Peer-review started: October 24, 2014
First decision: November 3, 2014
Revised: December 1, 2014
Accepted: December 16, 2014
Article in press: December 17, 2014
Published online: February 6, 2015
Processing time: 104 Days and 3.1 Hours
Venous thromboembolism (VTE) encompasses deep vein thrombosis and pulmonary embolism and is a major health burden, both medically and economically. Anticoagulation is the primary treatment and can be divided into three stages: initial, long term and extended treatment. Initial anticoagulation is given to reduce the risk of complications including fatal pulmonary embolism, while long term and extended treatment are aimed at prevention of recurrent VTE. Until recently, initial anticoagulation has only been achievable with administration of parental agents such as unfractionated or low molecular weight heparin, while vitamin K antagonists such as warfarin, have been the mainstay of long term and extended treatment. Factor-Xa inhibitors and direct thrombin inhibitors are oral anticoagulants that are being increasingly utilized as an alternative form of anticoagulation. This article aims to review the current guidelines in the management of VTE, the recent literature regarding novel anticoagulants in VTE, suggested treatment regimes and limitations.
Core tip: Novel oral anticoagulants (NOACs) are emerging as viable alternatives to Vitamin K antagonist (VKA) in the treatment of venous thromboembolism. Trials have shown that they are as efficacious as current standard treatment with low-molecular-weight heparin followed by VKA, and have potentially less bleeding associated with them. The regimes are simple and no monitoring is required and therefore it has the potential to reduce the burden of anticoagulation. Caution is required however, as testing of anticoagulant effect is limited and patient selection is important as many of the NOACs are metabolized in the liver and cleared by the kidney.
- Citation: Jo HE, Barnes DJ. Role of novel oral anticoagulants in the management and prevention of venous thromboembolism. World J Hematol 2015; 4(1): 1-9
- URL: https://www.wjgnet.com/2218-6204/full/v4/i1/1.htm
- DOI: https://dx.doi.org/10.5315/wjh.v4.i1.1
Venous thromboembolism (VTE) is a common condition[1] that may be lethal or lead to chronic disease and disability[2,3]. Warfarin has been the “gold standard” in oral anticoagulation for more than 50 years however its slow onset of action necessitates treatment with parenteral anticoagulation for at least 5 d. This means that hospital admission is often required for the immediate treatment phase while warfarin reaches therapeutic levels. The therapeutic dose of warfarin for individuals also varies, reflecting differences in dietary vitamin K consumption, drug interactions and genetic polymorphisms in enzymes of warfarin metabolism. As a result, patient education, compliance and frequent monitoring are essential to ensure anticoagulation remains in the narrow therapeutic window. Sub therapeutic levels can increase the risk of thrombosis while excessive anticoagulation can result in bleeding[4]. Also, while rare, warfarin skin necrosis can be a devastating complication of warfarin therapy[5].
The novel oral anticoagulants (NOACs) have a fast onset of action and more predictable anticoagulation effect. Some of the NOACs, rivaroxaban and apixaban, can be administered without the need for initial parenteral anticoagulation and they can all be given in fixed doses with no, or little need for monitoring of anticoagulant effect. This has the potential to reduce the burden of anticoagulation for the patient, physician and the healthcare system by introducing regimes that are easy to administer and monitor. Without the need for parental anticoagulation for some of the NOACs, there is potential for patients with low risk pulmonary embolisms to be discharged early and managed at home[6].
The decision regarding length of treatment is based on the benefits and harms of continuing anticoagulation as assessed by the physician, as well as on the patient’s preference. There have been many clinical trials evaluating various durations of anticoagulant treatment for VTE[7-10]. These studies showed that anticoagulation should be continued for at least 3 mo, as treatment for shorter durations resulted in a significant increase in recurrent VTE. There was a similar rate of recurrent VTE after longer treatments of 6 to 12 mo. Studies have also shown that indefinite treatment reduces the risk of recurrent VTE by about 90% but there was also an increase in major bleeding by 1% or higher annually[11,12]. The risk of recurrent VTE was lowest in those with an isolated below knee DVT and was similar after proximal DVT and PE. It was also noted that recurrence was lower if the thrombosis was provoked by a temporary risk factor compared to an unprovoked thrombosis (HR = 0.55, 95%CI: 0.41-0.74)[13].
These results are reflected in existing guidelines in VTE management[6,14,15]. Anticoagulation for 3 mo is recommended for PE secondary to a transient, reversible risk factor. The guidelines regarding extended treatment however are less clear and promote extended treatment in patients with unprovoked PE that have low bleeding risk. They also recommend the risk-benefit ratio for anticoagulation be re-assessed at regular intervals.
With the advent of novel oral anticoagulants, the risk-benefit ratio has potentially shifted, with many of the new agents associated with lower bleeding complications than traditional treatment with warfarin. They also have simpler regimes that do not require frequent monitoring. We aim to review the current literature and examine the pharmacology of the novel anticoagulants as well as their efficacy and safety in the immediate, long term and extended treatment of VTE.
Dabigatran, an oral direct thrombin inhibitor, is the active form of the double prodrug, dabigatran etelixate. Once absorbed from the gastrointestinal tract, bioconversion of dabigatran occurs in the gut and is completed in the liver. Cytochrome P450 does not play any role in its metabolism and therefore the risk of drug interactions is low[16,17]. The bioavailability of dabigatran is however, only 8% and absorption from the gut is reliant on an acid environment, meaning that proton pump inhibitors can reduce drug absorption. Peak concentrations occur in about 2 h and the half life is 12-17 h. About 80% of dabigatran is excreted unchanged by the kidneys and therefore plasma concentrations can increase in renal impairment.
The pivotal studies looking at the role of dabigatran in VTE were the RE-COVER[18] and RECOVER II studies[19]. RE-COVER was a randomized double blind, non-inferiority study of 2539 patients comparing dabigatran with warfarin. In this phase three study, 69% of patients had DVT, 21% had PE and 10% had both PE and DVT. Both the dabigatran and warfarin groups received a full 5 d course of low molecular weight heparin before starting the drug. The dabigatran dose was 150 mg twice daily and the target INR in the warfarin group was 2-3, with intended treatment length of 6 mo.
Recurrent VTE occurred in 2.4% in the dabigatran group and 2.1% in warfarin (HR = 1.10, 95%CI: 0.65-1.84), indicating that dabigatran was non-inferior to warfarin. There was no statistically significant difference in major bleeding with 1.6% in dabigatran compared with 1.9% in warfarin (HR = 0.82, 95%CI: 0.45-1.48) having major bleeding events. There was however a reduction in major or clinically significant bleeding in the dabigatran group (5.6%) compared with warfarin (8.8%) (HR = 0.63, 95%CI: 0.47-0.84).
RE-COVER II had a similar design and tested an additional 2589 patients with acute VTE. The population was similar to the RE-COVER trial and had 68% of patients with DVT, 23% with PE and 9% with both DVT and PE. Results were similar to the previous trial and showed that dabigatran was non-inferior to warfarin. In this trial, VTE occurred in 2.3% of the dabigatran group compared with 2.2% in warfarin group (HR = 1.08, 95%CI: 0.64-1.80). There was again no difference in major bleeding with 1.2% in dabigatran and 1.7% in warfarin (HR = 0.69, 95%CI: 0.36-1.32) but again there was a reduction in major or clinically relevant non-major bleeding with 5% in dabigatran compared with 7.9% in warfarin (HR = 0.62, 95%CI: 0.45-0.84).
Dabigatran in extended therapy was evaluated in RE-MEDY[20] and RE-SONATE[20]. RE-MEDY was a phase III trial comparing extended therapy with dabigatran 150 mg twice daily with warfarin (INR 2-3). There were 2866 patients with VTE who had completed 3-12 mo of anticoagulation with either warfarin or dabigatran as part of the RE-COVER and RE-COVER II trials enrolled. Dabigatran was non-inferior to warfarin with recurrent VTE occurring in 1.8% of patients treated with dabigatran compared with 1.3% of patient treated with warfarin (HR = 1.44, 95%CI: 0.78-2.64). There seemed to be an almost 50% reduction in major bleeding in the dabigatran group compared with warfarin; (0.9%) vs (1.8%) respectively, however the confidence interval was wide and was not statistically significant (HR = 0.52, 95%CI: 0.27-1.02). There was however, a significant reduction in major or clinically relevant bleeding with almost double the amount of bleeding in warfarin group (10.2%) compared with dabigatran (5.6%) (HR = 0.54, 95%CI: 0.41-0.71).
RE-SONATE was a phase III study in 1353 patients that compared dabigatran 150 mg twice daily to placebo for 6 mo. All patients in this study had completed 6 to 18 mo of treatment for VTE with dabigatran or warfarin. Recurrent VTE was less frequent in the dabigatran group with an incidence of 0.4% vs 5.6% in the placebo arm (HR = 0.08, 95%CI: 0.02-0.25). This however, was at the expense of increased bleeding, with major bleeding occurring in 2 patients (0.3%) in dabigatran compared with none in placebo. Major or clinically significant bleeding was also more common in the dabigatran group (5.3%) compared with placebo (1.8%) (HR = 2.92, 95%CI: 1.52-5.60).
These results highlight that dabigatran, a direct thrombin inhibitor, are non-inferior to warfarin in the long term and extended treatment for VTE. There is also the added benefit of a reduction in clinically relevant and overall bleeding in dabigatran compared with warfarin. While dabigatran was effective at reducing recurrent VTE in extended therapy compared to placebo, this was, not surprisingly, associated with increased bleeding. Treatment with dabigatran however still necessitates immediate treatment with parenteral therapy, making treatment regimes with this drug more complex than some of the other novel anticoagulants. The predictable anticoagulation profile means that it does not need to be monitored and therefore would be less cumbersome than warfarin therapy. As the majority of dabigatran is cleared by the kidney, use of this drug is contra-indicated in severe renal impairment.
Rivaroxaban is a direct, selective inhibitor of factor Xa. It is well absorbed from the gut and has a bioavailability of greater than 80%[21]. Peak concentrations occur in about 3 h and its half life is 5-9 h in the young and 11-13 h in the elderly. It is metabolized in the liver via CYP3A4, CYP2C8 as well as CYP-independent mechanisms[22] and is therefore contraindicated in severe liver disease. It has few drug interactions, however drugs that have potent affects on CYP3A4 may alter plasma concentrations. Rivaroxaban is excreted via the kidney as unchanged drug (30%-40%) and as its metabolites (30%-40%), with the remainder excreted as unchanged drug in faeces. Intestinal excretion of rivaroxaban is partly mediated by P-glycoprotein, and potent inhibitors can increase drug concentrations. Because of its renal clearance, rivaroxaban should be used with caution in patients with renal impairment.
Unlike studies in other NOACs, the efficacy of rivaroxaban was assessed in DVT and PE separately in the EINSTEIN–DVT and EINSTEIN-PE studies.
EINSTEIN-DVT[23] was a phase III, open label, event driven study comparing rivaroxaban (15 mg twice daily for 3 wk, followed by 20 mg once daily) with subcutaneous enoxaparin followed by a vitamin K antagonist for 3, 6 or 12 mo in patients with acute, symptomatic DVT. There were 3449 patients included in the study, most of whom received treatment for 6 mo (63%). Rivaroxaban was non-inferior to warfarin with recurrent VTE occurring in 2.1% of the rivaroxaban group and 3.0% in the warfarin group (HR = 0.68, 95%CI: 0.44-1.04). While there was a trend towards less major bleeding with rivaroxaban (0.8%) compared with warfarin (1.2%), this did not reach statistical significance (HR = 0.65; 95%CI: 0.33-1.30). There was also no significant difference in clinically relevant non major bleeding between the two groups (rivaroxaban 7.3% and warfarin 7.0%).
EINSTEIN-PE[24] had the same design in patients with acute PE, with or without DVT. There were 4832 patients enrolled, making this the largest cohort of patients with PE to be studied across the various phase III NOAC studies. Most patients were treated for 6 mo (57.4%) with a large proportional treated for 12 mo (37.5%) and a smaller portion for 3 mo (5.2%). Rivaroxaban was non-inferior to warfarin, with recurrent VTE occurring in 2.1% in the rivaroxaban group and 1.8% in the warfarin group (HR = 1.12, 95%CI: 0.75-1.68). Major bleeding was halved in the rivaroxaban group (1.1%) compared with standard therapy (2.2%) (HR = 0.49; 95%CI: 0.31-0.79). There was however again, no significant difference in the major or clinically significant non major bleeding; 10.3% in rivaroxaban compared with 11.4% in warfarin (HR = 0.90; 95%CI: 0.76-1.07).
Rivaroxaban in extended treatment was tested in EINSTEIN-extension[23] and included 1196 patient who had completed at least 6 mo of treatment in either the EINSTEIN-DVT or EINSTEIN PE study. It was a phase III, randomized, double blind, placebo controlled study comparing an additional 6-12 mo of rivaroxaban 20 mg daily to placebo. Rivaroxaban significantly reduced the occurrence of recurrent VTE (1.3%) compared to placebo (7.1%) (HR = 0.18; 95%CI: 0.09-0.39), indicating superiority. This however, was at the expense of a small increase in bleeding with major bleeding occurring in 4 patients (0.7%) taking rivaroxaban compared with none in the placebo group. Clinically relevant non-major bleeding was also significantly greater with 5.4% in rivaroxaban and 1.2% in placebo.
These results highlight that rivaroxaban is non-inferior to warfarin with a potential reduction in major bleeding. As rivaroxaban does not require initial parenteral anticoagulation, it has the added benefit of simplicity and potential for early discharge and management in the community. In extended therapy, rivaroxaban was superior to placebo for the prevention of recurrent VTE, but also resulted in increased major and non-major bleeding.
Apixaban is small molecule inhibitor of factor Xa. It has an oral bioavailability of about 50%. Peak plasma levels are reached in about 3 h and like all other factor Xa inhibitors, the half life is short at about 12 h. Like rivaroxaban, apixaban is metabolized in the liver by CYP3A4 and CYP independent pathways. About 25% of apixaban is excreted renally with the rest excreted in faeces[22,25].
AMPLIFY[26] was the pivotal study that compared apixaban to standard therapy, with subcutaneous enoxaparin followed by warfarin, in the treatment of VTE. Apixiban was given at a higher dose of 10 mg twice daily for 7 d for initial treatment, followed by a lower dose of 5 mg twice daily for 6 mo. This was a phase III, randomized, double blind study, on 5395 patients, most of whom had DVT (65.5%) with fewer having PE (25.2%) and PE with DVT (8.8%). Apixaban was non-inferior to standard therapy with recurrent VTE occurring in 2.3% in apixaban compared with 2.7% in the conventional group (HR = 0.84; 95%CI: 0.60-1.18). Major bleeding was reduced significantly in the apixaban group (0.6%) compared with standard treatment (1.8%) (HR = 0.31 95%CI: 0.17-0.55). There was also a significant reduction in major and clinically relevant non-major bleeding with a greater than 50% reduction in the apixaban group (4.3%) compared to standard treatment (9.7%) (HR = 0.44, 95%CI: 0.36-0.55).
AMPLIFY-EXT[27] was a randomized, double blind, placebo controlled study in 2486 patients who had completed at least 6 mo of anticoagulation as part of the AMPLIFY study. Two doses of apixaban (2.5 mg twice daily and 5 mg twice daily) were compared to placebo for 12 mo Recurrent VTE occurred in 8.8% of patients on placebo compared with 1.7% on apixaban 2.5 mg and 1.7% on apixaban 5 mg. The rates of major bleeding were low in all groups with placebo (0.5%), apixaban 2.5 mg (0.2%) and apixaban 5 mg (0.1%). Rates of clinically relevant non-major bleeding were also comparable in all groups with placebo (2.3%), apixaban 2.5 mg (3.0%) and apixaban 5 mg (4.2%).
These results highlight that, not only has apixaban been shown to be non-inferior compared with standard therapy with regards to efficacy, it is also associated with less major and non-major bleeding in the treatment of VTE. In fact, the risk of major and non-major bleeding appears to be comparable to placebo in extended therapy, making apixaban a unique and attractive option. Similar to rivaroxaban, there is no need for initial treatment with parental anticoagulation and thus may help facilitate early discharge and treatment in the community in low risk PEs.
Edoxaban is an oral, direct, selective FXa inhibitor. It is rapidly absorbed with a peak concentration occurring within 1-3 h and is short acting with a half life of 9 to 11 h. It has linear pharmacokinetics within the therapeutic dose range and is not altered by food intake. It has a high oral bioavailability of about 50% which is comparable to the other FXa inhibitors. Edoxaban, like the other factor Xa inhibitors has a dual mechanism of excretion with 50% of edoxaban and its metabolites excreted renally; the remaining 50% is excreted in faeces[28].
Hokusai-VTE[29] was the pivotal study of edoxaban in VTE and was a phase III randomized, double blind study of 8240 patients with DVT (60%) and PE (40%). All patients received a heparin bridge prior to commencing edoxaban 60 mg, edoxaban 30 mg (CrCl 30-50 mL or weight < 60 kg) or warfarin for 3 to 12 mo. Recurrent symptomatic VTE occurred in 3.2% in the edoxaban group compared with 3.5% in the warfarin group (HR = 0.89 95%CI: 0.70-1.13), proving edoxaban is non-inferior to warfarin. There was no significant difference in major bleeding, with 1.4% in edoxaban compared with 1.6% in warfarin (HR = 0.84, 95%CI: 0.59-1.21). There was however, statistically less non-major bleeding with 7.2% in edoxaban and 8.9% in warfarin (HR = 0.80 95%CI: 0.68-0.93).
This was the largest single phase III study in novel oral anticoagulants in VTE and showed that edoxaban was non-inferior to warfarin in the prevention of recurrent symptomatic VTE. Edoxaban also caused less bleeding overall however there was no difference in major bleeding compared with warfarin. Like dabigatran, this study used a heparin bridge as initial treatment, rendering treatment regimes more complex and less amenable for early discharge (Table 1, Table 2, Table 3).
| Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |
| Target | IIa (thrombin) | Xa | Xa | Xa |
| Bioavailability | 8% | 66% without food | 50% | 62% |
| 100% with food | ||||
| Time to peak level (h) | 1.25-3 | 2-4 | 1-4 | 1-2 |
| Half life (h) | 12-17 | 5-9 young | 12 | 9-11 |
| 11-13 elderly | ||||
| Trough | 12-24 | 16-24 | 12-24 | 12-24 |
| Drug interactions | Proton pump inhibitors | Potent CYP3A4 and P- glycoprotein inhibitors | Potent CYP3A4 and P- glycoprotein inhibitors | P- glycoprotein inhibitors |
| Renal excretion | 80% | 35% | 25% | 50% |
| Coagulation test effect | aPPT > 2 × ULN at trough → increased bleeding | Prolonged PT may indicate excess bleeding risk but local calibration required | PT and aPTT prolonged but no known relation to bleeding risk | PT and aPTT prolonged but no known relation to bleeding risk |
| dTT at trough > 200 ng/mL or > 65 s → excess bleeding | Anti-FXa chromogenic assays: quantitative but no data on threshold values for bleeding or thrombosis | Anti-FXa chromogenic assays: quantitative but no data on threshold values for bleeding or thrombosis | Anti-FXa chromogenic assays: quantitative but no data on threshold values for bleeding or thrombosis |
| RECOVER and RECOVER II | EINSTEIN DVT | EINSTEIN PE | AMPLIFY | Hokusai VTE | |
| Drug | Dabigatran | Rivaroxaban | Rivaroxaban | Apixaban | Edoxaban |
| N | 5128 | 3449 | 4832 | 5395 | 8240 |
| Indication | VTE | DVT | PE | VTE | VTE |
| Heparin bridge | Yes | No | No | No | Yes |
| Duration (mo) | 6 | 3, 6, 12 | 3, 6, 12 | 6 | 3, 6, 12 |
| Incidence of recurrent VTE | Incidence of major bleeding | |||||
| NOAC | Warfarin | HR (95%CI) | NOAC | Warfarin | HR (95%CI) | |
| RE-COVER | 2.4% | 2.1% | 1.10 (0.65-1.84) | 1.6% | 1.9% | 0.82 (0.45-1.48) |
| RE-COVER II | 2.3% | 2.2% | 1.08 (0.45-1.48) | 1.2% | 1.9% | 0.69 (0.36-1.32) |
| RE-MEDY1 | 1.8% | 1.3% | 1.44 (0.78-2.64) | 0.9% | 1.8% | 0.52 (0.27-1.02) |
| EINSTEIN DVT | 2.1% | 3.0% | 0.68 (0.45-1.48) | 0.8% | 1.2% | 0.65 (0.33-1.30) |
| EINSTEIN PE | 2.1% | 1.8% | 1.12 (0.75-1.68) | 1.1% | 2.2% | 0.49 (0.31-0.79) |
| AMPLIFY | 2.3% | 2.7% | 0.84 (0.60-1.18) | 0.6% | 1.8% | 0.31 (0.17-0.55) |
| HOKUSAI-VTE | 3.2% | 3.5% | 0.89 (0.70-1.13) | 1.4% | 1.6% | 0.84 (0.59-1.21) |
Routine monitoring is not required for the NOACs and dosing should not be changed in response to laboratory coagulation results. There may however, be special circumstances, such as urgent surgery or serious bleeding, whereby the quantitative assessment of anticoagulant effect may be required. Measures of drug exposure may also be useful if there is concern regarding poor compliance and recurrent thrombotic effects, as well as in the presence of renal or hepatic dysfunction.
Unlike VKA monitoring, it is critical that the timing of NOAC use relative to blood testing is known, as all the NOACs have a short duration of action with maximal effect occurring at the maximal plasma concentration. Traditional anticoagulation markers such as aPTT and PT may provide a qualitative assessment however, they cannot provide a quantitative assessment. For most clinical scenarios however, qualitative information may be sufficient as clinicians are mostly concerned about extremely high or low levels of anticoagulation.
Dabigatran has almost no effect on PT and INR at clinically important concentrations and therefore these tests are not useful in determining the anticoagulant effect of direct thrombin inhibitors (DTIs). The aPTT may provide a qualitative assessment however the relationship between aPTT and dabigatran is curvilinear and results need to be interpreted with caution. In the presence of DTIs, there is a larger change in aPTT at lower concentrations but less significant change at higher concentrations. This is further complicated by the fact that the sensitivity of different aPTT reagents varies greatly. That said, a normal aPTT indicates no clinically relevant anticoagulation effect and an aPTT trough level that is above two times the upper limit of normal warrants caution, as it may be associated with a higher risk of bleeding[30].
Quantitative tests do exist but are not widely available in most hospitals as yet. Ecarin clotting time assay is a test that directly measures the activity of DTIs. When dabigatran is given twice daily, a trough level greater than 3 times normal is associated with a higher risk of bleeding[31]. A diluted thrombin test (dTT) is a test that requires calibration, but can predict the level of anticoagulation more accurately. Hemoclot is a dTT that, when used with the appropriate calibrators for dabigatran, provides a direct linear relationship between the anticoagulant effect and dabigatran concentration. A normal dTT implies no significant anticoagulant effect of dabigatran while a Hemoclot of > 200 ng/mL (equal to dTT > 65 s) at trough dabigatran plasma concentration, is associated with an increased risk of bleeding[31]. There is however no current data on a cut-off dTT below which surgery can be undertaken safely.
The different FXa-inhibitors affect PT and aPTT to a varying extent, however neither provides quantitative results. There is a weak prolongation of aPTT with paradoxical response at low concentrations as well as significant variability with different assays, thus rendering aPTT an unacceptable measure of FXa inhibitory effect[32]. The effect on PT is concentration dependent and is more reflective of FXa inhibitory effect however this also depends on both the assay and the type of FXa inhibitor. Assay specific calibrators and calibration curves can be made for rivaroxaban, providing qualitative information on anticoagulant effect[33]. No such data is currently available for apixaban or edoxaban.
Anti-FXa “chromogenic assays” are new tests that have been developed to assess plasma concentrations of the FXa-inhibitors. Theses tests require validated calibrators and are becoming increasing available commercially. Biophen DiXal is an example of such an assay and gives in vitro quantitative measurements of direct FXa inhibitors on human citrated blood plasma. It is based on the inhibition of a constant amount of exogenous FXa and the hydrolysis of a FXa specific chromogenic substrate by the residual FXa. It is suitable to measure rivaroxaban plasma concentrations in a wide range and there is acceptable inter-laboratory precision[34]. Similar studies have also been performed with apixaban showing linear dose-response curves[35,36].
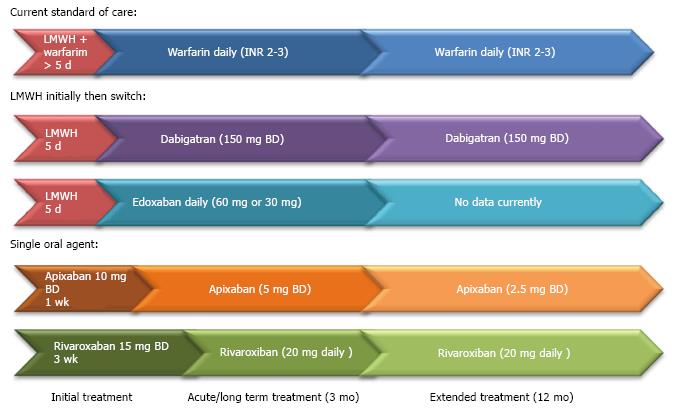
We can see that the thrombin times (aPTT and dTT) are affected by the DTIs whereas the Factor Xa inhibitors tend to prolong PT. These tests can provide qualitative assessment of anticoagulation but there are significant limitations in interpretation. While quantitative tests do exist, the major limitation, other than the availability of these tests, is that information regarding harmful and therapeutic ranges are currently lacking. The practical guide to use can see Table 1. Figure 1 shows regimes for anticoagulation in pulmonary embolism (Table 4).
| When considering the use of a NOAC, there are important steps that should be considered: |
| (1) Consideration as to whether anticoagulation is necessary |
| Does the patient have a confirmed indication for anticoagulation? |
| Did the patient have a transient risk factor for VTE that has resolved or did they have an unprovoked VTE and should be considered for extended treatment? |
| (2) Consideration as to whether a NOAC is the most appropriate choice |
| Does the patient have normal renal and liver function? |
| Does the patient have an underlying malignancy for which LMWH may be a more appropriate alternative? |
| (3) Review of any other medications that may be contra-indicated or pose unfavourable drug-drug interactions |
| Potent inhibitors: ketoconazole, itraconazole, voriconazole, posaconazole |
| Potent inducers: rifampicin, carbamazepine, phenytoin, phenobarbital, HIV protease inhibitors |
| (4) Education regarding the importance of compliance and bleeding risk |
| Due to the short half life, there is a rapid decline in protective anticoagulation |
| (5) Regular follow-up to assess: |
| Therapy adherence |
| Potential thromboembolic event |
| Any adverse events |
| Bleeding events |
| Co-medications |
| Blood tests for haemoglobin, renal and hepatic function |
| (6) Assessment to determine whether ongoing anticoagulation is necessary and beneficial |
Overall, 27048 patients have been involved in phase 3 trials into the efficacy and safety of NOACs in VTE. We can seen from the studies outlined above that all the NOACs are non inferior to warfarin with efficacy ranging between 1.8%-3.2% in the incidence of recurrent VTE in the NOAC arms and between 1.3%-3.5% in the warfarin arms. The studies have also shown either a trend or statistically significant reduction in bleeding compared with standard therapy with heparin and warfarin, with major bleeding ranging from 0.6%-1.4% in NOACs compared with 1.2%-2.2% with warfarin. Apixaban appeared to be associated with the lowest bleeding risk of all the NOACs and was comparable to placebo in extended treatment in terms of bleeding. Given that the length of anticoagulation beyond 3 mo should be determined by the risk of bleeding with anticoagulation, these results have the potential to significantly influence decisions regarding the length of treatment.
Another concept introduced by some of the NOACs is the option for no initial parenteral anticoagulation. NOACs have a fast onset of action so, unlike warfarin, parenteral anticoagulation is not a necessity. Rivaroxaban and apixaban were the only NOACs to provide an alternative regime, where instead a heparin, patients were given an increased dose of the same drug in the initial phase of treatment. This has the potential to simplify treatment and facilitate early discharge and is reflected in the most recent ESC guideline, with early discharge and treatment at home recommended in patients with low risk PE.
While the results of these trials are very enticing, it important to remember that patient selection is critical when deciding to use these agents. Patients included in these trials were younger, with less co-morbidity and lower risks of bleeding than the patients usually seen in clinical practice. Patients with significant renal and hepatic dysfunction were excluded and there were few patients with strong indications for extended anticoagulation, namely cancer, antiphopholipid syndrome and recurrent VTE, included in these studies. Also, testing of anticoagulant effect is limited in its availability and correlation to risk. Careful consideration and more real world experience are therefore needed when using these new but promising treatments.
P- Reviewer: Lazo-Langner A S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 550] [Cited by in RCA: 511] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 2. | Klok FA, van Kralingen KW, van Dijk AP, Heyning FH, Vliegen HW, Kaptein AA, Huisman MV. Quality of life in long-term survivors of acute pulmonary embolism. Chest. 2010;138:1432-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Fanikos J, Piazza G, Zayaruzny M, Goldhaber SZ. Long-term complications of medical patients with hospital-acquired venous thromboembolism. Thromb Haemost. 2009;102:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:204S-233S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 766] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 5. | Nazarian RM, Van Cott EM, Zembowicz A, Duncan LM. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs JS, Huisman MV, Humbert M, Kucher N. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)Endorsed by the European Respiratory Society (ERS). Eur Heart J. 2014;35:3033-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1883] [Cited by in RCA: 1896] [Article Influence: 172.4] [Reference Citation Analysis (0)] |
| 7. | Schulman S, Rhedin AS, Lindmarker P, Carlsson A, Lärfars G, Nicol P, Loogna E, Svensson E, Ljungberg B, Walter H. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of Anticoagulation Trial Study Group. N Engl J Med. 1995;332:1661-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 578] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 8. | Kearon C, Gent M, Hirsh J, Weitz J, Kovacs MJ, Anderson DR, Turpie AG, Green D, Ginsberg JS, Wells P. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. 1999;340:901-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 751] [Cited by in RCA: 712] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 9. | Agnelli G, Prandoni P, Santamaria MG, Bagatella P, Iorio A, Bazzan M, Moia M, Guazzaloca G, Bertoldi A, Tomasi C. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med. 2001;345:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 400] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 10. | Pinede L, Ninet J, Duhaut P, Chabaud S, Demolombe-Rague S, Durieu I, Nony P, Sanson C, Boissel JP. Comparison of 3 and 6 months of oral anticoagulant therapy after a first episode of proximal deep vein thrombosis or pulmonary embolism and comparison of 6 and 12 weeks of therapy after isolated calf deep vein thrombosis. Circulation. 2001;103:2453-2460. [PubMed] |
| 11. | Schulman S, Granqvist S, Holmström M, Carlsson A, Lindmarker P, Nicol P, Eklund SG, Nordlander S, Lärfars G, Leijd B. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. The Duration of Anticoagulation Trial Study Group. N Engl J Med. 1997;336:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 372] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Schulman S. The effect of the duration of anticoagulation and other risk factors on the recurrence of venous thromboembolisms. Duration of Anticoagulation Study Group. Wien Med Wochenschr. 1999;149:66-69. [PubMed] |
| 13. | Boutitie F, Pinede L, Schulman S, Agnelli G, Raskob G, Julian J, Hirsh J, Kearon C. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ. 2011;342:d3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 266] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 14. | Howard LS, Hughes RJ. NICE guideline: management of venous thromboembolic diseases and role of thrombophilia testing. Thorax. 2013;68:391-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S-e494S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2565] [Cited by in RCA: 2568] [Article Influence: 197.5] [Reference Citation Analysis (0)] |
| 16. | Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008;36:386-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 513] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 17. | Stangier J, Stähle H, Rathgen K, Fuhr R. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet. 2008;47:47-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 318] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 18. | Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, Baanstra D, Schnee J, Goldhaber SZ. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1838] [Cited by in RCA: 1804] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 19. | Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, Christiansen AV, Friedman J, Le Maulf F, Peter N. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129:764-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 660] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 20. | Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, Kvamme AM, Friedman J, Mismetti P, Goldhaber SZ. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 686] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 21. | Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005;78:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 478] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 22. | Gross PL, Weitz JI. New anticoagulants for treatment of venous thromboembolism. Arterioscler Thromb Vasc Biol. 2008;28:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499-2510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2218] [Cited by in RCA: 2266] [Article Influence: 151.1] [Reference Citation Analysis (0)] |
| 24. | Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, Chlumsky J, Verhamme P, Wells P, Agnelli G. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1646] [Cited by in RCA: 1691] [Article Influence: 130.1] [Reference Citation Analysis (0)] |
| 25. | Pinto DJ, Orwat MJ, Koch S, Rossi KA, Alexander RS, Smallwood A, Wong PC, Rendina AR, Luettgen JM, Knabb RM. Discovery of 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide (apixaban, BMS-562247), a highly potent, selective, efficacious, and orally bioavailable inhibitor of blood coagulation factor Xa. J Med Chem. 2007;50:5339-5356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 294] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 26. | Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Masiukiewicz U, Pak R, Thompson J, Raskob GE. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1533] [Cited by in RCA: 1639] [Article Influence: 136.6] [Reference Citation Analysis (0)] |
| 27. | Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Porcari A, Raskob GE, Weitz JI. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 908] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 28. | Bathala MS, Masumoto H, Oguma T, He L, Lowrie C, Mendell J. Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans. Drug Metab Dispos. 2012;40:2250-2255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 29. | Büller HR, Décousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, Raskob GE, Schellong SM, Schwocho L, Segers A. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1341] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 30. | van Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feuring M, Clemens A. Dabigatran etexilate--a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 990] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 31. | Huisman MV, Lip GY, Diener HC, Brueckmann M, van Ryn J, Clemens A. Dabigatran etexilate for stroke prevention in patients with atrial fibrillation: resolving uncertainties in routine practice. Thromb Haemost. 2012;107:838-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 32. | Lindhoff-Last E, Samama MM, Ortel TL, Weitz JI, Spiro TE. Assays for measuring rivaroxaban: their suitability and limitations. Ther Drug Monit. 2010;32:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 33. | Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2013;15:625-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 552] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 34. | Douxfils J, Mullier F, Loosen C, Chatelain C, Chatelain B, Dogné JM. Assessment of the impact of rivaroxaban on coagulation assays: laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb Res. 2012;130:956-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 35. | Hillarp A, Gustafsson KM, Faxälv L, Strandberg K, Baghaei F, Fagerberg Blixter I, Berndtsson M, Lindahl TL. Effects of the oral, direct factor Xa inhibitor apixaban on routine coagulation assays and anti-FXa assays. J Thromb Haemost. 2014;12:1545-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Tripodi A, Padovan L, Testa S, Legnani C, Chantarangkul V, Scalambrino E, Ludovici S, Bassi L, Peyvandi F. How the direct oral anticoagulant apixaban affects hemostatic parameters. Results of a multicenter multiplatform study. Clin Chem Lab Med. 2014;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |