Peer-review started: August 13, 2015
First decision: September 17, 2015
Revised: December 12, 2015
Accepted: January 5, 2016
Article in press: January 7, 2016
Published online: February 6, 2016
Processing time: 169 Days and 17.7 Hours
Acquired bone marrow failure diseases (ABMFD) are a class of hematopoietic stem cell diseases with a commonality of non-inherited disruption of hematopoiesis that results in pancytopenia. ABMFDs also are a group of heterogeneous diseases with different etiologies and treatment options. The three most common ABMFDs are aplastic anemia, myelodysplastic syndrome, and paroxysmal nocturnal hemoglobinuria. Stem cell transplantation is the only treatment that can cure these diseases. However, due to high therapy-related mortality, stem cell transplantation has rarely been used as a first line treatment in treating ABMFD. With the advance of personalized medicine and precision medicine, various novel cellular therapy strategies are in trial to increase the efficiency and efficacy of ABMFD treatment. This article aims to review current available stem cell transplantation protocols and promising cellular therapy research in treating ABMFD.
Core tip: Stem cell transplantation is the only method can cure acquired bone marrow failure diseases (ABMFD). However, due to the high mortality rate of stem cell transplantation itself, this method is not usually used as the first line treatment for ABMFD. With the advance of current cellular therapy technology, it is becoming possible to cure ABMFD without significant treatment related complications.
- Citation: Sun XS, Liu X, Xu KL, Chen A, Rybka WB, Pu JJ. Advances and perspectives on cellular therapy in acquired bone marrow failure diseases. World J Hematol 2016; 5(1): 31-36
- URL: https://www.wjgnet.com/2218-6204/full/v5/i1/31.htm
- DOI: https://dx.doi.org/10.5315/wjh.v5.i1.31
Acquired bone marrow failure diseases (ABMFD) are a group of rare hematologic disorders manifested by insufficient hematopoiesis to produce a sufficient amount of red blood cells, white blood cells, or thrombocytes. ABMFD can occur after exposure to viral infections, toxins, chemicals, or radiation. ABMFD includes aplastic anemia (AA), myelodysplastic syndrome (MDS), and paroxysmal nocturnal hemoglobinuria (PNH). Though the pathogenesis of these diseases is heterogeneous, the high similarity of their clinical manifestation and their bone marrow pathophysiological presentation makes them hard to distinguish from each other. ABMFD can be cured by stem cell transplantation. However, because of the high mortality rate of this therapy, stem cell transplantation has not usually been used as a first line treatment for ABMFD. Currently, there is a lack of literature that offers insight into ABMFD as a class of disorders. This review offers a comprehensive overview of many of the standard and novel treatment options.
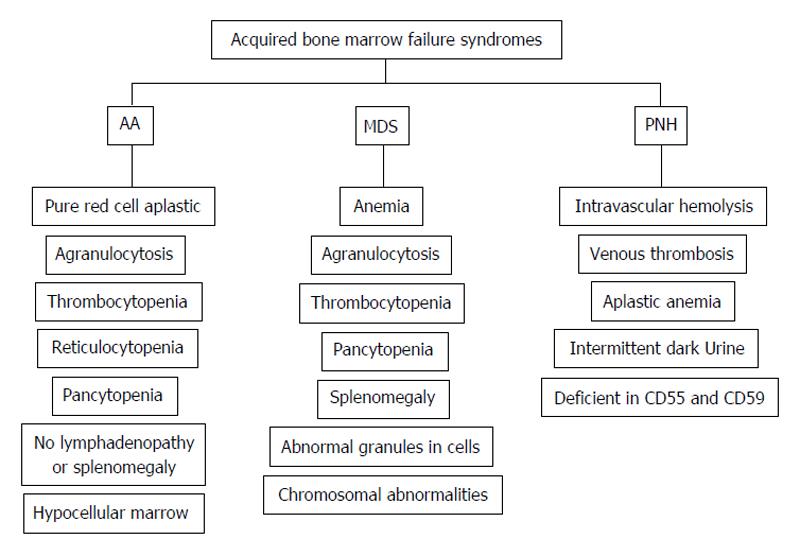
The common clinical presentations for AA, MDS and PNH are cytopenia in single or multiple hematological lineages, together with signs of impaired quality of life such as fatigue, dizziness, headache, shortness of breath, and other symptoms that are associated with prolonged anemia. The individual clinical presentations of AA, MDS and PNH are illustrated as Figure 1. Due to nearly indistinguishable clinical presentation, peripheral blood smear and bone marrow biopsies are used in the diagnosis of ABMFD.
MDS is the most common form of ABMFD, affecting around 15000 Americans each year[1]. The risk of MDS increases with age[2]: It typically affects people at age 60 years or older. In MDS, myeloid stem cell dysfunction in the bone marrow leads to ineffective hematopoiesis[3]. If left untreated, some of MDS can progress into acute myeloid leukemia. Cancer drugs such as chlorambucil, cyclophosphamide, doxorubicin, ifosfamide, mechlorethanmine, melphalan, procarbazine, and etoposide are associated with onset of treatment related MDS[3].
AA is the second most common form of ABMFD, with an incidence rate of 2.0/million to 7.4/million worldwide[4], and can be triggered by toxins, radiation, chemotherapy, viruses, medicines, autoimmune disorders, or pregnancy[4]. In AA, the bone marrow is injured and the hematopoiesis is interrupted. In most cases, AA is secondary to immune system dysfunction and subsequent premature turnover of hematopoietic cells. AA is commonly seen in young adults.
PNH is a rare hemolytic disease caused by complement system attack on cells with surface membrane glycosylphosphatidylinositol (GPI)-anchor protein deficiency. PNH affects roughly 6000 Americans each year. The clinical presentation of PNH includes hemolytic anemia, thrombosis in large blood vessels, and cytopenia or pancytopenia, depending on severity[5]. PNH appears in suddenly, but recurring episodes can be triggered by stress or physical exertion of the body. Attack on both hematopoietic cells and mature blood cells leads to formation of abnormal blood cells[6]. Abnormally weakened red blood cells will rupture. The ruptured red blood cells will release free hemoglobin that is then excreted through the kidney and stains the urine dark-colored.
Transfusion therapy is recommended as a part of supportive therapy for all ABMFDs[7,8]. The current transfusion guidelines suggests transfusion for those patients with platelet counts below 10 × 109/L (or < 20 × 109/L in febrile patients), though the ultimate decision for transfusion should be based on the patient’s overall clinical condition. Transfusions should be used cautiously because it can induce alloimmunization and autoimmunization that will complicate future treatments, such as hematopoietic cell transplantation (HCT).
Transfusion using blood from family members may induce sensitization against human leukocyte antigens (HLA) of potential HCT donors. The blood units should be carefully screened for common viruses (such as cytomegalovirus, human immunodeficiency virus, human T-lymphotropic retroviruses, hepatitis B and C, and West Nile virus), undergo leukocyte reduction, and be irradiated to avoid graft-vs-host disease (GVHD). Platelet transfusion is useful to prevent or stop thrombocytopenic bleeding. Platelet units are useful only for 3-7 d. Platelet shall be stored at room temperature to keep its activity, which, on the otherside, increases the risk of transfusion related infection. White blood cell transfusion is not highly recommended due to efficacy issue.
For AA and PNH, immunosuppressive therapy (IST) is a front line management to treat immune system dysfunction[9,10]. The complement system is a part of immune system that facilitates leukocytes and antibodies in removing pathogens. However, the over activated complement system attacking GPI-anchor protein deficient stem cells in bone marrow is the mechanism of PNH[11,12]. Different types of immunosuppressive agents, such as antithymocyte globulin (ATG), cyclosporine-A (CSA), or various anti-complement anti-bodies or complement blockers, are used with high degree of response and survival[10,13,14].
Androgens (naturally occurring male hormones) have long been used as supportive treatment for many forms of anemia, including ABMFD[10]. Either injection of androgen (testosterone) or giving medications to increase endogenous androgen production are the common approaches to increase serum androgen level. The elevated androgen levels in patient’s body may have gender-specific side effects: Men may experience enlargement of breasts or prostate, while women may experience facial hair growth, development of muscles, deepening of voice, or enlargement of the clitoris. Other side effects such as acne, jaundice due to increases in liver enzymes, and liver damage may occur. Due to the wide range of side effects, androgen therapy is limited and is typically used in combination with blood transfusions.
Hematopoietic stem cell transplantation (HSCT) is the process of treating with a conditioning regimen followed by infusion of a healthy donor’s mononuclear cells rich in hematopoietic stem cells and progenitor cells. In general, HSCT can be autologous (obtained from the patient’s own cells), syngeneic (obtained from the patient’s identical twin), or allogeneic (obtained from another individual); however, autologous HSCT is usually not a choice for ABMFD because the patient’s lack of hematopoietic stem cells. The hematopoietic stem cells can be derived from either bone marrow, peripheral blood, or umbilical cord blood. The advantages and disadvantages of each type of hematopoietic stem cell transplantation are shown in Table 1. GVHD is one of the most common and serious complications. The risk and the severity of GVHD are largely related with the degree of HLA tissue type match between the donor and the recipient. Typically, a sibling has a 25% probability of being a perfect match for the recipient’s eight major HLA antigens. The chance of finding an unrelated match ranges from 10% for some minority groups, to around 60%-70% for Caucasians in the United States.
| Sources of stem cells for transplantation | Peripheral blood | Bone marrow | Cord blood |
| Advantages | Abundant supply | Abundant supply | Rapid procedure |
| Easy to collect and differentiate | Easy to storage | Less GVHD | |
| No surgical procedure | Relatively fast engraftment | Tolerance of HLA-mismatching | |
| Short recovery period | Autologous cells are immune compatible | ||
| Fastest engraftment | |||
| Low rates of morbidity and mortality | |||
| Disadvantages | High risk of GVHD | Surgical procedure | Limited number of stem cells |
| Requirement of close HLA-matching | Long recovery period | Difficult to grow and differentiate | |
| High risk of GVHD | Slow engraftment | ||
| Requirement of close HLA-matching | Tissue rejection |
Quite often, ABMFD occurs in patients that receive high doses of radiation therapy and/or chemotherapy. HSCT is often used, following cancer treatments, to facilitate recovery from high doses of radiation therapy and/or chemotherapy by replacing damaged or destroyed stem cells in the bone marrow and restoring hematopoiesis. HSCT for ABMFD has showed promising results[15].
Peripheral blood stem cell transplantation (PBSCT) involves harvesting stem cells from the peripheral blood cells (peripheral blood is composed of erythrocytes, leukocytes and platelets) of the donor[16]. Before harvesting, donors are usually injected with granulocyte colony-stimulating factor to promote stem cell growth and release into the peripheral blood[17]. Currently, PBSCT is the most commonly performed HSCT due to easy access to peripheral blood stem cells and quick donor peripheral blood cell recovery[16].
Harvesting bone marrow stem cells is particularly complex procedure, compared to harvesting peripheral blood and umbilical cord blood. The donor must be given a general anesthetic and placed in an operation room. During the procedure, an aspiration needle is inserted at multiple points of the iliac crest region to collect approximately one liter (10-15 mL/kg) of bone marrow targeting a harvest of 2-4 × 108 nucleated cells per kilogram of recipient weight. The marrow is then filtered prior to infusion into the recipient. In the past, bone marrow stem cell transplantation was the only option available for HSCT, but due to the many obstacles in harvesting and health risks to the donor, other HSCT sources are becoming more frequently used. However, bone marrow stem cell transplantation is still a preferred option for ABMFD partially due to fewer amounts of lymphocytes in bone marrow reducing the risk and intensity of GVHD.
Umbilical cord blood collections are typically obtained from allogeneic, unrelated donors[18]. Cord blood is harvested from the leftover blood of the placenta and umbilical cord after a birth. The hematopoietic stem cells are filtered from the cord blood and kept frozen in storage. Total cord blood stem cell content is usually less than that obtained from peripheral blood or bone marrow, but the cord blood stem cells have higher hematopoietic potential and are able to produce more blood per cell than their counterparts. Due to the lesser quantity of cord blood stem cells, this type of transplantation is given to children or adults of smaller stature. There does not seem to be a strong association between HLA matching and acquiring GVHD and only one-third of patients can find a HLA-identical donor[19,20]. Thus, cord blood transplantation is beneficial for patients that cannot find an acceptable donor based on their HLA loci[21].
As of yet, there have been no clinical trials that have compared IST and HSCT. However, many cohort studies have been completed to analyze overall survival, quality of life and failure-free survival. Survival using HSCT is highly dependent upon the age of patients and donor matching (HLA-identical donor transplants showed the highest proportion of survival). While in general, studies reported that for IST and HSCT overall survival and event-free survival were similar in the two groups, HSCT in patients that received HLA-identical transplants resulted in higher survival than patients receiving IST. Adjusting for quality of life, HSCT patients enjoyed longer periods without symptoms or drug toxicity than IST patients. In the past, most patients received IST due to the inability to find an HLA-identical donor, but with scientific advancement in combatting GVHD and rejection and improved survival in transplants involving unrelated donors, HSCT is being more frequently used.
Clinicians and researchers are working towards developing novel therapies to cure ABMFD. The goal of novel cellular therapies is to increase patient accessibility, improve feasibility, and reduce procedure related complications. The methods range from improvements upon traditional methods, such as haploidentical transplantation, amplified umbilical blood transplantation, and mesenchymal cell transplantation, to novel ideas such as thrombocyte stimulator and chimeric antigen receptor T-cells.
Haploidentical HSCT has been used frequently in the past, for patients that are unable to find a HLA identical donor[22]. Haploidentical HSCT, by itself, leads to the great amount of complications due to unmatched HLA, such as GVHD, graft failure, or infection, resulting in significant morbidity or mortality[23]. Recently, haploidentical HSCT has been used in combination with immunosuppressive techniques to counteract the side effects of unmatched HLA. The overall goal of these combination therapies is to induce acceptance of unmatched donor stem cells in the recipient’s bone marrow via conditioning.
Full or partial T-cell depletion in combination with non-myeloablative haploidentical HSCT has shown good preliminary results[24,25]. Immunotoxins are used to fully or partially eliminate the T-cells of the HSCT recipient before the transplant. After the transplant, immunosuppressive agents such as ATG, CSA, or various anti-complement anti-bodies or complement blockers are given on a regular basis to prevent GVHD or rejection of the stem cells. “Megadose” haploidentical HSCT along with full T-cell depletion has also been explored[24,25]. Patients showed success in stem cell engraftment, but they experienced delayed immune reconstitution and higher rate of rejection compared with using partial T-cell depletion with normal HSCT. Variations of these types of therapies are currently being explored; some of them have showed impressive result comparable with HLA matched donor stem cell transplantation. The advantages of haploidentical HSCT combination therapy are the short waiting period in finding a donor and the brevity of the entire HSCT procedure, compared with other methods.
Umbilical cord blood HSCT offers an option to patients without a HLA matched donor. The recipients of HLA unmatched umbilical cord HSCT have significantly decreased risk of GVHD or graft failure compared to matched unrelated donor HSCT. Typically, cord blood HSCT from one donor is only sufficient to treat children or small adults. Larger adults must receive amplified cord blood from two or more donors.
Mesenchymal stem cells (MSC) are found in the bone marrow and fat and are capable of differentiating into hematopoietic cells. MSCs represent a very small proportion of all adult bone marrow cells (< 0.1%), and their exact anatomical location within the bone marrow has yet to be determined[26]. These cells are multipotent and can differentiate into osteoblasts, fat and cartilage, in addition to hematopoietic cells. When transfused into a recipient, MSCs have a tendency to migrate to areas of injury or inflammation and proliferate into resident progenitor cells, but do not induce lymphocyte differentiation, thus immune cells such as T-cells or natural killer cells do not target MSC cells. The MSCs tendency to migrate to injured and inflammatory areas also represents a downside of using this transplantation, leading to poor engraftment. MSC can be used to enhance engraftments after HSCT. Efforts have been made to overcome these difficulties by selecting homogeneous populations of MSCs that exhibit strong osteoblastic potential, through identifying and selecting cells expression of certain surface antigens (such as STRO-1 or STRO-3)[27].
For AA and PNH patients, drugs that stimulate thrombocyte production have been shown to have clinical benefits by improving blood clotting and raising blood cell levels for patients that have failed all standard therapies. This therapy provides a salvage option for AA or PNH patients, who are ineligible for immunosuppression and HSCT[28,29]. The drugs mimic thrombopoietin, which is the principal regulator of thrombocyte production, by binding of the receptor c-MPL on megakaryocytes. Initial clinical trials have shown a median increase in platelet count of 44000 per cubic millimeter for patients receiving the drug. Interestingly, it was observed that 8 of the 11 patients sensitive to the drug kept their response in a median of 10 mo. These drugs have been shown to stimulate erythrocyte and thrombocyte production[28] and are very helpful for patients who are unable to receive stem cell transplantation.
ABMFD is a group of rare but serious hematological diseases with a manifestation of insufficient blood cell formation. There are three main forms of ABMFD that share a similar clinical presentation and bone marrow histological appearance. The primary goals in treating ABMFD are to remove the underlining etiologic factors and to rebuild a healthy bone marrow for normal hematopoiesis. Stem cell transplantation is the ideal method to treatment ABFD. However, the high treatment related mortality, long-term complications such as GVHD, and lack of HLA matched donor sources hinder the practical use of this treatment option. With advances in cellular therapy, immunotherapy, and personalized medical therapy, novel gene modification/targeting therapy under precision medicine model opens a new frontier for ABFD therapy.
P- Reviewer: Fukuda S, Georgescu A, Imashuku S, Schattner M, Teimourian S S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
| 1. | Cazzola M, Malcovati L. Myelodysplastic syndromes--coping with ineffective hematopoiesis. N Engl J Med. 2005;352:536-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 2. | Gattermann N, Aul C. [Diagnosis and therapy strategy in myelodysplastic syndromes]. Praxis (Bern 1994). 1996;85:39-44. [PubMed] |
| 3. | Kurtin SE. Advances in the management of low- to intermediate-risk myelodysplastic syndrome: Integrating the National Comprehensive Cancer Network guidelines. Clin J Oncol Nurs. 2006;10:197-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 4. | Young NS, Kaufman DW. The epidemiology of acquired aplastic anemia. Haematologica. 2008;93:489-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Brodsky RA. Advances in the diagnosis and therapy of paroxysmal nocturnal hemoglobinuria. Blood Rev. 2008;22:65-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Parker C, Omine M, Richards S, Nishimura J, Bessler M, Ware R, Hillmen P, Luzzatto L, Young N, Kinoshita T. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699-3709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 517] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 7. | Danieli G. [Transfusion therapy in aplastic anemia]. G Clin Med. 1974;55:390-400. [PubMed] |
| 8. | Chan LS, Shapiro R, Buckstein R, Lin Y, Callum J, Chodirker L, Lee CD, Prica A, Lam A, Mamedov A. Initial transfusion intensity predicts survival in myelodysplastic syndrome. Leuk Lymphoma. 2014;55:2296-2300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Pu JJ, Mukhina G, Wang H, Savage WJ, Brodsky RA. Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur J Haematol. 2011;87:37-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Marsh JC, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC, Keidan J, Laurie A, Martin A, Mercieca J. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147:43-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 397] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 11. | Ben-Bassat I, Brok-Simoni F, Ramot B. Complement-sensitive red cells in aplastic anemia. Blood. 1975;46:357-361. [PubMed] |
| 12. | Parker CJ. The pathophysiology of paroxysmal nocturnal hemoglobinuria. Exp Hematol. 2007;35:523-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | DeZern AE, Uknis M, Yuan X, Mukhina GL, Varela J, Saye J, Pu J, Brodsky RA. Complement blockade with a C1 esterase inhibitor in paroxysmal nocturnal hemoglobinuria. Exp Hematol. 2014;42:857-861.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Pu JJ, Brodsky RA. Paroxysmal nocturnal hemoglobinuria from bench to bedside. Clin Transl Sci. 2011;4:219-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Santarone S, Bacigalupo A, Risitano AM, Tagliaferri E, Di Bartolomeo E, Iori AP, Rambaldi A, Angelucci E, Spagnoli A, Papineschi F. Hematopoietic stem cell transplantation for paroxysmal nocturnal hemoglobinuria: long-term results of a retrospective study on behalf of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Haematologica. 2010;95:983-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Anderlini P, Rizzo JD, Nugent ML, Schmitz N, Champlin RE, Horowitz MM. Peripheral blood stem cell donation: an analysis from the International Bone Marrow Transplant Registry (IBMTR) and European Group for Blood and Marrow Transplant (EBMT) databases. Bone Marrow Transplant. 2001;27:689-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Vadhan-Raj S, Broxmeyer HE, Spitzer G, LeMaistre A, Hultman S, Ventura G, Tigaud JD, Cork MA, Trujillo JM, Gutterman JU. Stimulation of nonclonal hematopoiesis and suppression of the neoplastic clone after treatment with recombinant human granulocyte-macrophage colony-stimulating factor in a patient with therapy-related myelodysplastic syndrome. Blood. 1989;74:1491-1498. [PubMed] |
| 18. | Schoemans H, Theunissen K, Maertens J, Boogaerts M, Verfaillie C, Wagner J. Adult umbilical cord blood transplantation: a comprehensive review. Bone Marrow Transplant. 2006;38:83-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Gluckman E, Koegler G, Rocha V. Human leukocyte antigen matching in cord blood transplantation. Semin Hematol. 2005;42:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Gluckman E, Rocha V, Arcese W, Michel G, Sanz G, Chan KW, Takahashi TA, Ortega J, Filipovich A, Locatelli F. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004;32:397-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 301] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 21. | Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, Berkowitz RL, Cabbad M, Dobrila NL, Taylor PE. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 965] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 22. | Hansen JA, Petersdorf E, Martin PJ, Anasetti C. Hematopoietic stem cell transplants from unrelated donors. Immunol Rev. 1997;157:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Eapen M, Le Rademacher J, Antin JH, Champlin RE, Carreras J, Fay J, Passweg JR, Tolar J, Horowitz MM, Marsh JC. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618-2621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | Huang XJ. Current status of haploidentical stem cell transplantation for leukemia. J Hematol Oncol. 2008;1:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Koh LP, Chao N. Haploidentical hematopoietic cell transplantation. Bone Marrow Transplant. 2008;42 Suppl 1:S60-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Battiwalla M, Hematti P. Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy. 2009;11:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Leyva-Leyva M, Barrera L, López-Camarillo C, Arriaga-Pizano L, Orozco-Hoyuela G, Carrillo-Casas EM, Calderón-Pérez J, López-Díaz A, Hernandez-Aguilar F, González-Ramírez R. Characterization of mesenchymal stem cell subpopulations from human amniotic membrane with dissimilar osteoblastic potential. Stem Cells Dev. 2013;22:1275-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Olnes MJ, Scheinberg P, Calvo KR, Desmond R, Tang Y, Dumitriu B, Parikh AR, Soto S, Biancotto A, Feng X. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 404] [Article Influence: 31.1] [Reference Citation Analysis (1)] |
| 29. | Kelly RJ, Hill A, Arnold LM, Brooksbank GL, Richards SJ, Cullen M, Mitchell LD, Cohen DR, Gregory WM, Hillmen P. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117:6786-6792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 373] [Article Influence: 26.6] [Reference Citation Analysis (0)] |