Peer-review started: May 23, 2016
First decision: July 27, 2016
Revised: October 12, 2016
Accepted: October 25, 2016
Article in press: October 27, 2016
Published online: November 6, 2016
Processing time: 166 Days and 7.6 Hours
We present an 18-year-old female with sickle cell disease, who presented with an extensive lower leg ulcer over a 12-year course of the disease. Definitive reconstruction was made using a free latissimus dorsi flap and split-skin grafts. One week before the surgery, the plasmapheresis protocol and blood transfusion were administered, in order to achieve a hemoglobin S level of ≤ 30%. Intraoperatively, the flap pedicle was rinsed with plasminogen activator inhibitor-1 until the thrombolytic agent was obtained from the comitant vein; after the arterial flow had been released, an intravenous bolus dose of heparin (2000 U) was administrated. No vascular complications occurred. Postoperatively, the patient received a 10-d course of hemodilution and a 14-d course of full-dose anticoagulation. After 8 mo postoperatively, the patient was able to walk and run, and showed complete wound healing. This case indicates that sickle cell disease is not a contraindication to free tissue transfer; however, the complications, their rate and overall outcomes for these cases are not yet clear. Herein, we provide an algorithm based on our clinical experience in this type of case and treatment, including several recommendations that may help to reduce thrombosis risk and systemic complications.
Core tip: This is a case report of a successful microsurgical reconstruction in a patient with sickle cell disease who presented with an extensive lower leg ulcer during a 12-year course of the disease. We provide several recommendations for plasmapheresis and blood transfusions before the surgical reconstruction, and the anticoagulation protocol during the procedure and the postoperative period. This case description is intended to increase our colleagues’ motivation to perform microsurgical reconstruction with a safer approach in the presence of hematologic diseases with elevated risk of thrombosis.
- Citation: Posso C, Cuéllar-Ambrosi F. Successful lower leg microsurgical reconstruction in homozygous sickle cell disease: Case report. World J Hematol 2016; 5(4): 94-98
- URL: https://www.wjgnet.com/2218-6204/full/v5/i4/94.htm
- DOI: https://dx.doi.org/10.5315/wjh.v5.i4.94
The introduction of microsurgery has opened new fields for the reconstructive surgeon, so that we are now prepared to face even more challenging cases. During vascular anastomosis in microsurgical procedures, there is always risk of thrombosis, even in experienced hands and at prestigious institutions[1,2], but especially in cases of lower leg reconstruction. Patients with hematologic diseases and hypercoagulability are usually associated with high rates of complications, including anastomotic thrombosis and flap loss[3]. Clinical experience with free flaps and sickle cell disease is limited, and clear recommendations are not available.
Herein, we describe our clinical experience with a patient with sickle cell disease, who presented with a chronic ischemic lower leg ulcer that was reconstructed successfully with a free flap.
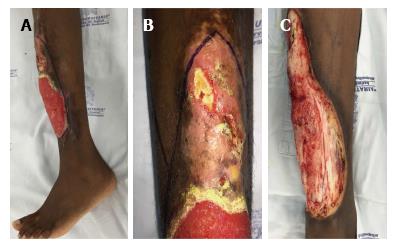
An 18-year-old Afro-American female from Chocó, Colombia, with known homozygous sickle cell disease consulted with our department regarding an extensive lower leg ulcer that had presented during a 12-year period. During that time, the patient had also presented with multiple episodes of limited osteomyelitis and soft tissue infections, for which she had been treated with systemic antibiotics; however, at no time had a real debridement and definitive reconstruction been performed because of the risk of complications (Figure 1).
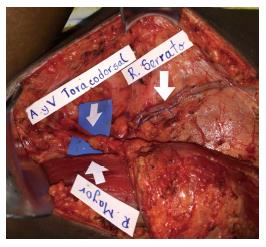
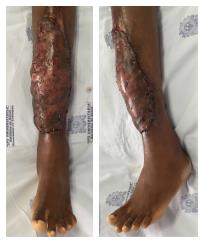
One week before the microsurgical reconstruction, we started the patient on a plasmapheresis protocol and blood transfusion, with the aim of achieving a hemoglobin S level of ≤ 30%. An extensive soft tissue debridement and an anterior tibial decortication were performed subsequently, and no obvious bone defect was created. Bone and soft tissue cultures were taken; the analysis of which provided negative results. One week later, a free latissimus dorsi flap surgery was performed to cover the soft tissue defect (20 cm × 8 cm; Figure 2). The anterior tibial vessels were determined to be compromised at the middle-third of the lower leg. Before the anastomosis, the flap pedicle was rinsed using plasminogen activator inhibitor-1 (commonly known as PAI-1), until the thrombolytic agent was obtained from the comitant vein. An end-to-end anastomosis was then made between the posterior tibial artery and the thoracodorsal artery, and only one comitant vein was anastomosed. The venous anastomosis was made first following the arterial anastomosis, and later on an intravenous bolus dose of heparin (2000 U) was administered. The total ischemia time was 40 min. A partial skin graft was made to cover the muscle (Figure 3).
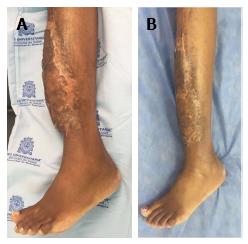
During the postoperative period, hemodilution was administered over a 10-d course; no vascular complications occurred. A 14-d course of full-dose anticoagulation (low-molecular-weight heparins) was administered as well. After 3 wk, the patient presented partial loss of the skin graft due to a superficial infection. The patient was admitted to the hospital for intravenous treatment with ciprofloxacin (400 mg/d). After 10 d, a second skin graft was made and complete healing was achieved. The final clinical result is shown in Figure 4, after 3 mo and 8 mo of follow-up.
The sickle cell trait (SCT) results from the inheritance of one normal hemoglobin gene (HbA) and one mutated beta-globin gene (a sickle hemoglobin gene, HbS). In sickle cell disease, however, two sickle hemoglobin genes are inherited. The incidence of SCT and sickle cell disease in individuals living in the geographic region of Chocó, a Northwest Colombian area with a high density of Afro-American peoples, is 11%; intriguingly, vascular events are less frequent in these cases due to geographic adaptations of the sickle gene[4]. Increased erythrocyte sickling occurs under conditions of hypoxia, acidosis, dehydration and hypothermia[5]. SCT has been associated with an increased rate of exercise-related deaths, fetal loss, pre-eclampsia and venous thromboembolism.
Patients with SCT or sickle cell disease are usually considered a higher risk group for microsurgical reconstructions, and there is some pessimism among microsurgeons when these procedures are required[6]. In particular, rates of free flap loss in lower extremity reconstructions are higher compared to those in head and neck reconstruction, where the accepted rate of failure can be up to 5%. Failure rates reported in lower leg reconstruction, in contrast, varies from 6% to 15%, depending on the case series[7].
There is growing evidence from reports of clinical experiences involving patients with hematologic diseases and microsurgery[8], but most of the case series have thus far included patients with very different types of disorders (i.e., various etiologies and risks of thrombosis or bleeding), such as an increased number of blood elements responsible for hemostasis, abnormal blood elements or abnormality within the coagulation cascade. If we consider patients with sickle cell disease exclusively, only a few cases have been reported to date, some of which include flap loss[9,10]; hence, the question remains: Can we compare different hematologic diseases and outcomes in microsurgery?
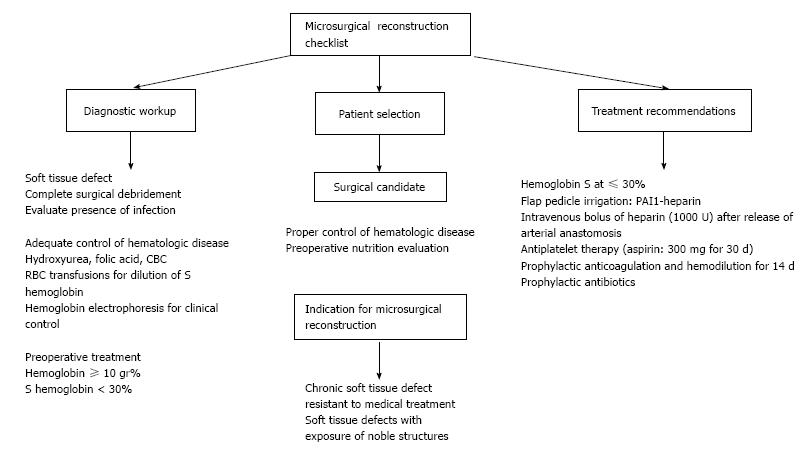
According to our experience, there are several recommendations that should be included in this particular group of patients undergoing microsurgical reconstruction (Figure 5). In the first place, control of the medical condition is essential and the hematologist should work closely with the reconstructive surgeon. Normally, the patient receives multiple transfusions to maintain the HbS level well below the recommended 30% level for a free flap surgery so that the risk of anastomosis occlusion can be minimized[11]. On the other hand, it has been strongly recommended to use prophylactic anticoagulation therapy, but there is no evidence that this treatment will avoid any type of vascular complication. The ideal duration and type of therapy are not yet known.
Regarding the microsurgical technique, Ozkan et al[12] reported some strategies that would help to avoid complications, but we disagree on some of them. First, proper flap selection does not depend on whether or not your patient has any hematologic disease; reliable anatomy is always desirable, but donor site morbidity, extension and location of the soft tissue defect should be always considered. Ozkan et al[12] also recommended large recipient vessels, but what it is really important is to perform the vascular anastomosis in a healthy zone, avoiding inflammation of the vessel wall.
In conclusion, microsurgery transfer provides a well-vascularized tissue to solve extensive soft-tissue defects in a single procedure. In patients with SCT or sickle cell disease, complications such as thrombosis, bleeding, infection or poor wound healing may occur. This particular group of patients should be evaluated carefully before surgery so as to modify any potential risk factor. We have now demonstrated that it is possible to perform a successful free flap in one of these patients, but more extensive and closer monitoring in the postoperative period and a longer regimen of anticoagulation agents might be indicated. Moreover, this is a single-case report and larger case series are needed to clearly establish outcomes and rate of complications.
An 18-year-old female with medical history of sickle cell disease presented with a 12-year history of skin ulcer in her left leg and multiple episodes of limited osteomyelitis and soft tissue infections. The ulcer was reconstructed using a free flap.
A soft tissue defect was located over the anterior surface of the lower leg with bone exposure (20 cm × 8 cm).
Chronic osteomyelitis, vascular ulcer.
The hemoglobin level at admission was consistent with severe anemia.
A vascular occlusion was present in the anterior tibial artery.
After surgical debridement, bone culture was negative.
Definitive soft tissue reconstruction was made using a free muscular flap and split-thickness skin graft.
Microsurgical reconstruction in patients with hematologic diseases has not been reported frequently in the medical literature. Furthermore, if the authors consider patients with sickle cell disease exclusively, very few case reports are available, some of which include flap loss. There are no clear recommendations on how to decrease the risk of pedicle thrombosis in this patient population.
Sickle cell disease is an inherited red blood cell disorder, in which two sickle hemoglobin genes (HbS) are inherited. Increased erythrocyte sickling and secondary thrombosis occurs under special conditions, such as hypoxia, acidosis, dehydration and hypothermia, causing vascular compromise and skin necrosis in some cases. Patients with the sickle cell trait or disease are usually considered a higher risk group for microsurgical reconstructions, and preoperative control of the disease and a different surgical protocol should be included on order to avoid vascular complications.
This is an interesting and well written clinical case report.
Manuscript source: Unsolicited manuscript
Specialty type: Hematology
Country of origin: Colombia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Carter WG, Siddiqui AH S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Spence RJ. The use of a free flap in homozygous sickle cell disease. Plast Reconstr Surg. 1985;76:616-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Khouri RK, Upton J. Bilateral lower limb salvage with free flaps in a patient with sickle cell ulcers. Ann Plast Surg. 1991;27:574-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Richards RS, Bowen CV, Glynn MF. Microsurgical free flap transfer in sickle cell disease. Ann Plast Surg. 1992;29:278-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Cuéllar-Ambrosi F, Mondragón MC, Figueroa M, Préhu C, Galactéros F, Ruiz-Linares A. Sickle cell anemia and beta-globin gene cluster haplotypes in Colombia. Hemoglobin. 2000;24:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | McAnneny A, Durden F, Pearson GD, Tiwari P. Intra-flap thrombosis secondary to acute sickle crisis: a case report. Microsurgery. 2012;32:585-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Hill JB, Vogel JE, Sexton KW, Guillamondegui OD, Corral GA, Shack RB. Re-evaluating the paradigm of early free flap coverage in lower extremity trauma. Microsurgery. 2013;33:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Lin PY, Cabrera R, Chew KY, Kuo YR. The outcome of free tissue transfers in patients with hematological diseases: 20-year experiences in single microsurgical center. Microsurgery. 2014;34:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Weinzweig N, Schuler J, Marschall M, Koshy M. Lower limb salvage by microvascular free-tissue transfer in patients with homozygous sickle cell disease. Plast Reconstr Surg. 1995;96:1154-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Platt AJ, Robertson A, Batchelor AG. Successful free flap transfer and salvage in sickle cell trait. Br J Plast Surg. 2000;53:707-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Koshy M, Weiner SJ, Miller ST, Sleeper LA, Vichinsky E, Brown AK, Khakoo Y, Kinney TR. Surgery and anesthesia in sickle cell disease. Cooperative Study of Sickle Cell Diseases. Blood. 1995;86:3676-3684. [PubMed] |
| 12. | Ozkan O, Chen HC, Mardini S, Cigna E, Hao SP, Hung KF, Chen HS. Microvascular free tissue transfer in patients with hematological disorders. Plast Reconstr Surg. 2006;118:936-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |