Peer-review started: July 21, 2016
First decision: August 5, 2016
Revised: August 31, 2016
Accepted: September 21, 2016
Article in press: September 23, 2016
Published online: November 6, 2016
Processing time: 106 Days and 1.4 Hours
To determine if activation of the ATP-gated P2X7 receptor channel induces phosphatidylserine (PS) exposure in erythrocytes from multiple dog breeds.
Peripheral blood was collected from 25 dogs representing 13 pedigrees and seven crossbreeds. ATP-induced PS exposure on canine erythrocytes in vitro was assessed using a flow cytometric Annexin V binding assay.
ATP induced PS exposure in erythrocytes from all dogs studied. ATP caused PS exposure in a concentration-dependent manner with an EC50 value of 395 μmol/L. The non-P2X7 agonists, ADP or AMP, did not cause PS exposure. The P2X7 antagonist, AZ10606120, but not the P2X1 antagonist, NF449, blocked ATP-induced PS exposure.
The results indicate that ATP induces PS exposure in erythrocytes from various dog breeds and that this process is mediated by P2X7 activation.
Core tip: Phosphatidylserine (PS) exposure in erythrocytes has potential roles in erythrocyte clearance and thrombus formation. Activation of the ATP-gated P2X7 receptor channel induces PS exposure in human erythrocytes, but whether this process occurs in erythrocytes from other mammals remained hitherto unknown. The current study shows that extracellular ATP causes PS exposure in dog erythrocytes from 13 pedigrees and seven crossbreeds. Notably, the current study shows that this process is mediated by P2X7 activation. These results suggest that P2X7-mediated PS exposure on erythrocytes may have important roles in red blood cell biology in dogs.
- Citation: Faulks M, Kuit TA, Sophocleous RA, Curtis BL, Curtis SJ, Jurak LM, Sluyter R. P2X7 receptor activation causes phosphatidylserine exposure in canine erythrocytes. World J Hematol 2016; 5(4): 88-93
- URL: https://www.wjgnet.com/2218-6204/full/v5/i4/88.htm
- DOI: https://dx.doi.org/10.5315/wjh.v5.i4.88
Exposure of the plasma membrane lipid, phosphatidylserine (PS), to the outer leaflet is an important physiological and pathophysiological signal[1]. In erythrocytes, PS exposure serves emerging roles in the clearance of senescent, damaged and diseased erythrocytes from the circulation[2]. Moreover, PS exposure can serve as a substrate for thrombin formation and incorporation of erythrocytes into thrombi[3]. PS exposure also serves as a parameter for stored erythrocyte integrity[4] and may be important in the removal of such cells following transfusion[5]. Thus, it remains important to understand the mechanisms by which PS becomes exposed on the surface of erythrocytes.
The P2X7 receptor is a trimeric ligand-gated channel activated by extracellular ATP[6] at concentrations at least 10-fold greater than that required for other purinergic receptors[7]. Functional P2X7 has been reported in humans, dogs, rodents and other species[8]. P2X7 and other purinergic receptors, namely P2X1, P2Y1 and P2Y13, are present on the plasma membrane of erythrocytes[9]. P2X7 activation induces PS exposure in human erythrocytes[10,11], but it remains unknown if P2X7 activation mediates PS exposure in erythrocytes from other species. ATP can induce PS exposure in erythrocytes obtained from English springer spaniels[12], but whether this process occurs in other dog breeds and whether it is mediated by P2X7 activation remains to be determined. P2X7, however, is present in leukocytes from various dog breeds[13,14] suggesting that P2X7 activation may mediate PS exposure in canine erythrocytes.
Using a flow cytometric Annexin V binding assay, the current study aimed to determine if ATP induces PS exposure in erythrocytes from multiple dog breeds and whether this process is mediated by P2X7 activation.
Nucleotides were from Sigma Chemical Co. (St. Louis, MO). AZ10606120 was from Tocris Bioscience (Ellisville, MO). NF499 was from Cayman Chemical (Ann Arbor, MI).
Peripheral blood was collected from either pedigree or crossbreed dogs into VACUETTE lithium heparin tubes (Greiner Bio-One, Frickenheisen, Germany). All samples were collected from privately owned dogs presenting at the Albion Park Veterinary Hospital (Albion Park, Australia), with informed consent of owners, and in accordance with and approval from the Animal and Human Ethics Committees of the University of Wollongong (Wollongong, Australia). The animal protocol was designed to minimize pain or discomfort to the animals, and conducted according to standard veterinary practices.
Erythrocytes from peripheral blood were isolated and resuspended in NaCl medium (147.5 mmol/L NaCl, 2.5 mmol/L KCl, 5 mmol/L glucose, 20 mmol/L HEPES, pH 7.4) at a final haematocrit of 2% as described[12]. Erythrocytes were then incubated in 96-well U-bottom plates (Greiner Bio-One) in the absence or presence of nucleotide (as indicated) for 24 h at 37 °C/5% CO2. In some experiments, erythrocytes were pre-incubated in the absence or presence of AZ10606120 or NF449 for 15 min at 37 °C prior to ATP addition. Following nucleotide incubation, 20 μL of resuspended erythrocytes were washed once in 1 mL Annexin V Binding Buffer (BioLegend, San Jose, CA) (450 × g for 3 min) and labeled with fluorescein isothiocyanate (FITC)-conjugated Annexin V (BioLegend, San Diego, CA) according to the manufacturer’s instructions. Data was collected using a BD (San Jose, CA) LSR II or LSRFortessa flow cytometer and FACSDiva software. The percentage of Annexin V+ cells (PS exposure) was determined using FlowJo software (Tree Star, Inc., Ashland, OR).
Data is presented as mean ± SD. Statistical comparisons were performed using Prism 5 for Mac OS X (GraphPad Software, San Diego, CA). Differences between two or more groups were compared using a paired student’s t-test or an ANOVA (using Tukey’s multiple comparison test), respectively. Concentrations curves were fitted using the log(agonist) vs normalized response (variable slope) method.
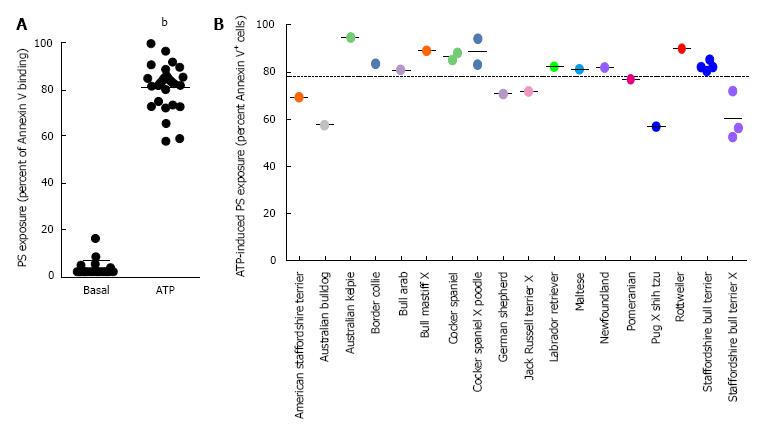
To determine if ATP could induce PS exposure in erythrocytes in dog breeds other than English springer spaniels, erythrocytes, from 25 dogs representing 13 pedigrees and seven crossbreeds, were incubated in the absence or presence of ATP and the percent of Annexin V+ cells (PS exposure) determined by flow cytometry. Incubation in the absence of ATP led to a mean PS exposure of 3.3% ± 3.2% (Figure 1A). In contrast, incubation with ATP caused a 25-fold increase in the mean PS exposure to 81.0% ± 10.4% (Figure 1A). Collectively, this resulted in an average ATP-induced PS exposure of 77.7% ± 11.8% (Figure 1B). Notably, ATP caused PS exposure in erythrocytes from all dogs studied (Figure 1B). ATP incubation also caused visible hemolysis compared to cells incubated in the absence of ATP (results not shown), but neither this nor other changes in erythrocyte morphology were investigated further.
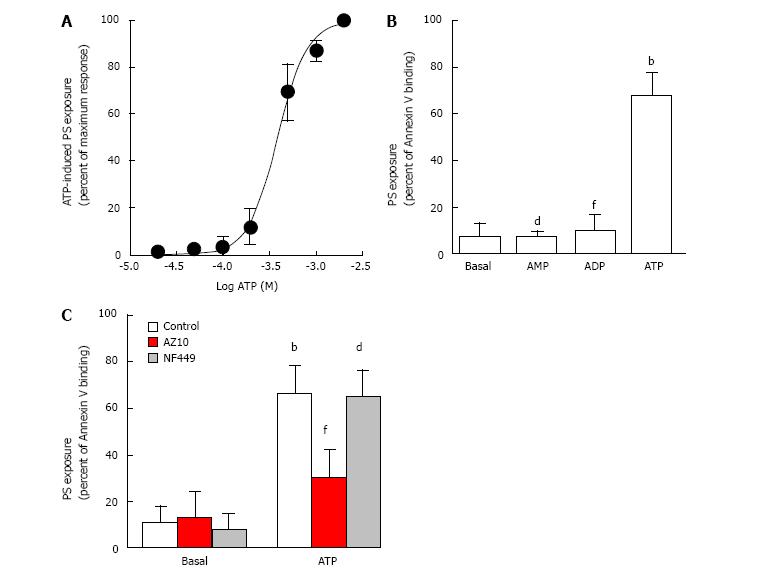
To determine if P2X7 activation mediates exposure of PS in canine erythrocytes, erythrocytes were incubated with increasing concentrations of ATP and subsequent PS exposure assessed as described above. ATP induced PS exposure in a concentration-dependent manner with a maximum response at 2 mmol/L ATP and with an EC50 value of 395 ± 45 μmol/L (Figure 2A).
To further establish if P2X7 activation mediates PS exposure in canine erythrocytes, erythrocytes were incubated with ATP, as well as ADP and AMP, which do not activate canine P2X7[12,15]. Again ATP caused robust PS exposure in erythrocytes compared to erythrocytes incubated in the absence of nucleotide (Figure 2B). In contrast, ADP and AMP did not induce PS exposure in erythrocytes, with binding of Annexin V similar to that of erythrocytes incubated in the absence of nucleotide (Figure 2B).
Finally, canine erythrocytes were pre-incubated in the absence or presence of AZ10606120, which impairs canine P2X7[15], or NF449, which impairs human and rodent P2X1[16,17], prior to ATP incubation. Pre-incubation with AZ10606120 impaired ATP-induced PS exposure by 79%, while pre-incubation with NF449 had minimal effect on ATP-induced PS exposure (Figure 2C). Neither AZ10606120 nor NF449 affected PS exposure in the absence of ATP (Figure 2C).
The current study demonstrated that ATP induces PS exposure in erythrocytes from 25 dogs representing 13 pedigrees and seven crossbreeds. On average, ATP caused PS exposure on 78% of erythrocytes from these dogs. This value is similar to that of ATP-induced PS exposure previously observed in erythrocytes from English springer spaniels (88%)[12]. Combined, these data indicate that ATP can induce PS exposure in erythrocytes from multiple dog breeds and suggests that this is likely to be a common phenomenon in all breeds of dogs. Moreover, these data confirm that ATP-induced PS exposure in canine erythrocytes is about six-fold greater than that observed for ATP-induced PS exposure in human erythrocytes[12], which corresponds to the increased expression and activity of P2X7 in canine erythrocytes compared to human erythrocytes[12,18].
Similar to human erythrocytes[10,11], the current study also demonstrates that ATP-induced PS exposure in canine erythrocytes is predominately mediated by P2X7 activation. First, the EC50 value for ATP-induced PS exposure (395 μmol/L) is similar to that observed for native and recombinant canine P2X7-mediated cation fluxes in English springer spaniel erythrocytes[12,18] and transfected HEK-293 cells[15,19], respectively; second, the non-P2X7 agonists, ADP and AMP, did not cause PS exposure; last, the P2X7 antagonist, AZ10606120, but not the P2X1 antagonist, NF449, impaired ATP-induced PS exposure. It should be noted that blockade with AZ10606120 was not complete indicating that either other purinergic receptors have an additional role in this process, or that AZ10606120 has limited efficacy in the conditions tested and that P2X7 remains solely responsible for ATP-induced PS exposure in canine erythrocytes. The latter is supported by at least three points. First, the concentration response curve for ATP-induced PS exposure revealed a simple, not biphasic, sigmoidal curve suggesting involvement of only one purinergic receptor subtype. Second, ATP concentrations below 100 μmol/L, which are sufficient to activate other ATP-responsive purinergic receptors[7], failed to cause PS exposure. Last, ADP, which can activate P2X1, P2Y1 and P2Y13, but not P2X7[7], all of which are present in human or rodent erythrocytes[20-22], did not induce PS exposure.
It remains unknown why the relative amounts of P2X7 differ between canine and human erythrocytes, but we have previously speculated[12] that this difference may be due to alterations in the proteolytic systems mediating maturation-associated degradation in reticulocytes between these two species. Differences in erythrocyte P2X7 activity between these two species are unlikely to be due to altered expression of splice variants. Previous immunoblotting studies using an antibody to the extracellular loop of P2X7, which is predicted to bind all known splice variants of canine P2X7 (URL: http://www.ncbi.nlm.nih.gov/gene/448778) and human P2X7[23,24], demonstrated only the full-length receptor in erythrocytes from both species[12]. Notably, the lifespans of canine and human erythrocytes are similar (approximately 115 d)[25] suggesting that P2X7-induced PS exposure in erythrocytes is unlikely to influence the removal of senescent cells.
In the current study, ATP caused visible hemolysis of canine erythrocytes, however this was not formally investigated. We have previously observed that 24 h ATP incubation induces a small but significant amount hemolysis of erythrocytes from English springer spaniels compared to those incubated in the absence of ATP (16% vs 1%, respectively)[12]. Future studies are required to explore if this ATP-induced hemolysis is mediated by P2X7 or other purinergic receptors, such as P2X1 or P2Y1, which can also mediate hemolysis[20,21]. Also, it remains unknown if 24 h ATP incubation causes other changes in erythrocyte morphology. Five minutes incubation with 1 mmol/L ATP of beagle erythrocytes increases cell viscosity as assessed by filterability of packed cells, but not changes in cell shape as observed by light microscopy[26]. Therefore, further studies could explore if activation of P2X7 or other purinergic receptors alters canine erythrocyte morphology.
In conclusion, the current study indicates that P2X7 activation induces PS exposure in canine erythrocytes and that this phenomenon is common to many, if not all, dog breeds. The physiological importance of P2X7-induced PS exposure in canine erythrocytes, as for human erythrocytes, remains to be established. The tendency of human erythrocytes to undergo ATP-induced PS exposure does not change with erythrocyte age[11], further supporting the concept that P2X7-induced PS exposure in erythrocytes is unlikely to be involved in the removal of aged cells. Instead, it remains plausible, that P2X7-induced PS exposure in erythrocytes is responsible for the clearance of these cells during cell stress, damage or disease. Alternatively, but not mutually exclusive to this point, P2X7-induced PS exposure in erythrocytes may facilitate thrombus formation to promote wound healing and immunity during tissue injury or infection, or to inadvertently cause vasocclusion in disorders such as malaria, sickle cell disease or diabetes. The robust PS exposure in canine erythrocytes following P2X7 activation will provide a valuable experimental model to understand further the role of this receptor in red blood cell biology. Finally, whilst PS exposure is routinely reported in canine platelets[27,28] and to some extent canine leukocytes[29,30], to the best of our knowledge PS exposure in canine erythrocytes is limited to our preliminary[12] and current observations. Thus, these studies support a rationale for exploring the physiological and pathophysiological roles and consequences of PS exposure in erythrocytes within dogs.
The authors are grateful to Vanessa Sluyter (University of Wollongong) for technical assistance and pet owners for samples.
Exposure of phosphatidylserine (PS) in erythrocytes has roles in erythrocyte clearance and thrombus formation. Activation of P2X7 by extracellular adenosine triphosphate (ATP) induces PS exposure in human erythrocytes, but whether this process occurs in erythrocytes from dogs was unknown. Therefore this study aimed to determine if ATP can induce PS exposure in erythrocytes from dogs and if so, whether this process is mediated by activation of P2X7.
The mechanisms by which PS exposure on dog erythrocytes and the function of P2X7 on these cells occurs remain poorly characterised. Moreover, there are limited reports of PS exposure on dog erythrocytes in any context.
This study demonstrated that extracellular ATP causes PS exposure in dog erythrocytes from multiple breeds and that this process is mediated by activation of P2X7.
This study suggests that P2X7-mediated PS exposure on erythrocytes may have important roles in red blood cell biology in dogs. This may have potential therapeutic or biomarker applications. Moreover, the relatively high amount of P2X7-mediated PS exposure on dog erythrocytes may provide a model to study this process, including its biological significance, in greater detail.
PS is a phospholipid that is predominately localized to the inner layer of the lipid bilayer of the plasma membrane of healthy cells, but can become localized to the outer layer (exposed) following cellular activation. Annexin V is a PS-binding protein that can be conjugated to a fluorescent label and used to study cellular PS exposure by fluorescent techniques such as flow cytometry. The P2X7 receptor is a plasma membrane ligand-gated channel activated by extracellular ATP.
It is a well written interesting paper studying ATP-induced PS exposure, which has potential roles in erythrocyte clearance and thrombus formation, from various dog breeds and showing that this process is mediated by P2X7 activation.
Manuscript source: Invited manuscript
Specialty type: Hematology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Classen CF, Moschovi MA S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Bevers EM, Williamson PL. Getting to the Outer Leaflet: Physiology of Phosphatidylserine Exposure at the Plasma Membrane. Physiol Rev. 2016;96:605-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 338] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 2. | Lang E, Lang F. Triggers, inhibitors, mechanisms, and significance of eryptosis: the suicidal erythrocyte death. Biomed Res Int. 2015;2015:513518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Du VX, Huskens D, Maas C, Al Dieri R, de Groot PG, de Laat B. New insights into the role of erythrocytes in thrombus formation. Semin Thromb Hemost. 2014;40:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Dinkla S, Peppelman M, Van Der Raadt J, Atsma F, Novotný VM, Van Kraaij MG, Joosten I, Bosman GJ. Phosphatidylserine exposure on stored red blood cells as a parameter for donor-dependent variation in product quality. Blood Transfus. 2014;12:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 5. | Bosman GJ. Survival of red blood cells after transfusion: processes and consequences. Front Physiol. 2013;4:376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Jiang LH, Baldwin JM, Roger S, Baldwin SA. Insights into the Molecular Mechanisms Underlying Mammalian P2X7 Receptor Functions and Contributions in Diseases, Revealed by Structural Modeling and Single Nucleotide Polymorphisms. Front Pharmacol. 2013;4:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Jacobson KA, Müller CE. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology. 2016;104:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 8. | Bartlett R, Stokes L, Sluyter R. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol Rev. 2014;66:638-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 332] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 9. | Sluyter R. P2X and P2Y receptor signaling in red blood cells. Front Mol Biosci. 2015;2:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Sluyter R, Shemon AN, Wiley JS. P2X(7) receptor activation causes phosphatidylserine exposure in human erythrocytes. Biochem Biophys Res Commun. 2007;355:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Sophocleous RA, Mullany PR, Winter KM, Marks DC, Sluyter R. Propensity of red blood cells to undergo P2X7 receptor-mediated phosphatidylserine exposure does not alter during in vivo or ex vivo aging. Transfusion. 2015;55:1946-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Sluyter R, Shemon AN, Hughes WE, Stevenson RO, Georgiou JG, Eslick GD, Taylor RM, Wiley JS. Canine erythrocytes express the P2X7 receptor: greatly increased function compared with human erythrocytes. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2090-R2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Jalilian I, Peranec M, Curtis BL, Seavers A, Spildrejorde M, Sluyter V, Sluyter R. Activation of the damage-associated molecular pattern receptor P2X7 induces interleukin-1β release from canine monocytes. Vet Immunol Immunopathol. 2012;149:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Spildrejorde M, Curtis SJ, Curtis BL, Sluyter R. Extracellular adenosine 5’-triphosphate and lipopolysaccharide induce interleukin-1β release in canine blood. Vet Immunol Immunopathol. 2014;157:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Spildrejorde M, Bartlett R, Stokes L, Jalilian I, Peranec M, Sluyter V, Curtis BL, Skarratt KK, Skora A, Bakhsh T. R270C polymorphism leads to loss of function of the canine P2X7 receptor. Physiol Genomics. 2014;46:512-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Braun K, Rettinger J, Ganso M, Kassack M, Hildebrandt C, Ullmann H, Nickel P, Schmalzing G, Lambrecht G. NF449: a subnanomolar potency antagonist at recombinant rat P2X1 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Hülsmann M, Nickel P, Kassack M, Schmalzing G, Lambrecht G, Markwardt F. NF449, a novel picomolar potency antagonist at human P2X1 receptors. Eur J Pharmacol. 2003;470:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Stevenson RO, Taylor RM, Wiley JS, Sluyter R. The P2X(7) receptor mediates the uptake of organic cations in canine erythrocytes and mononuclear leukocytes: comparison to equivalent human cell types. Purinergic Signal. 2009;5:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Roman S, Cusdin FS, Fonfria E, Goodwin JA, Reeves J, Lappin SC, Chambers L, Walter DS, Clay WC, Michel AD. Cloning and pharmacological characterization of the dog P2X7 receptor. Br J Pharmacol. 2009;158:1513-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Skals M, Jorgensen NR, Leipziger J, Praetorius HA. Alpha-hemolysin from Escherichia coli uses endogenous amplification through P2X receptor activation to induce hemolysis. Proc Natl Acad Sci USA. 2009;106:4030-4035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Tanneur V, Duranton C, Brand VB, Sandu CD, Akkaya C, Kasinathan RS, Gachet C, Sluyter R, Barden JA, Wiley JS. Purinoceptors are involved in the induction of an osmolyte permeability in malaria-infected and oxidized human erythrocytes. FASEB J. 2006;20:133-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Wang L, Olivecrona G, Götberg M, Olsson ML, Winzell MS, Erlinge D. ADP acting on P2Y13 receptors is a negative feedback pathway for ATP release from human red blood cells. Circ Res. 2005;96:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Cheewatrakoolpong B, Gilchrest H, Anthes JC, Greenfeder S. Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem Biophys Res Commun. 2005;332:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Feng YH, Li X, Wang L, Zhou L, Gorodeski GI. A truncated P2X7 receptor variant (P2X7-j) endogenously expressed in cervical cancer cells antagonizes the full-length P2X7 receptor through hetero-oligomerization. J Biol Chem. 2006;281:17228-17237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Rettig MP, Low PS, Gimm JA, Mohandas N, Wang J, Christian JA. Evaluation of biochemical changes during in vivo erythrocyte senescence in the dog. Blood. 1999;93:376-384. [PubMed] |
| 26. | Parker JC, Snow RL. Influence of external ATP on permeability and metabolism of dog red blood cells. Am J Physiol. 1972;223:888-893. [PubMed] |
| 27. | Jandrey KE, Norris JW, Tucker M, Brooks MB. Clinical characterization of canine platelet procoagulant deficiency (Scott syndrome). J Vet Intern Med. 2012;26:1402-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Wills TB, Wardrop KJ, Meyers KM. Detection of activated platelets in canine blood by use of flow cytometry. Am J Vet Res. 2006;67:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Singh SK, Dimri U, Sharma MC, Swarup D, Sharma B. Determination of oxidative status and apoptosis in peripheral blood of dogs with sarcoptic mange. Vet Parasitol. 2011;178:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Singh SK, Dimri U, Sharma MC, Swarup D, Sharma B, Pandey HO, Kumari P. The role of apoptosis in immunosuppression of dogs with demodicosis. Vet Immunol Immunopathol. 2011;144:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |