various organs or tissues such as the heart, contributing to organ dysfunction with dilation and hypertrophy, arrhythmia and heart failure[10]. The pathophysiological mechanisms described are characterized as adaptive actions against the aggressions produced by the disease and the persistence of the changes explains why it is uncommon for the physical examination of a patient with SCA to show no changes[9].
Pulmonary complications are the main causes of morbidity and mortality in patients with SCA in all age groups. It is estimated that 90% of these adult individuals have abnormal lung function. Chronic lung disease is likely a consequence of recurrent episodes of acute chest syndrome (ACS), infections, fat embolism and pulmonary infarction. It is believed that PH is one of the main causes of death in adult patients. However, only 10% of patients with SCA are monitored for early detection of PH. Its pathophysiological mechanism is complex and probably multifactorial[9,11]. Cardiopulmonary complications can develop independently and each one of them (cardiac or pulmonary) individually contributes to greater morbidity and mortality and the combination of both is an important aggravating factor in the worsening of the prognosis of these patients[11].
Given the high prevalence of cardiovascular and pulmonary abnormalities associated with SCA, the clinical impact caused in addition to compromising the quality of life of patients and the overcharge that it represents to the public health system, this study systematized and evaluated scientific publications on pulmonary complications and cardiovascular diseases in sickle cell patients from 1920 to 2020. This compilation aims to provide knowledge for health professionals and managers in order to draw attention to the importance of chronic diseases in SCA patients and in addition to providing elements that provide improvements in the management of useful resources that contribute to improve the quality and increase the life expectancy of these patients.
This is a systematic review conducted in accordance with the PRISMA recommendation on cardiopulmonary alterations in patients with SCA. We also cite high-quality articles in Reference Citation Analysis (https://www.referencecitationanalysis.com).
Types of studies: Articles that had as their object: Cardiac or pulmonary alterations in SCA published between 1990 and 2020 in English, Portuguese and Spanish were included. Also included were cross-sectional, descriptive, quantitative, cohort, meta-analysis, case-control and experimental studies.
Types of participants: Patients with SCA who have cardiopulmonary disorders.
Types of results: Scientific articles that include results of prevalence, relative risk, outcome or data analysis about cardiopulmonary complications in patients with SCA.
The search and evaluation of scientific articles took place between June and September 2020 in the Scientific Electronic Library Online (SCIELO), United States National Library of Medicine (PUBMED) and Bibliteca Virtual de Saúde (BVS) databases.
The search strategy in the PUBMED and VHL databases included the following keywords: “sickle cell anemia and cardiovascular complications”, “sickle cell anemia and lung complications”, “sickle cell anemia and cardiopulmonary anemia”, “sickle cell anemia and cardiopulmonary”, “sickle cell anemia and lung complications”, “sickle cell anemia and cardiac complications”, “sickle cell anemia and lung” and “sickle cell anemia and cardiac”. For the SCIELO database, the terms used were: “sickle cell anemia and cardiopulmonary”, “sickle cell anemia and lung changes” and “sickle cell anemia and lung”.
Two authors of this study carried out the selection of articles independently (Silva Lopes J, Garcia Viana Í). Subsequently, verification and exclusion of duplicates was performed with subsequent reading and selection of abstracts excluding those that did not directly address cardiopulmonary changes in patients with SCA. Finally, full reading of the articles was established including only those that met the eligibility criteria.
After reading the articles, the data of interest for this review were extracted using a collection instrument developed by the authors available in the supplementary material.
The information extracted from the studies included: Year of publication, title, journal/magazine, article objective and content synthesis.
Risk of bias in each study: The main bias found in the cross-sectional studies presented here was the establishment of causality, an inherent characteristic of this type of study. Reduced sampling was reported in three articles. In case-control studies, a selection bias prevailed, especially in the selection of controls that were properly matched to the cases. In clinical trials, the most reported limitation was the difficulty in extrapolating the results to clinical management, due to the limited number of participants. The inability to apply “gold standard” tests was also mentioned in two articles. The sample size, the retrospective characteristic and the inability to use “gold standard” tests were the main limitations found in the cohort studies.
After applying the uni-terms, 6767 articles were found, distributed as follows: 3948 in PUBMED, 2797 in BVS and 22 in SCIELO. After applying the eligibility criteria, reading titles, excluding duplicates, reading abstracts and full texts, 27 studies that make up this review were selected (Figure 1). The main results of this study are summarized in Table 1.
| Ref. | Location | Type of study | n | Main results |
| Dham et al[15], 2009 | Washington | Case control | 364 | Children with SCA have a mild increase in SCA, which correlates with increased cardiac output and left ventricular filling pressures |
| Cuervo et al[22], 2002 | Cuba | Case control | 53 | Restrictive ventilatory dysfunction was observed in all patients with sickle cell anemia |
| Fitzhugh et al[19], 2010 | North Carolina | Retrospective transversal | 240 | This study points out the main causes of death in patients with SCA. Among them are: Pulmonary emboli, stroke and multiple organ failure. In addition, it mentions the main pre-morbid conditions found: Pneumonia, congestive heart failure, myocardial infarction and arrhythmias |
| Parent et al[13], 2011 | France | Transversal | 398 | Patients with confirmed pulmonary hypertension were older and had worse functional capacity than other patients |
| Damy et al[32], 2016 | France | Transversal | 1.780 | TRV ≥ 2.5 m/s and left ventricular dysfunction predict mortality in patients with SCA |
| Arteta et al[27], 2014 | Michigan | Cohort | 146 | It is common for children with sickle cell anemia to have abnormal lung function, most often of the obstructive type |
| Lobo et al[18], 2015 | Rio de Janeiro | Prospective transversal | 125 | Patients over 32-years-old have mostly elevated LDH, severe anemia and creatinine clearance > 1, in addition to a poor prognosis, and may be at risk of developing pulmonary hypertension |
The decade from 2010 to 2020 concentrated the largest number of publications with a total of 21 (77.77%) studies, followed by the decade from 2000 to 2010, with 5 (18.51%) studies in this period. In the decade from 1990 to 2000 only one publication was found. In 2016, 5 studies (18.51%) were published, 2017, 4 (14.81%). In 2011 and 2015, 3 studies were published each year (11.11%). In 2008, 2012 and 2018, 2 articles.
The type of study most carried out in the period was cohort 10 (37.03%) followed by cross-sectional and case-control with 8 studies each (29.62%) and a multicenter study (3.70%). Regarding the language of publication, most were published in English 25 (92.59%), with one article in Spanish and another in Portuguese. As for the age range of the studies, 10 (37.03%) referred to adulthood, 9 (33.33%) referred to pediatric patients and 8 (29.62%) included both age groups.
In compiling the data from the 27 articles, it was possible to observe that the main cardiopulmonary changes described in sickle cell patients were: PH, acute thoracic syndrome (ATS), restrictive and obstructive respiratory dysfunctions, wheezing in children, increased tricuspid regurgitation velocity (TRV), enlargement of the left ventricle (LV) and left atrium (LA) and diastolic dysfunction with normal systolic function. It was also possible to observe that PH and ACS are among the most important causes of morbidity and mortality in patients with SCA.
PH can be diagnosed through right cardiac catheterization, an invasive method that is the gold standard for diagnosis and non-invasively by measuring the TRV with diagnostic confirmation defined as a TRV ≥ 2.5 m/s. Caughey et al[12] demonstrated that most studied patients with suspected PH had elevated TRV and 59% had values > 3.0 m/s. A higher PH detection rate was observed by measuring TRV, highlighting a greater possibility of false-positive results with this method[11,13,14].
Dham et al[15] found that children with SCA had significantly higher TRV, systolic, diastolic and mean pulmonary artery pressures than controls. The highest frequency of pediatric patients with ACS and PH found was in the age group of 5-7 years. The high prevalence of PH among younger children is an uncommon finding as this complication is known to progress with age[16]. TRV values ≥ 2.5 m/s were shown to be correlated with a history of acute chest syndrome and previous transfusions. Elevated left atrial pressure and right ventricular stroke volume were predictors of TRV in a multivariate regression model. Higher TRV was also associated with increased left ventricular and atrium chambers and higher levels of B-type natriuretic peptide, lactate dehydrogenase (LDH), amino aspartate transferase (AST), erythropoietin, urea, creatinine and reticulocytes. Another clinical finding reported in the studies found was the presence of the second heart sound with greater intensity. This change was statistically associated with PH in children with ACS and individuals with this clinical finding demonstrated a 3.4 times greater chance of having PH[12,13,17-20].
However, PVR and Hb levels were indirectly related to the increase in TRV. For every 1.0 g/dL increase in Hb, TRV decreased by 13%. The data found suggested that most adult patients with ACS and suspected PH had normal PVR. Caughey et al[12] demonstrated that the mean PVR index was significantly higher in patients with suspected PH than in those without PH although they were still below the cutoff point for elevated PVR. Only 2 individuals with suspected PH (6%) with TRV values between 3.0 and 3.9 m/s, respectively, had a high PVR index[14,15].
The pathogenesis of PH in patients with SCD is complex. The compensatory state of high CO due to chronic anemia may contribute to increased pulmonary arterial pressure (PAP) in the presence of normal PVR. PH as a manifestation of left ventricular dilatation and eccentric hypertrophy may be significant in some patients. Hemolysis is also believed to play an important role leading to nitric oxide depletion, endothelin-1 release and platelet activation. Ultimately, they result in vasculopathy characterized by endothelial dysfunction, increased vascular tone, inflammation, hypercoagulability and vascular remodeling[12].
The strong and independent associations of TRV with the velocity-time integral of the right ventricular outflow tract and the left atrial pressure index support the importance of high CO in the pathogenesis of PH in this population. A possible role for hemolysis is suggested by the negative correlation of TRV with Hb and reticulocyte counts. Circulating erythropoietin concentrations reflect the degree of tissue hypoxia and the association of a higher level of erythropoietin with higher TRV may serve as a marker of the degree of tissue hypoxia which appears to be associated with the development of PH in other conditions[12,16,17].
ATS was reported in the study by Vichinsky et al[21] in which more than two-thirds of participants with SCA had a history of ACS with multiple episodes. The cause of ACS was established in 38% of the episodes with infections and pulmonary emboli (bone marrow, fatty or thrombotic) being the main ones reported. Of the 27 different pathogens identified, Chlamydia pneumoniae was the most prevalent followed by Mycoplasma pneumoniae.
Maioli et al[11] and Cuervo et al[22] showed that the pulmonary function test in patients with ACS can identify different elements related to the evolutionary stage of the disease, including restrictive ventilatory dysfunction, observed in patients with SCA, regardless of a previous history of SCA. However, a history of 2 or more episodes of AST makes this clinical manifestation the most important risk factor for chronic lung damage and consequently, characteristic ventilatory changes. MacLean et al[23] and Cuervo et al[22] also reported that obstructive pulmonary abnormalities occur first followed by the development of restrictive abnormalities which become more prominent with increasing age in children and adolescents with SCA. A history of asthma or wheezing, bronchopulmonary dysplasia, cystic fibrosis, bronchiolitis and a higher concentration of LDH were associated with obstructive pulmonary disease reflecting lower TFP values. It was also observed that low forced expiratory volume in 1 s (FEV1%) was considered an independent predictor of early death in adults with SCA, with a decrease in FEV1% being associated with an increase in the measurement of TRV[23,24].
Throughout life, patients with SCA have the lung parenchyma subject to episodes of ischemia during vaso-occlusion crises. These events sometimes lead to necrosis and subsequent regeneration with formation of fibrotic tissue. These pathophysiological mechanisms can occur during ACS or in the course of a vaso-occlusive chest crisis so that with advancing age, the lung parenchyma starts to present more fibrotic tissue contributing to the onset of the restrictive change which justifies the increase in the percentage of restrictive disorders from the age of 25 onwards. An association between restrictive changes and increased left ventricular size was also observed. LV dilation can reduce lung volume due to pulmonary congestion and the direct effect of heart compression on the lung parenchyma. In obstructive changes, they reported that increased capillary blood volume and hemolysis may contribute to increased airway obstruction in children with SCA[11,23-28].
Other pulmonary alterations associated with SCA have been described: Mosaic attenuation pattern on computed tomography (CT) associated with increased TRV, decreased hemoglobin levels and reduced respiratory muscle strength in a ground-glass pattern. Furthermore, it has been reported that children with sickle cell have more frequent wheezing compared to children without SCA and that leukocytosis is considered a risk factor for early decline in pediatric lung volumes[11,29,30]. Several mechanisms may be involved in the decrease in respiratory muscle strength in these patients: Shallow breathing due to chest pain, vaso-occlusion that affects muscle performance and chest cavity deformities resulting from successive bone infarctions. The results of the study by Maioli et al[11] suggested that the partial collapse of airway spaces after inspiration, due to respiratory muscle weakness, may explain the matte pattern in the CT of these patients. The association between elevated TRV and the appearance of a mosaic attenuation pattern on CT is indicative of occlusive vascular disease and small airway obstructive disease.
The finding of wheezing on pulmonary auscultation also supports the appearance of obstructive disease. However, the mechanisms by which leukocytes can affect lung volumes are not clear. Leukocytes are able to adhere to blood vessel walls and obstruct the lumen. They also stimulate the vascular endothelium resulting in a cascade of events that lead to tissue damage and an inflammatory reaction that further favors the phenomenon of vaso-occlusion[29,30].
The most reported cardiac alteration indicated is an enlargement of the LV and atrium and prolongation of the corrected QT (QTc) interval on the electrocardiogram. Patients with SCA also had diastolic dysfunction with increasing age (with preservation of systolic function) and, in some cases, systolic dysfunction[31,32]. The pattern of diastolic dysfunction, left atrial dilation and normal systolic function observed in these patients is consistent with an aspect of restrictive cardiomyopathy. Elevated TRV is correlated with increased PAP, being the result of pulmonary arterial endothelial dysfunction due to intravascular hemolysis. However, restrictive physiology also increases PAP and TRV secondary to increased LA pressure[31,33].
Diastolic dysfunction may also result from a combination of myocardial fibrosis, microvascular occlusions by sickle cells, ischemic events, cardiomyocyte loss and oxidative stress. Niss et al[33] reported that individuals with advanced fibrosis had concomitant diastolic dysfunction. These progressive myocardial injuries promote dilatation and increased pressure in the LA and a slight increase in retrograde pulmonary venous pressure[33,34]. The increase in LV is mainly due to hyperdynamic circulation related to anemia. In addition, abnormal loading conditions associated with anemia also lead to its dilation and consequent increase in stroke volume. Other factors, including iron overload, immunogenic factors, damage to the microcirculation from vaso-occlusive crisis and associated valvular disease may contribute to the remodeling process and cardiac dysfunction[31,33,34].
Indik et al[32] reported that prolongation of the QTc interval on the electrocardiogram was present in 39% of men and 27% of women with SCA and associated with higher values of TRV. A QTc interval greater than 450 ms in men and 470 ms in women was associated with a higher risk of death. Thus, the presence of multiple vaso-occlusive episodes throughout life may also contribute to QTc interval prolongation, coronary microvascular dysfunction and increase the risk of sudden death in SCD.
The studies compiled herein showed an important frequency of pulmonary and cardiac impairment in patients with SCA. The treatments now available have contributed to increase the life expectancy of patients. The increase in the average age of patients may imply an increase in the prevalence of cardiopulmonary alterations, in addition to other comorbidities associated with the disease. This scenario suggests the need to improve specialized and early care for these patients, especially with a view to early diagnosis of dysfunctions and monitoring of cardiopulmonary function from childhood, aiming to promote a decrease in the worsening of cases of pulmonary and cardiac dysfunction, contributing to improvement of the cardiopulmonary function of these patients and above all, allow guarantees of a better quality and expansion of life expectancy for this population.
The findings presented here suggest that cardiopulmonary alterations have an important negative clinical repercussion in patients with SCA. These changes are the result of multiple etiologies: Inflammatory, restrictive, obstructive, remodeling and vaso-occlusive. Among the changes, it is worth highlighting the high prevalence of PH, ventricular hypertrophy and diastolic dysfunction. The increased survival of patients with SCA highlights the need to develop strategies aimed at improving the quality of life of these patients. These interventions involve improving the early diagnosis of cardiopulmonary changes with specific tests and family guidance in the face of the first signs of these complications (wheezing, dyspnea and chest pain). Public health strategies for the diagnosis and monitoring of cardiopulmonary dysfunction in patients with SCA must necessarily offer specialized medical care with a specialist and complementary diagnostic tests that are sufficient for the conclusive diagnosis and treatment of clinical complications secondary to cardiopulmonary alterations.
Early diagnosis, follow-up and specialized treatment may contribute to reducing the episodes of hospital admissions due to complications which may impact on cost reduction to the health system since it is a disease that can generate long periods of hospital admissions in situations of worsening of the condition, in addition to the human impact caused. Basic health care through more frequent follow-up at basic health units, blood centers and other secondary care units and immediate seeking of medical assistance in possibly serious situations such as episodes of pain crises can determine, in the long term, less need for hospital admission.
Sickle cell anemia (SCA) is part of a group of hemoglobinopathies called sickle cell disease (SCD) in which individuals inherit hemoglobin variants derived from single-point mutations that result in morphological abnormalities in red blood cells.
Illness related to SCD is an important public health problem worldwide as it has a great impact on morbidity and mortality in the affected population which in Brazil is estimated at 30000 patients with an annual increase of 3500 new cases. Furthermore, about 20% of children do not reach the first 5 years of life, especially when they do not have adequate medical care.
This study aimed to systematically compile information about cardiopulmonary changes in patients with SCA.
A systematic literature review was performed based on the PRISMA recommendation including scientific articles indexed in the Scientific Electronic Library Online databases, United States National Library of Medicine and Biblioteca Virtual de Saúde. The search period was delimited between 1990 and 2020 and selected in Portuguese, English and Spanish. Three sets of descriptors were used for each database including only research carried out with human beings. After reading the articles, those useful for this review were extracted using a collection instrument designed for this purpose. The final selection included 27 studies.
The year with the highest number of publications was 2016 with 5 studies (18.51%), followed by 2017 with 4 (14.81%). The type of study most carried out in the period was cohort 10 (37.03%) followed by cross-sectional and case-control with 8 studies in each (29.62%). Regarding the language of publication, the distribution was as follows: 25 (92.59%) in English, 1 (3.70%) in Spanish and 1 (3.70%) in Portuguese.
The findings of the present study suggest that cardiopulmonary alterations represent a serious clinical repercussion of SCA. Of the analyzed studies, the high occurrence of pulmonary hypertension, ventricular hypertrophy and diastolic dysfunction stands out as the main cardiopulmonary complications.
In view of the increased survival in SCA, there is a need for surveillance and the development of strategies aimed at preserving the cardiopulmonary function and, consequently, improving the quality of life of these patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kainickal CT, India A-Editor: Lin FY, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Brunetta DM, Clé DV, Haes TM, Roriz-Filho JS, Moriguti JC. Manejo das complicações agudas da doença falciforme. Medicina (Ribeirão Preto). 2010;43:231-237. [DOI] [Full Text] |
| 2. | da Guarda CC, Yahouédéhou SCMA, Santiago RP, Neres JSDS, Fernandes CFL, Aleluia MM, Figueiredo CVB, Fiuza LM, Carvalho SP, Oliveira RM, Fonseca CA, Ndidi US, Nascimento VML, Rocha LC, Goncalves MS. Sickle cell disease: A distinction of two most frequent genotypes (HbSS and HbSC). PLoS One. 2020;15:e0228399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Sundd P, Gladwin MT, Novelli EM. Pathophysiology of Sickle Cell Disease. Annu Rev Pathol. 2019;14:263-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 417] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 4. | da Saúde M. Doença falciforme: atenção e cuidado: a experiência brasileira: 2005-2010 /Sickle cell disease: attention and care: Brazil's experience: 2005-2010. Brasília: Ministério da Saúde, 2014. |
| 5. | de Paiva e Silva RB, Ramalho AS, Cassorla RM. [Sickle cell disease as a public health problem in Brazil]. Rev Saude Publica. 1993;27:54-58. [PubMed] |
| 6. | Martins PRJ, Moraes-Souza H, Silveira TB. Morbimortalidade em doença falcifome. Rev Bras Hematol Hemoter. 2010;32. |
| 7. | de Araujo OM, Ivo ML, Ferreira Júnior MA, Pontes ER, Bispo IM, de Oliveira EC. Survival and mortality among users and non-users of hydroxyurea with sickle cell disease. Rev Lat Am Enfermagem. 2015;23:67-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Gladwin MT. Cardiovascular complications in patients with sickle cell disease. Hematology Am Soc Hematol Educ Program. 2017;2017:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Rojas-Jiménez S, Lopera-Valle J, Yabur-Espítia M. [Cardiopulmonary complications in sickle cell anemia]. Arch Cardiol Mex. 2013;83:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Cançado RD. Sobrecarga e quelação de ferro na anemia falciforme. Rev Bras Hematol Hemoter. 2007;29:316-326. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Maioli MC, Soares AR, Bedirian R, Alves UD, de Lima Marinho C, Lopes AJ. Relationship between pulmonary and cardiac abnormalities in sickle cell disease: implications for the management of patients. Rev Bras Hematol Hemoter. 2016;38:21-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Caughey MC, Hinderliter AL, Jones SK, Shah SP, Ataga KI. Hemodynamic characteristics and predictors of pulmonary hypertension in patients with sickle cell disease. Am J Cardiol. 2012;109:1353-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Parent F, Bachir D, Inamo J, Lionnet F, Driss F, Loko G, Habibi A, Bennani S, Savale L, Adnot S, Maitre B, Yaïci A, Hajji L, O'Callaghan DS, Clerson P, Girot R, Galacteros F, Simonneau G. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 371] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 14. | Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA. 2012;307:1254-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 15. | Dham N, Ensing G, Minniti C, Campbell A, Arteta M, Rana S, Darbari D, Nouraie M, Onyekwere O, Lasota M, Kato GJ, Gladwin MT, Castro O, Gordeuk V, Sable C. Prospective echocardiography assessment of pulmonary hypertension and its potential etiologies in children with sickle cell disease. Am J Cardiol. 2009;104:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 16. | Onyekwere OC, Campbell A, Teshome M, Onyeagoro S, Sylvan C, Akintilo A, Hutchinson S, Ensing G, Gaskin P, Kato G, Rana S, Kwagyan J, Gordeuk V, Williams J, Castro O. Pulmonary hypertension in children and adolescents with sickle cell disease. Pediatr Cardiol. 2008;29:309-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Sachdev V, Kato GJ, Gibbs JS, Barst RJ, Machado RF, Nouraie M, Hassell KL, Little JA, Schraufnagel DE, Krishnamurti L, Novelli EM, Girgis RE, Morris CR, Rosenzweig EB, Badesch DB, Lanzkron S, Castro OL, Taylor JG 6th, Hannoush H, Goldsmith JC, Gladwin MT, Gordeuk VR; Walk-PHASST Investigators. Echocardiographic markers of elevated pulmonary pressure and left ventricular diastolic dysfunction are associated with exercise intolerance in adults and adolescents with homozygous sickle cell anemia in the United States and United Kingdom. Circulation. 2011;124:1452-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Lobo CL, do Nascimento EM, Abelha R, Queiroz AM, Connes P, Cardoso GP, Ballas SK. Risk Factors of Pulmonary Hypertension in Brazilian Patients with Sickle Cell Anemia. PLoS One. 2015;10:e0137539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Fitzhugh CD, Lauder N, Jonassaint JC, Telen MJ, Zhao X, Wright EC, Gilliam FR, De Castro LM. Cardiopulmonary complications leading to premature deaths in adult patients with sickle cell disease. Am J Hematol. 2010;85:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Mekontso Dessap A, Leon R, Habibi A, Nzouakou R, Roudot-Thoraval F, Adnot S, Godeau B, Galacteros F, Brun-Buisson C, Brochard L, Maitre B. Pulmonary hypertension and cor pulmonale during severe acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 2008;177:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Vichinsky EP, Neumayr LD, Earles AN, Williams R, Lennette ET, Dean D, Nickerson B, Orringer E, McKie V, Bellevue R, Daeschner C, Manci EA. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med. 2000;342:1855-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 794] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 22. | Cuervo JRM, Leyva OC, Miyares JH, Ternblóm AP, Buchillón RL, Rodríguez LR, Agramonte O, Martínez1 EE. Modificaciones funcionales ventilatorias en pacientes con anemia drepanocítica y antecedentes de síndrome torácico agudo. Rev Cubana Hematol Inmunol Hemoter. 2002;18. [DOI] [Full Text] |
| 23. | MacLean JE, Atenafu E, Kirby-Allen M, MacLusky IB, Stephens D, Grasemann H, Subbarao P. Longitudinal decline in lung volume in a population of children with sickle cell disease. Am J Respir Crit Care Med. 2008;178:1055-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Chaturvedi S, Labib Ghafuri D, Kassim A, Rodeghier M, DeBaun MR. Elevated tricuspid regurgitant jet velocity, reduced forced expiratory volume in 1 second, and mortality in adults with sickle cell disease. Am J Hematol. 2017;92:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Sylvester KP, Patey RA, Milligan P, Dick M, Rafferty GF, Rees D, Thein SL, Greenough A. Pulmonary function abnormalities in children with sickle cell disease. Thorax. 2004;59:67-70. [PubMed] |
| 26. | Adekile AD, Azab AF, Owayed A, Khadadah M. Correlates of Pulmonary Function in Children with Sickle Cell Disease and Elevated Fetal Hemoglobin. Med Princ Pract. 2018;27:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Arteta M, Campbell A, Nouraie M, Rana S, Onyekwere OC, Ensing G, Sable C, Dham N, Darbari D, Luchtman-Jones L, Kato GJ, Gladwin MT, Castro OL, Minniti CP, Gordeuk VR. Abnormal pulmonary function and associated risk factors in children and adolescents with sickle cell anemia. J Pediatr Hematol Oncol. 2014;36:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Galadanci NA, Liang WH, Galadanci AA, Aliyu MH, Jibir BW, Karaye IM, Inusa BP, Vermund SH, Strunk RC, DeBaun MR. Wheezing is common in children with sickle cell disease when compared with controls. J Pediatr Hematol Oncol. 2015;37:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Tassel C, Arnaud C, Kulpa M, Fleurence E, Kandem A, Madhi F, Bernaudin F, Delacourt C. Leukocytosis is a risk factor for lung function deterioration in children with sickle cell disease. Respir Med. 2011;105:788-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Niss O, Quinn CT, Lane A, Daily J, Khoury PR, Bakeer N, Kimball TR, Towbin JA, Malik P, Taylor MD. Cardiomyopathy With Restrictive Physiology in Sickle Cell Disease. JACC Cardiovasc Imaging. 2016;9:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Indik JH, Nair V, Rafikov R, Nyotowidjojo IS, Bisla J, Kansal M, Parikh DS, Robinson M, Desai A, Oberoi M, Gupta A, Abbasi T, Khalpey Z, Patel AR, Lang RM, Dudley SC, Choi BR, Garcia JG, Machado RF, Desai AA. Associations of Prolonged QTc in Sickle Cell Disease. PLoS One. 2016;11:e0164526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Damy T, Bodez D, Habibi A, Guellich A, Rappeneau S, Inamo J, Guendouz S, Gellen-Dautremer J, Pissard S, Loric S, Wagner-Ballon O, Godeau B, Adnot S, Dubois-Randé JL, Hittinger L, Galactéros F, Bartolucci P. Haematological determinants of cardiac involvement in adults with sickle cell disease. Eur Heart J. 2016;37:1158-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Niss O, Fleck R, Makue F, Alsaied T, Desai P, Towbin JA, Malik P, Taylor MD, Quinn CT. Association between diffuse myocardial fibrosis and diastolic dysfunction in sickle cell anemia. Blood. 2017;130:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 34. | Whipple NS, Naik RJ, Kang G, Moen J, Govindaswamy SD, Fowler JA, Dowdy J, Penkert R, Joshi VM, Hankins JS. Ventricular global longitudinal strain is altered in children with sickle cell disease. Br J Haematol. 2018;183:796-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |