Revised: January 27, 2014
Accepted: March 17, 2014
Published online: May 6, 2014
Processing time: 188 Days and 15.7 Hours
Over the past decade, the administration of anti-CD20 monoclonal antibodies such as rituximab has demonstrated various degrees of effectiveness and has improved patients’ outcomes during the treatment of autoimmune hematological disorders and hematological malignancies. However, the depletion of B-cells, the distribution of T-cell populations, and the reconstruction of host immunity resulting from the use of anti-CD20 monoclonal antibodies potentially lead to severe viral infections, such as hepatitis B virus (HBV), hepatitis C virus (HCV), parvovirus B19, and herpes viruses, in patients who are undergoing immune therapy or immunochemotherapy. Of these infections, HBV- and HCV-related hepatitis are a great concern in endemic areas because of the high morbidity and mortality rates in untreated patients. As a result, prophylaxis against HBV infection is becoming a standard of care in these areas. Parvovirus B19, a widespread pathogen that causes red blood cell aplasia in immunocompromised hosts, also causes hepatitis in healthy individuals. Recently, its association with hepatitis was recognized in a patient treated with rituximab. In addition, adenovirus, varicella-zoster virus, hepatitis E virus, and rituximab itself have been linked to the occurrence of hepatitis during or after rituximab treatments. The epidemiologies and pathogeneses of these etiologies remain unknown. Because of the increasing use of anti-CD20 monoclonal antibodies for the treatment of hematological malignancies or autoimmune hematological disorders, it is imperative that physicians understand and balance the risks of hepatotropic virus-associated hepatitis against the benefits of using anti-CD20 monoclonal antibodies.
Core tip: Anti-CD20 monoclonal antibodies are widely used for the treatment of hematological malignancies and autoimmune disorders. These agents produce prolonged B-cell depletion and significant immune suppression. In this review, we summarized the clinical use of anti-CD20 monoclonal antibodies and the reports of acute or chronic hepatitis associated with the use of these agents. Most of these hepatitis cases had viral etiologies. We discuss the mechanisms of the hepatitis caused by these drugs. These infections not only interrupted the immunotherapy but are also associated with high mortality and morbidity. This review may prompt physicians to monitor patients’ liver function more closely and to provide adequate prophylaxis while using these agents.
- Citation: Yang SH, Hsu C, Cheng AL, Kuo SH. Anti-CD20 monoclonal antibodies and associated viral hepatitis in hematological diseases. World J Hematol 2014; 3(2): 29-43
- URL: https://www.wjgnet.com/2218-6204/full/v3/i2/29.htm
- DOI: https://dx.doi.org/10.5315/wjh.v3.i2.29
The nonglycosylated transmembrane phosphoprotein CD20 is a differentiation marker of B cells that was characterized over 30 years ago[1]. CD20 is expressed from early pre-B cells to mature B cells but not in plasma cells[2]. The majority of B-cell lymphomas variably express the CD20 surface marker[3]. CD20 can form multimeric complexes[4,5], interact with B-cell receptors in lipid rafts[5-7], mediate calcium influx[4,7], and regulate the cell cycle and apoptosis[8-11].
Because of the ubiquitous expression of CD20 in normal and malignant B cells, CD20 is an excellent target molecule for the treatment of B cell-related diseases. Rituximab, a genetically engineered chimeric murine/human monoclonal antibody (mAb)-targeting CD20, contains murine light- and heavy-chain variable regions and human constant regions[12]. Rituximab is used to exert cytotoxic effects on B cells by 3 mechanisms: antibody-dependent cell-mediated cytotoxicity (ADCC), complement-mediated lysis (CDC), and a direct apoptosis-inducing effect on CD20+ cells[12,13].
Based on a pivotal trial, rituximab was first approved by the United States Food and Drug Administration in 1997 for relapsed or refractory follicular and low-grade B-cell non-Hodgkin’s lymphoma (NHL)[14]. New anti-CD20 mAbs have been developed over the last few years, and these mAbs have been classified into 2 groups (type I and type II) according to their different activities in inducing CDC, ADCC, apoptosis, and lipid raft redistribution while binding to CD20[15,16]. Because anti-CD20 mAbs have optimized antibody structures and sometimes conjugated radioisotopes, they are used and indicated for the treatment of not only hematological malignancies but also autoimmune diseases[17]. However, following the widespread use of these agents, reports of infections related to B-cell depletion began to appear increasingly. Here, we discuss the clinical applications, the immunocompromising effects, and the association with hepatitis of anti-CD20 mAbs.
Rituximab, the first mAb approved for the treatment of malignancies, was initially indicated for relapsed or refractory indolent B-cell NHL[14]. In this pivotal trial, the schedule consisted of 4 weekly doses of 375 mg/m2. Nearly half of the 166 patients responded, with a projected median time-to-progression of 13 mo. Infusion reactions were the most frequently encountered acute adverse event[14]. The promising response and excellent tolerance to rituximab in indolent B-cell NHL cases were further demonstrated in clinical trials involving extended use and retreatment[18,19]. The excellent single-agent activity of rituximab was demonstrated not only in relapsed or refractory cases but also in newly diagnosed indolent B-cell NHL cases. In a phase II trial of single-agent rituximab for patients with low-grade NHL, the initial response rate (RR) after 4 weekly doses of rituximab was 54%, and the RR improved to 64% when rituximab retreatment was administered[20]. In addition to rituximab monotherapy, several studies have demonstrated that the combination of rituximab and chemotherapeutic agents provided high RRs and long time-to-progression in relapsed, refractory, or newly diagnosed indolent B-cell NHL cases[21-25]. To prolong disease control after the initial treatment, maintenance therapies after initial chemotherapy alone[21,26] or rituximab in addition to chemotherapy[21,27,28] have been tested in relapsed, refractory, or newly diagnosed indolent B-cell NHL cases. All of these studies demonstrated considerable improvement in the response duration or progression-free survival[21,26-28]. Moreover, the clinical successes of rituximab were partially recapitulated in diffuse large B-cell lymphoma (DLBCL)[29-35]. The benefits of maintenance therapy were not observed after first-line chemotherapy with or without rituximab or autologous stem-cell transplantation used for treating cases of relapsed DLBCL[34,35]. The difference may reflect the distinct nature of the indolent and aggressive B-cell NHLs.
Because of the clinical benefits demonstrated in rituximab-based regimens, efforts have been made to improve the efficacy of rituximab through the development of new anti-CD20 mAbs or the conjugation of radioisotopes (Table 1). Overall, the administration of yttrium-90 ibritumomab tiuxetan or iodine-131 tositumomab radioimmunotherapy (RIT) has not consistently yielded improved efficacy and survival in relapsed, refractory, or newly diagnosed B-cell NHL cases[36-44]. However, the use of RIT, which offers the theoretical benefits of radiotherapy, can be a viable option for patients who have not responded to prior rituximab treatments or as an alternative or a supplemental method to stem-cell transplantation. Second- and third-generation humanized anti-CD20 mAbs were designed with improved binding affinities for CD20 or the FcγRIIIa receptor for enhanced CDC or ADCC. The efficacy of these agents was also investigated with or without chemotherapy in relapsed, refractory, or newly diagnosed B-cell NHL cases[45-59]. Details regarding these anti-CD20 mAbs are summarized in Table 1.
| Antibody | Structure | Clinical applications | Associated hepatitis | Ref. |
| Rituximab | IgG1, chimeric murine/human mAb | B-cell NHL, RA, SLE, MS, AIHA, TTP, ITP, acquired hemophilia, cryoglobulinemia | HBV, HCV, parvovirus B19, VZV, adenovirus, HEV, drug-related? | [14,18-35,60-65,71, 73,74,78,85,89-93,102-111, 140-147,172,174-179] |
| Y-90 ibritumomab tiuxetan | IgG1, mouse mAb, conjugated with tiuxetan to yttrium-90 | B-cell NHL | HBV | [36-38,112] |
| I-131 tositumomab | IgG2, mouse mAb, covalently bound to iodine-131 | B-cell NHL | None | [39-44] |
| Ofatumumab | IgG1, human mAb | CLL, B-cell NHL, RA, MS, AIHA | HBV or drug-related? | [45-50,68,114-116] |
| Veltuzumab | IgG1, humanized mAb | B-cell NHL, ITP | Drug-related? | [51,69] |
| Ocrelizumab | IgG1, humanized mAb | B-cell NHL, RA, SLE, MS | None | [52] |
| Obinutuzumab | IgG1, humanized mAb, modified Fc | CLL, B-cell NHL | Drug-related? | [53-57] |
| PRO131921 | IgG1, humanized mAb, modified Fc | CLL, B-cell NHL | None | [58] |
| Ocaratuzumab | IgG1, humanized mAb, modified Fc | B-cell NHL | None | [59] |
The essential mechanism of autoimmune diseases is the loss of self-tolerance, which enables the immune system to evoke autoreactive humoral and cellular responses. Elimination of B cells with rituximab is effective in the treatment of autoimmune hematological diseases such as autoimmune hemolytic anemia (AIHA)[60], immune thrombocytopenic purpura (ITP)[61], acquired hemophilia[62], thrombotic thrombocytopenic purpura (TTP)[63], and cryoglobulinemia[64,65]. B cells produce autoantibodies and cytokines, act as antigen-presenting cells, promote naïve CD4+ T-cell differentiation, and affect dendritic cell homeostasis[66]. However, the responses of these autoimmune diseases to the off-label use of rituximab varied widely[67]. The varied results may be partially attributed to the underlying heterogeneity in the etiologies and pathogeneses of these diseases.
The data about the use of non-rituximab anti-CD20 mAbs for the treatment of autoimmune diseases are extremely limited. RIT may also be a valuable option, but its use for the treatment of autoimmune hematological diseases has not been reported. Ofatumumab, approved by the US Food and Drug Administration for treating chronic lymphocytic leukemia (CLL) refractory to fludarabine and alemtuzumab, was demonstrated to be effective in CLL complicated with AIHA[68]. In a phase I trial, veltuzumab demonstrated a RR of 55% in relapsed ITP cases, and long durations of response were observed in some patients[69]. Second- and third-generation humanized anti-CD20 mAbs are being developed for treating nonhematological autoimmune disorders, such as rheumatoid arthritis, systemic lupus erythematosus (SLE), and multiple sclerosis.
In general, peripheral blood B cells are depleted rapidly and effectively after anti-CD20 mAb treatment. The level of peripheral B cells remains extremely low and recovers gradually until 6 to 12 mo after the last dose of rituximab[61-65,70,71]. The depletion or recovery of B cells is not uniform among the various subsets and locations of B cells and may also depend on the baseline B-cell counts, the nature of the disease, and the dose, duration and type of anti-CD20 mAbs used[36,40,45,46,49-53,55,56,65,71,72]. The recovery of B cells starts with the immature or transitional B cells (CD38+, CD24+, CD10+, CD27-, IgD+) followed by naïve B cells (CD38+, CD27-, IgD+); however, memory B cells (CD27+, CD38-, IgD+) may remain considerably depleted for at least 2 years[73-76]. The pattern of B-cell repopulation is similar between autoimmune disease and B-cell NHL cases[73-76]. The effects of B-cell depletion on plasma immunoglobulin levels and blunted responses to immunization are individualized and heterogeneous among the different diseases treated with rituximab[18,19,21,29,60-65,70,77-80]. Typically, the levels of complements do not change substantially[64,70], but a major increase of C4 levels in the serum was noted after rituximab treatment in type II mixed cryoglobulinemia cases[65]. The levels, subsets, and functional status of T cells and natural killer (NK) cells after rituximab treatment are more complex because these factors depend on the expression of CD20 on T and NK cells[74] and the nature of the disease process[17,18,45,46,61,62,64,74,81-87]. Early and persistent reduction of peripheral CD4+/CD40L+ T cells was observed after treatment of SLE with rituximab[84]. The abnormalities of T-cell homeostasis can be reversed after rituximab administration[81-83,87], accompanied by increased CD4+CD25+ regulatory T cells[82,84,85,87], CD8+CD25+ T cells[85], or decreased autoreactive CD4+ T cells[81]. In a mouse model, B-cell depletion inhibited CD4+ but not CD8+ T-cell activation and clonal expansion in response to new exogenous antigens. Therefore, adequate antigen-specific CD4+ T-cell responses still required the presence of B cells[88]. The limited data on T cell, NK cell, complement, and immunoglobulin levels following treatments with anti-CD20 mAbs other than rituximab were highly similar compared with those with rituximab[36,51-54,69]. Because of the immunodeficiency induced by anti-CD20 mAbs, hepatitis and other infections have become a growing concern since the approval of rituximab.
Acute hepatitis and even fulminant hepatic failure are a well-documented threat in patients receiving chemotherapy, particularly in lymphoma cases[89]. In a prospective study, 44% (34/78) of hepatitis B virus (HBV; hepatitis B surface antigen, HBsAg+) carriers developed some form of hepatitis. Of these cases, 44% (15/34) were attributed to HBV reactivation[89]; 6 of these 15 were patients with lymphoma, and all of them had been treated with adriamycin, cyclophosphamide, vincristine, and prednisolone, also known as the CHOP regimen[89]. In addition, 4 of these 6 patients with lymphoma were seropositive for hepatitis B e antigen (HBeAg) at baseline and developed HBV reactivation sooner than the HBeAg-negative carriers[89]. In addition to those observations in lymphoma cases, HBV reactivation has been frequently reported in other hematological malignancies[90]. Corticosteroids, which are immunosuppressive agents frequently used in hematological malignancies and autoimmune diseases, may increase the risk of liver injury in HBV carriers[90,91]. Steroid-sparing regimens can be used to reduce the risk of HBV reactivation[92]. Additionally, adriamycin, a component of the CHOP regimen, can stimulate the replication of HBV[93]. Because the use of anti-CD20 mAb is common, designing a treatment plan that prevents the risk of HBV reactivation may be more complex than administrating chemotherapy for hematological malignancies or immunosuppressants for autoimmune diseases.
The natural history of HBV infection depends on the interaction of the host immunity, hepatocytes, and viral replication. Chronic HBV infections acquired early in life have 3 phases: the immune tolerance phase, the immune active phase, and the low-replication phase[94-96]. Some inactive HBV carriers (HBeAg seroconversion) can unexpectedly reenter the immune clearance phase and experience HBV reactivation with elevated HBV DNA and/or HBeAg reversion[97]. The incidence of HBV flares varies among studies and may depend on sex, HBV genotype, age at HBeAg seroconversion, and HBV DNA levels[96,97]. In adults, NK cells and type I interferon responses induced by HBV infection were observed before HBV-specific CD4+ and CD8+ T-cell responses[98]. In chronic HBV infections, HBV-specific CD8+ T cells presented HBV antigens regardless of the status of antibody to hepatitis B core antigen (anti-HBc)[99]. Both anti-HBc+ patients and inactive HBV carriers exhibited strong memory CD8+ T-cell responses[99]. However, the levels of FoxP3+, CD25+, and CD4+ regulatory T cells that inhibit HBcAg-specific responses were also higher in chronic HBV infection cases[100].
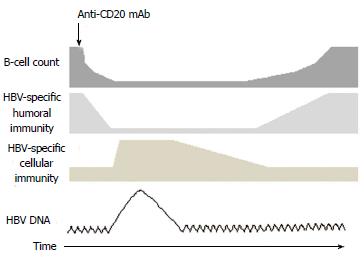
HBV replication within the liver and the subsequent spread of virions into the circulation may occur in cancer patients with immunosuppression induced by the diseases or treatments. Hepatitis flares can develop early or late, during immunosuppressive therapy or after its completion. The effects can range from asymptomatic elevation of HBV DNA to fulminant hepatitis. HBV reactivation has been reported not only in patients undergoing chemotherapy or steroid therapy but also in patients undergoing rituximab treatment, regardless of their HBV status[101-104]. In HBsAg+ carriers with B-cell NHL, HBV reactivation occurred in 80% (8/10) of patients without prophylaxis[105]. Significantly fewer cases of HBV reactivation were consistently observed in HBsAg+ carriers with prophylaxis[101]. In patients with resolved HBV (HBsAg-/anti-HBc+) and B-cell NHL, the rituximab-CHOP regimen led to more cases of HBV reactivation (reverse seroconversion or elevated HBV DNA) than the CHOP regimen alone did (23.8% vs 0%)[102]. The incidence rates of HBV reactivation and HBV hepatitis flare were reported as 10.4 and 6.4 per person-year in this group of patients, respectively[106]. The timing of HBV reactivation is closely associated with the lymphopenic state. The reconstruction of host immunity induced by rituximab and the recovery of B- and T-lymphocytes after rituximab may result in damage to HBV-infected hepatocytes by cytotoxic T-lymphocytes[90,104,106,107]. The impairment of the immune system appears to be more severe in patients treated with rituximab-CHOP than in those treated with rituximab alone[107].
Rituximab treatments, with or without steroids, were generally well-tolerated by patients with autoimmune hematological disorders such as AIHA[60,108], ITP[61,109], acquired hemophilia[62], TTP[63], and cryoglobulinemia[64,65]. No HBV reactivation was reported, most likely because of the exclusion of patients with positive HBV serology, a limited number of cases, or lesser immunosuppression in these autoimmune diseases compared with hematological malignancies. However, HBV reactivation is a major threat in patients with autoimmune disorders who are undergoing immunosuppressive therapy[110]; therefore, concurrent antiviral treatments are often prescribed to reduce the risk of reactivation[111].
Patients with chronic or resolved HBV receiving RIT or anti-CD20 mAbs are subject to the same or an even greater risk of HBV reactivation, and although these effects were not fully characterized in most prospective trials[36-59], scattered cases have been reported[111,112], both of which were successfully treated with lamivudine[112,113]. The United States Food and Drug Administration announced that physicians should be alert to potential HBV reactivation caused by ofatumumab[114]. Liver toxicities had been reported with ofatumumab with or without chemotherapy in hematological malignancies. However, the data on HBV reactivation in these cases were lacking[45,50,115,116].
HBV is endemic in the Asia-Pacific region with a prevalence of more than 10% in Taiwan, southern China, and certain areas of Southeast Asia[117]. In clinical practice, a greater than 10-fold increase above the baseline in serum HBV DNA levels, an absolute increase of more than 9 log10 copies/mL, a reappearance of HBV DNA in HBsAg, or a new onset of HBV DNA viremia in HBsAg-negative patients can be considered as an indication of HBV reactivation. In addition, an hepatitis flare is considered if the serum alanine transaminase (ALT) level is 3 times greater than the normal upper limit, or if an ALT level of more than 100 IU/L with a concomitantly increased HBV DNA level of more than 10 times the typical level is detected[89,118]. In high-risk endemic areas, HBV screening must be considered before implementing anti-CD20 mAb treatment[119-123]. In a cost-effectiveness study, screening for HBsAg in all patients about to receive rituximab-CHOP treatment considerably reduced the rate of HBV reactivation and cost the least[124]. The recommended tests for screening include HBsAg, anti-HBc, and anti-HBs (according to the CDC)[122], HBsAg and anti-HBc (AASLD and EASL)[121,125], and HBsAg with or without anti-HBc (ASCO)[123]. A more complex algorithm for screening in different at-risk populations was proposed[126]. Currently, oral agents approved for HBV treatment include lamivudine, adefovir, telbivudine, tenofovir, and entecavir. Based on research findings, the optimal start time and duration of HBV prophylaxis has not yet been determined[119-123]; however, initiating short-term antiviral therapy before starting anti-CD20 mAb treatment appears to be beneficial and safe. In a randomized trial involving HBsAg+ patients with NHL, the prophylactic use of lamivudine reduced considerably the occurrence of HBV reactivation and hepatitis flares[127]. In a meta-analysis, all-cause mortality, HBV reactivation, HBV-related mortality, and interruption of anti-CD20 mAb therapy were considerably reduced with lamivudine prophylaxis[128]. One major problem of lamivudine, telbivudine, and adefovir is that drug resistance and hepatitis flares increase with continuous use[125,129]; therefore, the use of entecavir and tenofovir was suggested for longer duration of prophylaxis[125]. In addition to HBsAg+ patients, HBV prophylaxis must be considered in patients with resolved HBV because the risk of reactivation remains (Figure 1)[106,130]. In a recent retrospective study of HBV reactivation by the Asia Lymphoma Study Group, the authors showed that patients receiving entecavir prophylaxis had a lesser incidence of HBV reactivation than those with lamivudine. Prospective studies to validate these findings are warranted.
The epidemiology of hepatitis C virus (HCV) differs from that of HBV, particularly because of the existence of a wide variation of HCV genotypes worldwide[131]. The most common types in Taiwan, China, Japan, and Korea are genotypes 1b and 2; by contrast, more diversity exists in North America and Europe[131]. The natural history of HCV infection consists of ramp-up and plateau phases in acute infections and various spontaneous clearances in chronic phases depending on several viral and host factors, including HCV genotypes, host immunity, and the genetic polymorphism of IFNL3 (IL-28B)[131]. The treatment of chronic HCV consists primarily of interferon-α, ribavirin, and protease inhibitors that are accompanied by major toxicities[131].
During acute infections, HCV-specific T cells activated by CD8+ and CD4+ are generated readily against multiple epitopes within 10 wk[132]. In patients with chronic infections and persistent viremia, HCV-specific CD8+ T cells are rarer and responsive to fewer epitopes than in those without viremia, where even HCV-specific CD8+ T cell responses are mounted[132]. In addition, CD8+ T cells are exhausted in chronic HCV with increased expression of programmed death-1 and cytotoxic T-lymphocyte-associated antigen-4[133]. HCV-specific CD8+ T cells are suppressed by increased CD25+ and CD4+ regulatory T cells[134]. In addition to evading CD8+ T-cell responses, HCV also evades CD4+ T-cell responses and humoral immunity with escape mutants because of its error-prone RNA polymerase[135-137].
HCV itself is highly associated with the development of lymphoproliferative disorders, particularly with B-cell NHL with or without mixed cryoglobulinemia[138,139]. HCV-related fulminant hepatitis occurring after chemotherapy with or without corticosteroids was rare in patients with lymphoma[140,141]. Liver dysfunction after chemotherapy was less common in HCV than in HBV patients (18.2% vs 75.0%) with hematological malignancies[142]. The incidence of HCV-associated liver dysfunction appeared to be higher in rituximab-containing regimens[143,144]. In addition, increased HCV RNA levels were common during or after rituximab-based chemotherapy and were followed by hepatitis flares and decreased HCV RNA levels in various intervals[144-146]. In contrast to persistent B-cell depletion for several months after rituximab treatments, the HCV viral load and the number of regulatory T cells were elevated initially, then decreased, indicating that B-cell depletion and HCV-specific T-cell responses participate in the mechanism of HCV reactivation in patients treated with rituximab-based regimens[64,147,148]. Although the elevation of HCV RNA load was not the major problem for lymphoma patients treated with standard courses of immunochemotherapy, the persistent elevation of HCV RNA load and subsequent liver cirrhosis can occur in follicular lymphoma patients treated with rituximab-maintenance therapy[147]. However, these studies evaluated HCV reactivation during and after rituximab regimens according to different criteria. The HCV RNA levels may increase up to 10 times above the baseline in chronic HCV infections[149]. In a retrospective study involving cancer patients, the acute exacerbation of chronic HCV was defined as a 3-fold or greater increase in ALT levels without tumor infiltration within the liver, without the use of hepatotoxic drugs or blood transfusion, and no concomitant systemic infections[150]. In addition, an at least 1 log10 IU/mL increase in HCV RNA levels after treatment with immunosuppressive agents was considered for HCV reactivation[150]. The same criteria may apply for HCV reactivation in anti-CD20 mAb treatments. A simple algorithm for monitoring ALT and HCV RNA in patients undergoing immunosuppressive therapy was proposed[151]. Although rituximab was also widely used in autoimmune hematological disorders such as HCV-related cryoglobulinemia, HCV reactivation with hepatitis flares was rare[65,152,153]. No data were available for RIT and non-rituximab anti-CD20 mAbs.
Traditionally, anti-HCV therapy is not considered in patients receiving immunosuppressive agents because of potential drug-drug interactions, the major side effects of anti-HCV therapy, and the rarity of severe HCV-related hepatitis flares. Currently, there is no consensus regarding the optimal strategy for the treatment and prevention of HCV reactivation in patients undergoing immunosuppressive therapy even though ribavirin with or without interferon-α has been successfully administered to patients with hematological malignancies[154,155].
Parvovirus B19 infections are common infections that spread through respiratory droplets or blood, and seropositivity rates are increasing in people of all ages[156]. The disease spectrum can range from asymptomatic disease to hydrops fetalis, fifth disease, arthropathy, aplastic anemia, autoimmune disorders, meningitis, encephalitis, and even fulminant hepatitis[156,157]. This virus has a tropism for erythroid progenitors in the blood, bone marrow, and fetal liver[156]. In healthy adults, acute infections result in viremia within 2 wk, immediately followed by virus-specific IgM and IgG responses and clearance of the virus in the serum[158]. Humoral immunity appears to be critical for controlling this virus, and patients with immunodeficiency disorders can have chronic infections[159]. Virus-specific CD8+ T cell responses toward multiple epitopes also develop soon after acute infection, and these striking CD8+ T-cell responses may have long durations with continuous viremia[160]. In addition, interferon-γ-secreting, virus-specific CD62L+ and CD4+ T cells developed within 3 mo of acute infection[161].
Parvovirus B19 infection is a major problem in immunocompromised hosts[162-164]. The seropositivity rate was high in cancer patients receiving chemotherapy[162,165], and half of patients exhibited detectable viral DNA in their serum[165]. Several studies have demonstrated that acute parvovirus B19 infections are associated with fever, arthralgia, hepatitis, myocarditis, pneumonia, pancytopenia, and even graft dysfunction[162-164]. Most importantly, immunosuppressive therapies can impair humoral immunity, exposing patients to a high risk of parvovirus B19 infection[164,166]. Several case studies have reported that parvovirus B19 infection-related symptoms can develop in B-cell NHL or immune thrombocytopenia patients treated with rituximab-containing regimens or after being treated with rituximab-containing regimens[167-172]. The major parvovirus B19 infection-related symptom was cytopenia in the erythroid lineage, but neutropenia or thrombocytopenia without anemia occurred in some patients. The onset was preceded by fever and skin eruptions in 2 patients[169,171]. Our group identified the first case with acute hepatitis[172]. Most patients developed the clinical manifestations at least 2 mo after the initiation of rituximab. The patients in 2 cases recovered without treatment, and the others responded positively to intravenous immunoglobulin (IVIG). However, the hepatitis flare in our patient persisted for 7 mo and was paralleled with cytopenia, which was correlated with the recovery of B cells after rituximab treatment[172]. The effects of other anti-CD20 mAbs on parvovirus B19 are unknown.
The diagnosis of parvovirus B19 reactivation or infection is based on serology and viral DNA analysis[156]. However, conducting virus-specific serology may be problematic in immunocompromised hosts[164,166]. The pathogenesis of parvovirus B19-related hepatitis is largely unknown, and either direct cytopathic or indirect immunity-related mechanisms are possible. In addition, the pathology images obtained during liver biopsies are nonspecific. Although viral DNA or RNA can be detected in hepatocytes, the clinical significance remains to be defined[173]. The most reasonable diagnostic sequence may be to exclude the other common hepatotropic viruses first, such as HBV or HCV, and then to analyze serum serology and viral DNA if the tests for hepatotropic viruses are negative. Because liver biopsy is invasive, it must be considered last. The most effective treatment and prevention methods for parvovirus B19-related hepatitis remain unknown; however, IVIG can be used for treating severe cases because of the clinical success achieved in using it in other parvovirus B19-related diseases[156,166-169,171].
In addition to HBV, HCV, and parvovirus B19, critical viral infections from cytomegalovirus, varicella-zoster virus (VZV), herpes simplex virus, echovirus, enterovirus, influenza A virus, or BK/JC virus can occur in lymphoma patients treated with rituximab regimens[174]. Most of them did not induce hepatitis. However, there are reports of 2 cases of hepatitis associated with adenovirus, one case of hepatic necrosis associated with disseminated VZV, and one of chronic hepatitis E virus infection that developed after rituximab treatment[175-178]. These pathogens are rarely connected to the use of rituximab, and the underlying mechanisms of these pathogeneses associated with hepatitis are less characterized. However, the actual incidence rates of these uncommon viral infections in lymphoma patients treated with rituximab regimens may be underestimated. One patient with ITP experienced drug-induced acute hepatitis, and the pathogen was not identified; however, the patient recovered soon after rituximab treatment was stopped[179]. There were also scattered reports of anti-CD20 mAb-related liver function abnormalities in patients with hematological disorders. However, the etiology was most likely related to anti-CD20 mAb[45,50,51,55,69,115,116].
There are numerous reports of animal lymphoma models to test the preclinical activity of anti-CD20 mAbs[180-182]. However, the safety data in liver toxicities of these animal models are very limited[180-182]. In addition, no animal or in vitro models of viral hepatitis-induced by anti-CD20 mAbs has been established. It would be very difficult to establish the animal model with viral hepatitis reactivated by anti-CD20 mAbs because most of previous studies used tumor xenografts implanted into mice with severe combined immunodeficiency in their animal model. However, immune-mediated liver damage is important for anti-CD20 mAb-associated viral hepatitis. Most likely, this may work in evaluating the precise mechanism of hepatitis caused by the aforementioned viruses during and after anti-CD20 mAbs alone when using the immunocompetent animal model.
This review summarized the clinical use of anti-CD20 mAbs and reports of acute or chronic hepatitis associated with the use of these agents. Most data are from cases where rituximab was used to treat hematological malignancies. The majority of diseases studied were caused by viral infections. These infections must be clinically recognized soon after their occurrence because they not only interrupt immunotherapy but are also associated with high mortality and morbidity. Close monitoring of HBV and HCV infections before and during anti-CD20 mAb treatment is highly recommended in endemic areas. The prophylactic therapy for HBV has become standard of care, but patient selection and the optimal regimen or duration remain to be defined. Physicians must monitor patients being treated with rituximab-based regimens for the risks of hepatotropic viruses, including HBV, HCV, parvovirus B19, and other viruses. Nevertheless, this review has limitations. Some pathogens that rarely induce hepatitis may not be reported in the literature; therefore, the causal relation, epidemiology, and pathogenesis of these pathogens are not the most accurate. Occasionally, the causal association between hepatitis and rituximab is difficult to confirm because of the concurrent use of multiple immunosuppressants or hepatotoxic drugs. The data on non-rituximab anti-CD20 mAbs are limited, likely because most clinical trials employ strict case selection criteria and exclude patients with hepatitis. Therefore, in vivo or in vitro studies are warranted to establish the actual roles of these viruses in the pathogenesis of hepatitis during and after anti-CD20 mAb treatments.
P- Reviewers: Bensussan A, Lee YY, Shi ZJ S- Editor: Gou SX L- Editor: A E- Editor: Wu HL
| 1. | Stashenko P, Nadler LM, Hardy R, Schlossman SF. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980;125:1678-1685. [PubMed] |
| 2. | Stashenko P, Nadler LM, Hardy R, Schlossman SF. Expression of cell surface markers after human B lymphocyte activation. Proc Natl Acad Sci USA. 1981;78:3848-3852. [PubMed] |
| 3. | Nadler LM, Ritz J, Hardy R, Pesando JM, Schlossman SF, Stashenko P. A unique cell surface antigen identifying lymphoid malignancies of B cell origin. J Clin Invest. 1981;67:134-140. [PubMed] |
| 4. | Bubien JK, Zhou LJ, Bell PD, Frizzell RA, Tedder TF. Transfection of the CD20 cell surface molecule into ectopic cell types generates a Ca2+ conductance found constitutively in B lymphocytes. J Cell Biol. 1993;121:1121-1132. [PubMed] |
| 5. | Polyak MJ, Li H, Shariat N, Deans JP. CD20 homo-oligomers physically associate with the B cell antigen receptor. Dissociation upon receptor engagement and recruitment of phosphoproteins and calmodulin-binding proteins. J Biol Chem. 2008;283:18545-18552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Petrie RJ, Deans JP. Colocalization of the B cell receptor and CD20 followed by activation-dependent dissociation in distinct lipid rafts. J Immunol. 2002;169:2886-2891. [PubMed] |
| 7. | Li H, Ayer LM, Lytton J, Deans JP. Store-operated cation entry mediated by CD20 in membrane rafts. J Biol Chem. 2003;278:42427-42434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Golay JT, Clark EA, Beverley PC. The CD20 (Bp35) antigen is involved in activation of B cells from the G0 to the G1 phase of the cell cycle. J Immunol. 1985;135:3795-3801. [PubMed] |
| 9. | Tedder TF, Forsgren A, Boyd AW, Nadler LM, Schlossman SF. Antibodies reactive with the B1 molecule inhibit cell cycle progression but not activation of human B lymphocytes. Eur J Immunol. 1986;16:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Holder M, Grafton G, MacDonald I, Finney M, Gordon J. Engagement of CD20 suppresses apoptosis in germinal center B cells. Eur J Immunol. 1995;25:3160-3164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Tedder TF, Engel P. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol Today. 1994;15:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 428] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 12. | Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, Newman RA, Hanna N, Anderson DR. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435-445. [PubMed] |
| 13. | Shan D, Ledbetter JA, Press OW. Apoptosis of malignant human B cells by ligation of CD20 with monoclonal antibodies. Blood. 1998;91:1644-1652. [PubMed] |
| 14. | McLaughlin P, Grillo-López AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825-2833. [PubMed] |
| 15. | Cragg MS, Glennie MJ. Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood. 2004;103:2738-2743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 248] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Beers SA, Chan CH, French RR, Cragg MS, Glennie MJ. CD20 as a target for therapeutic type I and II monoclonal antibodies. Semin Hematol. 2010;47:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Avivi I, Stroopinsky D, Katz T. Anti-CD20 monoclonal antibodies: beyond B-cells. Blood Rev. 2013;27:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Piro LD, White CA, Grillo-López AJ, Janakiraman N, Saven A, Beck TM, Varns C, Shuey S, Czuczman M, Lynch JW. Extended Rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol. 1999;10:655-661. [PubMed] |
| 19. | Davis TA, Grillo-López AJ, White CA, McLaughlin P, Czuczman MS, Link BK, Maloney DG, Weaver RL, Rosenberg J, Levy R. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: safety and efficacy of re-treatment. J Clin Oncol. 2000;18:3135-3143. [PubMed] |
| 20. | Hainsworth JD, Burris HA, Morrissey LH, Litchy S, Scullin DC, Bearden JD, Richards P, Greco FA. Rituximab monoclonal antibody as initial systemic therapy for patients with low-grade non-Hodgkin lymphoma. Blood. 2000;95:3052-3056. [PubMed] |
| 21. | van Oers MH, Klasa R, Marcus RE, Wolf M, Kimby E, Gascoyne RD, Jack A, Van’t Veer M, Vranovsky A, Holte H. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108:3295-3301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 444] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 22. | Forstpointner R, Dreyling M, Repp R, Hermann S, Hänel A, Metzner B, Pott C, Hartmann F, Rothmann F, Rohrberg R. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104:3064-3071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 440] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 23. | Marcus R, Imrie K, Belch A, Cunningham D, Flores E, Catalano J, Solal-Celigny P, Offner F, Walewski J, Raposo J. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105:1417-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 719] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 24. | Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, Reiser M, Metzner B, Harder H, Hegewisch-Becker S. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725-3732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1020] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 25. | Herold M, Haas A, Srock S, Neser S, Al-Ali KH, Neubauer A, Dölken G, Naumann R, Knauf W, Freund M. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group Hematology and Oncology Study. J Clin Oncol. 2007;25:1986-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 391] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 26. | Hochster H, Weller E, Gascoyne RD, Habermann TM, Gordon LI, Ryan T, Zhang L, Colocci N, Frankel S, Horning SJ. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 Study. J Clin Oncol. 2009;27:1607-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 27. | Forstpointner R, Unterhalt M, Dreyling M, Böck HP, Repp R, Wandt H, Pott C, Seymour JF, Metzner B, Hänel A. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG). Blood. 2006;108:4003-4008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 333] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 28. | Salles G, Seymour JF, Offner F, López-Guillermo A, Belada D, Xerri L, Feugier P, Bouabdallah R, Catalano JV, Brice P. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 804] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 29. | Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, Johnson P, Lister A, Feuring-Buske M, Radford JA. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92:1927-1932. [PubMed] |
| 30. | Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3975] [Cited by in RCA: 4042] [Article Influence: 175.7] [Reference Citation Analysis (0)] |
| 31. | Pfreundschuh M, Trümper L, Osterborg A, Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani PL. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1509] [Cited by in RCA: 1534] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 32. | Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, Reiser M, Nickenig C, Clemens M, Peter N. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 844] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 33. | Ketterer N, Coiffier B, Thieblemont C, Fermé C, Brière J, Casasnovas O, Bologna S, Christian B, Connerotte T, Récher C. Phase III study of ACVBP versus ACVBP plus rituximab for patients with localized low-risk diffuse large B-cell lymphoma (LNH03-1B). Ann Oncol. 2013;24:1032-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, Dakhil SR, Woda B, Fisher RI, Peterson BA. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121-3127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1009] [Cited by in RCA: 1041] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 35. | Gisselbrecht C, Schmitz N, Mounier N, Singh Gill D, Linch DC, Trneny M, Bosly A, Milpied NJ, Radford J, Ketterer N. Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20(+) diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol. 2012;30:4462-4469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 36. | Witzig TE, Gordon LI, Cabanillas F, Czuczman MS, Emmanouilides C, Joyce R, Pohlman BL, Bartlett NL, Wiseman GA, Padre N. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20:2453-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 791] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 37. | Esmaeli B, McLaughlin P, Pro B, Samaniego F, Gayed I, Hagemeister F, Romaguera J, Cabanillas F, Neelapu SS, Banay R. Prospective trial of targeted radioimmunotherapy with Y-90 ibritumomab tiuxetan (Zevalin) for front-line treatment of early-stage extranodal indolent ocular adnexal lymphoma. Ann Oncol. 2009;20:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 38. | Morschhauser F, Radford J, Van Hoof A, Vitolo U, Soubeyran P, Tilly H, Huijgens PC, Kolstad A, d’Amore F, Gonzalez Diaz M. Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J Clin Oncol. 2008;26:5156-5164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 314] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 39. | Vose JM, Wahl RL, Saleh M, Rohatiner AZ, Knox SJ, Radford JA, Zelenetz AD, Tidmarsh GF, Stagg RJ, Kaminski MS. Multicenter phase II study of iodine-131 tositumomab for chemotherapy-relapsed/refractory low-grade and transformed low-grade B-cell non-Hodgkin’s lymphomas. J Clin Oncol. 2000;18:1316-1323. [PubMed] |
| 40. | Kaminski MS, Tuck M, Estes J, Kolstad A, Ross CW, Zasadny K, Regan D, Kison P, Fisher S, Kroll S. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med. 2005;352:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 486] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 41. | Kaminski MS, Radford JA, Gregory SA, Leonard JP, Knox SJ, Kroll S, Wahl RL. Re-treatment with I-131 tositumomab in patients with non-Hodgkin’s lymphoma who had previously responded to I-131 tositumomab. J Clin Oncol. 2005;23:7985-7993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Press OW, Unger JM, Braziel RM, Maloney DG, Miller TP, LeBlanc M, Gaynor ER, Rivkin SE, Fisher RI. A phase 2 trial of CHOP chemotherapy followed by tositumomab/iodine I 131 tositumomab for previously untreated follicular non-Hodgkin lymphoma: Southwest Oncology Group Protocol S9911. Blood. 2003;102:1606-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 43. | Press OW, Unger JM, Rimsza LM, Friedberg JW, LeBlanc M, Czuczman MS, Kaminski M, Braziel RM, Spier C, Gopal AK. Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus (131)iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016. J Clin Oncol. 2013;31:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 44. | Vose JM, Carter S, Burns LJ, Ayala E, Press OW, Moskowitz CH, Stadtmauer EA, Mineshi S, Ambinder R, Fenske T. Phase III randomized study of rituximab/carmustine, etoposide, cytarabine, and melphalan (BEAM) compared with iodine-131 tositumomab/BEAM with autologous hematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: results from the BMT CTN 0401 trial. J Clin Oncol. 2013;31:1662-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 45. | Coiffier B, Lepretre S, Pedersen LM, Gadeberg O, Fredriksen H, van Oers MH, Wooldridge J, Kloczko J, Holowiecki J, Hellmann A. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1-2 study. Blood. 2008;111:1094-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 286] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 46. | Hagenbeek A, Gadeberg O, Johnson P, Pedersen LM, Walewski J, Hellmann A, Link BK, Robak T, Wojtukiewicz M, Pfreundschuh M. First clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: results of a phase 1/2 trial. Blood. 2008;111:5486-5495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 47. | Lemery SJ, Zhang J, Rothmann MD, Yang J, Earp J, Zhao H, McDougal A, Pilaro A, Chiang R, Gootenberg JE. U.S. Food and Drug Administration approval: ofatumumab for the treatment of patients with chronic lymphocytic leukemia refractory to fludarabine and alemtuzumab. Clin Cancer Res. 2010;16:4331-4338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 48. | Wierda WG, Kipps TJ, Dürig J, Griskevicius L, Stilgenbauer S, Mayer J, Smolej L, Hess G, Griniute R, Hernandez-Ilizaliturri FJ. Chemoimmunotherapy with O-FC in previously untreated patients with chronic lymphocytic leukemia. Blood. 2011;117:6450-6458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 49. | Czuczman MS, Fayad L, Delwail V, Cartron G, Jacobsen E, Kuliczkowski K, Link BK, Pinter-Brown L, Radford J, Hellmann A. Ofatumumab monotherapy in rituximab-refractory follicular lymphoma: results from a multicenter study. Blood. 2012;119:3698-3704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 50. | Coiffier B, Radford J, Bosly A, Martinelli G, Barca G, Davies A, Decaudin D, Gallop-Evans E, Padmanabhan-Iyer S, Van Eygen K. A multicentre, phase II trial of ofatumumab monotherapy in relapsed/progressive diffuse large B-cell lymphoma. Br J Haematol. 2013;163:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Morschhauser F, Leonard JP, Fayad L, Coiffier B, Petillon MO, Coleman M, Schuster SJ, Dyer MJ, Horne H, Teoh N. Humanized anti-CD20 antibody, veltuzumab, in refractory/recurrent non-Hodgkin’s lymphoma: phase I/II results. J Clin Oncol. 2009;27:3346-3353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 52. | Morschhauser F, Marlton P, Vitolo U, Lindén O, Seymour JF, Crump M, Coiffier B, Foà R, Wassner E, Burger HU. Results of a phase I/II study of ocrelizumab, a fully humanized anti-CD20 mAb, in patients with relapsed/refractory follicular lymphoma. Ann Oncol. 2010;21:1870-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 53. | Salles G, Morschhauser F, Lamy T, Milpied N, Thieblemont C, Tilly H, Bieska G, Asikanius E, Carlile D, Birkett J. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood. 2012;119:5126-5132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 54. | Sehn LH, Assouline SE, Stewart DA, Mangel J, Gascoyne RD, Fine G, Frances-Lasserre S, Carlile DJ, Crump M. A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood. 2012;119:5118-5125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 55. | Salles GA, Morschhauser F, Solal-Céligny P, Thieblemont C, Lamy T, Tilly H, Gyan E, Lei G, Wenger M, Wassner-Fritsch E. Obinutuzumab (GA101) in patients with relapsed/refractory indolent non-Hodgkin lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31:2920-2926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 56. | Morschhauser FA, Cartron G, Thieblemont C, Solal-Céligny P, Haioun C, Bouabdallah R, Feugier P, Bouabdallah K, Asikanius E, Lei G. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large b-cell lymphoma or mantle-cell lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31:2912-2919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 57. | Radford J, Davies A, Cartron G, Morschhauser F, Salles G, Marcus R, Wenger M, Lei G, Wassner-Fritsch E, Vitolo U. Obinutuzumab (GA101) plus CHOP or FC in relapsed/refractory follicular lymphoma: results of the GAUDI study (BO21000). Blood. 2013;122:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 58. | Friedberg JW, Vose J, Kahl BS, Brunvand M, Goy A, Kasamon Y, Brington B, Li J, Ho W, Cheson BD. A Phase I Study of PRO131921, a Novel Anti-CD20 Monoclonal Antibody in Patients with Relapsed/Refractory CD20 Indolent NHL: Correlation Between Clinical Responses and AUC Pharmacokinetics. ASH Annual Meeting Abstracts. 2009;114:3472. |
| 59. | Wayne JL, Ganjoo KN, Pohlman BL, De Vos S, Flinn IW, Dang NH, Mapara MY, Smith MR, O’Reilly AM, Marulappa SY. Efficacy of ocaratuzumab (AME-133v) in relapsed follicular lymphoma patients refractory to prior rituximab. ASCO Meeting Abstracts. 2012;30:8081. |
| 60. | Barcellini W, Zaja F, Zaninoni A, Imperiali FG, Battista ML, Di Bona E, Fattizzo B, Consonni D, Cortelezzi A, Fanin R. Low-dose rituximab in adult patients with idiopathic autoimmune hemolytic anemia: clinical efficacy and biologic studies. Blood. 2012;119:3691-3697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 61. | Stasi R, Pagano A, Stipa E, Amadori S. Rituximab chimeric anti-CD20 monoclonal antibody treatment for adults with chronic idiopathic thrombocytopenic purpura. Blood. 2001;98:952-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 396] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 62. | Stasi R, Brunetti M, Stipa E, Amadori S. Selective B-cell depletion with rituximab for the treatment of patients with acquired hemophilia. Blood. 2004;103:4424-4428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 63. | Fakhouri F, Vernant JP, Veyradier A, Wolf M, Kaplanski G, Binaut R, Rieger M, Scheiflinger F, Poullin P, Deroure B. Efficiency of curative and prophylactic treatment with rituximab in ADAMTS13-deficient thrombotic thrombocytopenic purpura: a study of 11 cases. Blood. 2005;106:1932-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 64. | Sansonno D, De Re V, Lauletta G, Tucci FA, Boiocchi M, Dammacco F. Monoclonal antibody treatment of mixed cryoglobulinemia resistant to interferon alpha with an anti-CD20. Blood. 2003;101:3818-3826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 243] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 65. | Zaja F, De Vita S, Mazzaro C, Sacco S, Damiani D, De Marchi G, Michelutti A, Baccarani M, Fanin R, Ferraccioli G. Efficacy and safety of rituximab in type II mixed cryoglobulinemia. Blood. 2003;101:3827-3834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 331] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 66. | LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 852] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 67. | Barcellini W, Zanella A. Rituximab therapy for autoimmune haematological diseases. Eur J Intern Med. 2011;22:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 68. | Nader K, Patel M, Ferber A. Ofatumumab in rituximab-refractory autoimmune hemolytic anemia associated with chronic lymphocytic leukemia: a case report and review of literature. Clin Lymphoma Myeloma Leuk. 2013;13:511-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Liebman HA, Saleh MN, Bussel JB, Negrea OG, Horne H, Wegener WA, Goldenberg DM. Low-dose anti-CD20 veltuzumab given intravenously or subcutaneously is active in relapsed immune thrombocytopenia: a phase I study. Br J Haematol. 2013;162:693-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Maloney DG, Grillo-López AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, Janakiraman N, Foon KA, Liles TM, Dallaire BK. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188-2195. [PubMed] |
| 71. | Nakou M, Katsikas G, Sidiropoulos P, Bertsias G, Papadimitraki E, Raptopoulou A, Koutala H, Papadaki HA, Kritikos H, Boumpas DT. Rituximab therapy reduces activated B cells in both the peripheral blood and bone marrow of patients with rheumatoid arthritis: depletion of memory B cells correlates with clinical response. Arthritis Res Ther. 2009;11:R131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 72. | Kamburova EG, Koenen HJ, Boon L, Hilbrands LB, effects of rituximab on the proliferation, activation and Joosten I. In vitro differentiation of human B cells. Am J Transplant. 2012;12:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 73. | Roll P, Palanichamy A, Kneitz C, Dorner T, Tony HP. Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis Rheum. 2006;54:2377-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 255] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 74. | Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 429] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 75. | Anolik JH, Friedberg JW, Zheng B, Barnard J, Owen T, Cushing E, Kelly J, Milner EC, Fisher RI, Sanz I. B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin Immunol. 2007;122:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 76. | Abulayha AM, Tabal SA, Shawesh EI, Elbasir MA, Elbanani AS, Lamami YM, Bredan A. Depletion of peripheral blood B cells with Rituximab and phenotype characterization of the recovering population in a patient with follicular lymphoma. Leuk Res. 2010;34:307-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 77. | Tsuda M, Moritoki Y, Lian ZX, Zhang W, Yoshida K, Wakabayashi K, Yang GX, Nakatani T, Vierling J, Lindor K. Biochemical and immunologic effects of rituximab in patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Hepatology. 2012;55:512-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 78. | Pescovitz MD, Torgerson TR, Ochs HD, Ocheltree E, McGee P, Krause-Steinrauf H, Lachin JM, Canniff J, Greenbaum C, Herold KC. Effect of rituximab on human in vivo antibody immune responses. J Allergy Clin Immunol. 2011;128:1295-1302.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 79. | Bedognetti D, Zoppoli G, Massucco C, Zanardi E, Zupo S, Bruzzone A, Sertoli MR, Balleari E, Racchi O, Messina M. Impaired response to influenza vaccine associated with persistent memory B cell depletion in non-Hodgkin’s lymphoma patients treated with rituximab-containing regimens. J Immunol. 2011;186:6044-6055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 80. | van Vollenhoven RF, Emery P, Bingham CO, Keystone EC, Fleischmann RM, Furst DE, Tyson N, Collinson N, Lehane PB. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann Rheum Dis. 2013;72:1496-1502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 279] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 81. | Eming R, Nagel A, Wolff-Franke S, Podstawa E, Debus D, Hertl M. Rituximab exerts a dual effect in pemphigus vulgaris. J Invest Dermatol. 2008;128:2850-2858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 82. | Stasi R, Cooper N, Del Poeta G, Stipa E, Laura Evangelista M, Abruzzese E, Amadori S. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. 2008;112:1147-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 314] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 83. | Stasi R, Del Poeta G, Stipa E, Evangelista ML, Trawinska MM, Cooper N, Amadori S. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood. 2007;110:2924-2930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 233] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 84. | Sfikakis PP, Souliotis VL, Fragiadaki KG, Moutsopoulos HM, Boletis JN, Theofilopoulos AN. Increased expression of the FoxP3 functional marker of regulatory T cells following B cell depletion with rituximab in patients with lupus nephritis. Clin Immunol. 2007;123:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 85. | Vallerskog T, Gunnarsson I, Widhe M, Risselada A, Klareskog L, van Vollenhoven R, Malmström V, Trollmo C. Treatment with rituximab affects both the cellular and the humoral arm of the immune system in patients with SLE. Clin Immunol. 2007;122:62-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 86. | Feuchtenberger M, Müller S, Roll P, Waschbisch A, Schäfer A, Kneitz C, Wiendl H, Tony HP. Frequency of regulatory T cells is not affected by transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Open Rheumatol J. 2008;2:81-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Saadoun D, Rosenzwajg M, Landau D, Piette JC, Klatzmann D, Cacoub P. Restoration of peripheral immune homeostasis after rituximab in mixed cryoglobulinemia vasculitis. Blood. 2008;111:5334-5341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 88. | Bouaziz JD, Yanaba K, Venturi GM, Wang Y, Tisch RM, Poe JC, Tedder TF. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci USA. 2007;104:20878-20883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 89. | Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, Hui P, Leung NW, Zee B, Johnson PJ. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000;62:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 90. | Takai S, Tsurumi H, Ando K, Kasahara S, Sawada M, Yamada T, Hara T, Fukuno K, Takahashi T, Oyama M. Prevalence of hepatitis B and C virus infection in haematological malignancies and liver injury following chemotherapy. Eur J Haematol. 2005;74:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 91. | Tan J, Zhou J, Zhao P, Wei J. Prospective study of HBV reactivation risk in rheumatoid arthritis patients who received conventional disease-modifying antirheumatic drugs. Clin Rheumatol. 2012;31:1169-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 92. | Cheng AL, Hsiung CA, Su IJ, Chen PJ, Chang MC, Tsao CJ, Kao WY, Uen WC, Hsu CH, Tien HF. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology. 2003;37:1320-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 93. | Hsu CH, Hsu HC, Chen HL, Gao M, Yeh PY, Chen PJ, Cheng AL. Doxorubicin activates hepatitis B virus (HBV) replication in HBV-harboring hepatoblastoma cells. A possible novel mechanism of HBV reactivation in HBV carriers receiving systemic chemotherapy. Anticancer Res. 2004;24:3035-3040. [PubMed] |
| 94. | Chu CM, Karayiannis P, Fowler MJ, Monjardino J, Liaw YF, Thomas HC. Natural history of chronic hepatitis B virus infection in Taiwan: studies of hepatitis B virus DNA in serum. Hepatology. 1985;5:431-434. [PubMed] |
| 95. | Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1700] [Cited by in RCA: 1712] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 96. | Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 993] [Article Influence: 62.1] [Reference Citation Analysis (1)] |
| 97. | Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, Liaw YF. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 508] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 98. | Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N, Lascar RM, Brown D, Gilson RJ, Tedder RJ. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology. 2009;137:1289-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 280] [Article Influence: 17.5] [Reference Citation Analysis (1)] |
| 99. | Zerbini A, Pilli M, Boni C, Fisicaro P, Penna A, Di Vincenzo P, Giuberti T, Orlandini A, Raffa G, Pollicino T. The characteristics of the cell-mediated immune response identify different profiles of occult hepatitis B virus infection. Gastroenterology. 2008;134:1470-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 100. | Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, Janssen HL. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 407] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 101. | Kim SJ, Hsu C, Song YQ, Tay K, Hong XN, Cao J, Kim JS, Eom HS, Lee JH, Zhu J. Hepatitis B virus reactivation in B-cell lymphoma patients treated with rituximab: analysis from the Asia Lymphoma Study Group. Eur J Cancer. 2013;49:3486-3496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 102. | Yeo W, Chan TC, Leung NW, Lam WY, Mo FK, Chu MT, Chan HL, Hui EP, Lei KI, Mok TS. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 500] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 103. | Dervite I, Hober D, Morel P. Acute hepatitis B in a patient with antibodies to hepatitis B surface antigen who was receiving rituximab. N Engl J Med. 2001;344:68-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 104. | Yang SH, Kuo SH. Reactivation of hepatitis B virus during rituximab treatment of a patient with follicular lymphoma. Ann Hematol. 2008;87:325-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 105. | Pei SN, Chen CH, Lee CM, Wang MC, Ma MC, Hu TH, Kuo CY. Reactivation of hepatitis B virus following rituximab-based regimens: a serious complication in both HBsAg-positive and HBsAg-negative patients. Ann Hematol. 2010;89:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 106. | Hsu C, Tsou HH, Lin SJ, Wang MC, Yao M, Hwang WL, Kao WY, Chiu CF, Lin SF, Lin J, Chang CS, Tien HF, Liu TW, Chen PJ, Cheng AL; Taiwan Cooperative Oncology Group. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: A prospective study. Hepatology. 2013;Epub ahead of print. [RCA] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 107. | Kurokawa T, Hase M, Tokuman N, Yoshida T. Immune reconstitution of B-cell lymphoma patients receiving CHOP-based chemotherapy containing rituximab. Hematol Oncol. 2011;29:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 108. | Peñalver FJ, Alvarez-Larrán A, Díez-Martin JL, Gallur L, Jarque I, Caballero D, Díaz-Mediavilla J, Bustelos R, Fernández-Aceñero MJ, Cabrera JR. Rituximab is an effective and safe therapeutic alternative in adults with refractory and severe autoimmune hemolytic anemia. Ann Hematol. 2010;89:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 109. | Gudbrandsdottir S, Birgens HS, Frederiksen H, Jensen BA, Jensen MK, Kjeldsen L, Klausen TW, Larsen H, Mourits-Andersen HT, Nielsen CH. Rituximab and dexamethasone vs dexamethasone monotherapy in newly diagnosed patients with primary immune thrombocytopenia. Blood. 2013;121:1976-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 110. | Droz N, Gilardin L, Cacoub P, Berenbaum F, Wendling D, Godeau B, Piette AM, Dernis E, Ebbo M, Fautrel B. Kinetic profiles and management of hepatitis B virus reactivation in patients with immune-mediated inflammatory diseases. Arthritis Care Res (Hoboken). 2013;65:1504-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 111. | Pasquet F, Combarnous F, Macgregor B, Coppere B, Mausservey C, Ninet J, Hot A. Safety and efficacy of rituximab treatment for vasculitis in hepatitis B virus-associated type II cryoglobulinemia: a case report. J Med Case Rep. 2012;6:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 112. | Cil T, Altintas A, Tuzun Y, Pasa S, Isikdogan A. Hepatitis B virus reactivation induced by Yttrium-90-ibritumomab-tiuxetan. Leuk Lymphoma. 2007;48:1866-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 113. | Soong YL, Lee KM, Lui HF, Chow WC, Tao M, Li Er Loong S. Hepatitis B reactivation in a patient receiving radiolabeled rituximab. Ann Hematol. 2005;84:61-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 114. | Mitka M. FDA: Increased HBV reactivation risk with ofatumumab or rituximab. JAMA. 2013;310:1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 115. | Ogawa Y, Ogura M, Suzuki T, Ando K, Uchida T, Shirasugi Y, Tobinai K, Lee JH, Kase M, Katsura K. A phase I/II study of ofatumumab (GSK1841157) in Japanese and Korean patients with relapsed or refractory B-cell chronic lymphocytic leukemia. Int J Hematol. 2013;98:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 116. | Cortelezzi A, Sciumè M, Liberati AM, Vincenti D, Cuneo A, Reda G, Laurenti L, Zaja F, Marasca R, Chiarenza A. Bendamustine in combination with ofatumumab in relapsed or refractory chronic lymphocytic leukemia: a GIMEMA Multicenter Phase II Trial. Leukemia. 2014;28:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 117. | Chen CJ, Wang LY, Yu MW. Epidemiology of hepatitis B virus infection in the Asia-Pacific region. J Gastroenterol Hepatol. 2000;15 Suppl:E3-E6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 176] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 118. | Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology. 2006;43:209-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 367] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 119. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1794] [Cited by in RCA: 1778] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 120. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 743] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 121. | European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 1155] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 122. | Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, Neitzel SM, Ward JW. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1-20. [PubMed] |
| 123. | Artz AS, Somerfield MR, Feld JJ, Giusti AF, Kramer BS, Sabichi AL, Zon RT, Wong SL. American Society of Clinical Oncology provisional clinical opinion: chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. J Clin Oncol. 2010;28:3199-3202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 124. | Zurawska U, Hicks LK, Woo G, Bell CM, Krahn M, Chan KK, Feld JJ. Hepatitis B virus screening before chemotherapy for lymphoma: a cost-effectiveness analysis. J Clin Oncol. 2012;30:3167-3173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 125. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2171] [Article Influence: 135.7] [Reference Citation Analysis (0)] |
| 126. | Liu CJ, Chen PJ, Chen DS, Kao JH. Hepatitis B virus reactivation in patients receiving cancer chemotherapy: natural history, pathogenesis, and management. Hepatol Int. 2011;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 127. | Hsu C, Hsiung CA, Su IJ, Hwang WS, Wang MC, Lin SF, Lin TH, Hsiao HH, Young JH, Chang MC. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin’s lymphoma: a randomized trial. Hepatology. 2008;47:844-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 244] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 128. | Katz LH, Fraser A, Gafter-Gvili A, Leibovici L, Tur-Kaspa R. Lamivudine prevents reactivation of hepatitis B and reduces mortality in immunosuppressed patients: systematic review and meta-analysis. J Viral Hepat. 2008;15:89-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 129. | Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, Dienstag JL, Heathcote EJ, Little NR, Griffiths DA. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 589] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 130. | Huang YH, Hsiao LT, Hong YC, Chiou TJ, Yu YB, Gau JP, Liu CY, Yang MH, Tzeng CH, Lee PC. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013;31:2765-2772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 266] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 131. | Trembling PM, Tanwar S, Rosenberg WM, Dusheiko GM. Treatment decisions and contemporary versus pending treatments for hepatitis C. Nat Rev Gastroenterol Hepatol. 2013;10:713-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 132. | Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499-1512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1003] [Cited by in RCA: 1001] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 133. | Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 299] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 134. | Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 135. | Martell M, Esteban JI, Quer J, Genescà J, Weiner A, Esteban R, Guardia J, Gómez J. Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: quasispecies nature of HCV genome distribution. J Virol. 1992;66:3225-3229. [PubMed] |
| 136. | Chang KM, Rehermann B, McHutchison JG, Pasquinelli C, Southwood S, Sette A, Chisari FV. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. J Clin Invest. 1997;100:2376-2385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 241] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 137. | von Hahn T, Yoon JC, Alter H, Rice CM, Rehermann B, Balfe P, McKeating JA. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132:667-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 308] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 138. | Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 925] [Cited by in RCA: 861] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 139. | Dal Maso L, Franceschi S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2006;15:2078-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 140. | Vento S, Cainelli F, Mirandola F, Cosco L, Di Perri G, Solbiati M, Ferraro T, Concia E. Fulminant hepatitis on withdrawal of chemotherapy in carriers of hepatitis C virus. Lancet. 1996;347:92-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 134] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 141. | Zuckerman E, Zuckerman T, Douer D, Qian D, Levine AM. Liver dysfunction in patients infected with hepatitis C virus undergoing chemotherapy for hematologic malignancies. Cancer. 1998;83:1224-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 142. | Kawatani T, Suou T, Tajima F, Ishiga K, Omura H, Endo A, Ohmura H, Ikuta Y, Idobe Y, Kawasaki H. Incidence of hepatitis virus infection and severe liver dysfunction in patients receiving chemotherapy for hematologic malignancies. Eur J Haematol. 2001;67:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 143. | Nosotti L, D’Andrea M, Pitidis A, Pimpinelli F, Dessanti ML, Pisani F, Vignally P, Petti MC. Hepatitis C virus infection prevalence and liver dysfunction in a cohort of B-cell non-Hodgkin’s lymphoma patients treated with immunochemotherapy. Scand J Infect Dis. 2012;44:70-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 144. | Ennishi D, Maeda Y, Niitsu N, Kojima M, Izutsu K, Takizawa J, Kusumoto S, Okamoto M, Yokoyama M, Takamatsu Y. Hepatic toxicity and prognosis in hepatitis C virus-infected patients with diffuse large B-cell lymphoma treated with rituximab-containing chemotherapy regimens: a Japanese multicenter analysis. Blood. 2010;116:5119-5125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 145. | Ennishi D, Terui Y, Yokoyama M, Mishima Y, Takahashi S, Takeuchi K, Okamoto H, Tanimoto M, Hatake K. Monitoring serum hepatitis C virus (HCV) RNA in patients with HCV-infected CD20-positive B-cell lymphoma undergoing rituximab combination chemotherapy. Am J Hematol. 2008;83:59-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 146. | Coppola N, Pisaturo M, Guastafierro S, Tonziello G, Sica A, Iodice V, Sagnelli C, Ferrara MG, Sagnelli E. Increased hepatitis C viral load and reactivation of liver disease in HCV RNA-positive patients with onco-haematological disease undergoing chemotherapy. Dig Liver Dis. 2012;44:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 147. | Tsutsumi Y, Ichiki K, Shiratori S, Kawamura T, Tanaka J, Asaka M, Imamura M, Masauzi N. Changes in hepatitis C virus antibody titer and viral RNA load in non-Hodgkin’s lymphoma patients after rituximab chemotherapy. Int J Lab Hematol. 2009;31:468-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 148. | Lake-Bakaar G, Dustin L, McKeating J, Newton K, Freeman V, Frost SD. Hepatitis C virus and alanine aminotransferase kinetics following B-lymphocyte depletion with rituximab: evidence for a significant role of humoral immunity in the control of viremia in chronic HCV liver disease. Blood. 2007;109:845-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 149. | Halfon P, Bourlière M, Halimi G, Khiri H, Bertezene P, Portal I, Botta-Fridlund D, Gauthier AP, Jullien M, Feryn JM. Assessment of spontaneous fluctuations of viral load in untreated patients with chronic hepatitis C by two standardized quantitation methods: branched DNA and Amplicor Monitor. J Clin Microbiol. 1998;36:2073-2075. [PubMed] |
| 150. | Mahale P, Kontoyiannis DP, Chemaly RF, Jiang Y, Hwang JP, Davila M, Torres HA. Acute exacerbation and reactivation of chronic hepatitis C virus infection in cancer patients. J Hepatol. 2012;57:1177-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 151. | Torres HA, Davila M. Reactivation of hepatitis B virus and hepatitis C virus in patients with cancer. Nat Rev Clin Oncol. 2012;9:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 152. | D’Arena G, Laurenti L, Capalbo S, D’Arco AM, De Filippi R, Marcacci G, Di Renzo N, Storti S, Califano C, Vigliotti ML. Rituximab therapy for chronic lymphocytic leukemia-associated autoimmune hemolytic anemia. Am J Hematol. 2006;81:598-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 153. | Dufour JF, Pradat P, Ruivard M, Hot A, Dumontet C, Broussolle C, Trepo C, Sève P. Severe autoimmune cytopenias in treatment-naive hepatitis C virus infection: clinical description of 16 cases. Eur J Gastroenterol Hepatol. 2009;21:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 154. | Papaevangelou V, Varsami M, Papadakis V, Zellos A, Parcharidou A, Papargyri S, Karentzou O, Manolaki N, Roma E, Polychronopoulou S. Hepatitis C treatment concomitant to chemotherapy as “salvage” therapy in children with hematologic malignancies. Pediatr Infect Dis J. 2010;29:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 155. | Pellicelli AM, Zoli V. Role of ribavirin in hepatitis flare in HCV-infected patients with B cell non Hodgkin’s lymphoma treated with rituximab-containing regimens. Dig Liver Dis. 2011;43:501-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 156. | Young NS, Brown KE. Parvovirus B19. N Engl J Med. 2004;350:586-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 615] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 157. | Sokal EM, Melchior M, Cornu C, Vandenbroucke AT, Buts JP, Cohen BJ, Burtonboy G. Acute parvovirus B19 infection associated with fulminant hepatitis of favourable prognosis in young children. Lancet. 1998;352:1739-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 158. | Anderson MJ, Higgins PG, Davis LR, Willman JS, Jones SE, Kidd IM, Pattison JR, Tyrrell DA. Experimental parvoviral infection in humans. J Infect Dis. 1985;152:257-265. [PubMed] |
| 159. | Kurtzman GJ, Ozawa K, Cohen B, Hanson G, Oseas R, Young NS. Chronic bone marrow failure due to persistent B19 parvovirus infection. N Engl J Med. 1987;317:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 318] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 160. | Norbeck O, Isa A, Pöhlmann C, Broliden K, Kasprowicz V, Bowness P, Klenerman P, Tolfvenstam T. Sustained CD8+ T-cell responses induced after acute parvovirus B19 infection in humans. J Virol. 2005;79:12117-12121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 161. | Kasprowicz V, Isa A, Tolfvenstam T, Jeffery K, Bowness P, Klenerman P. Tracking of peptide-specific CD4+ T-cell responses after an acute resolving viral infection: a study of parvovirus B19. J Virol. 2006;80:11209-11217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 162. | Kuo SH, Lin LI, Chang CJ, Liu YR, Lin KS, Cheng AL. Increased risk of parvovirus B19 infection in young adult cancer patients receiving multiple courses of chemotherapy. J Clin Microbiol. 2002;40:3909-3912. [PubMed] |
| 163. | Heegaard ED, Schmiegelow K. Serologic study on parvovirus b19 infection in childhood acute lymphoblastic leukemia during chemotherapy: clinical and hematologic implications. J Pediatr Hematol Oncol. 2002;24:368-373. [PubMed] |
| 164. | Eid AJ, Brown RA, Patel R, Razonable RR. Parvovirus B19 infection after transplantation: a review of 98 cases. Clin Infect Dis. 2006;43:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 165. | Li Y, Dong Y, Jiang J, Yang Y, Liu K, Li Y. High prevelance of human parvovirus infection in patients with malignant tumors. Oncol Lett. 2012;3:635-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 166. | Poplar S, Allford S, Rooney N. Chronic parvovirus B19 infection leading to red cell aplasia following treatment of Hodgkin lymphoma. Br J Haematol. 2010;148:671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 167. | Sharma VR, Fleming DR, Slone SP. Pure red cell aplasia due to parvovirus B19 in a patient treated with rituximab. Blood. 2000;96:1184-1186. [PubMed] |
| 168. | Song KW, Mollee P, Patterson B, Brien W, Crump M. Pure red cell aplasia due to parvovirus following treatment with CHOP and rituximab for B-cell lymphoma. Br J Haematol. 2002;119:125-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 169. | Isobe Y, Sugimoto K, Shiraki Y, Nishitani M, Koike K, Oshimi K. Successful high-titer immunoglobulin therapy for persistent parvovirus B19 infection in a lymphoma patient treated with rituximab-combined chemotherapy. Am J Hematol. 2004;77:370-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 170. | Klepfish A, Rachmilevitch E, Schattner A. Parvovirus B19 reactivation presenting as neutropenia after rituximab treatment. Eur J Intern Med. 2006;17:505-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 171. | Hartmann JT, Meisinger I, Kröber SM, Weisel K, Klingel K, Kanz L. Progressive bicytopenia due to persistent parvovirus B19 infection after immunochemotherapy with fludarabine/cyclophosphamide and rituximab for relapsed B cell lymphoma. Haematologica. 2006;91:ECR49. [PubMed] |
| 172. | Yang SH, Lin LW, Fang YJ, Cheng AL, Kuo SH. Parvovirus B19 infection-related acute hepatitis after rituximab-containing regimen for treatment of diffuse large B-cell lymphoma. Ann Hematol. 2012;91:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 173. | Wong S, Young NS, Brown KE. Prevalence of parvovirus B19 in liver tissue: no association with fulminant hepatitis or hepatitis-associated aplastic anemia. J Infect Dis. 2003;187:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 174. | Aksoy S, Harputluoglu H, Kilickap S, Dede DS, Dizdar O, Altundag K, Barista I. Rituximab-related viral infections in lymphoma patients. Leuk Lymphoma. 2007;48:1307-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 199] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 175. | Iyer A, Mathur R, Deepak BV, Sinard J. Fatal adenoviral hepatitis after rituximab therapy. Arch Pathol Lab Med. 2006;130:1557-1560. [PubMed] |
| 176. | Ronan BA, Agrwal N, Carey EJ, De Petris G, Kusne S, Seville MT, Blair JE, Vikram HR. Fulminant hepatitis due to human adenovirus. Infection. 2014;42:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 177. | Ollier L, Tieulie N, Sanderson F, Heudier P, Giordanengo V, Fuzibet JG, Nicand E. Chronic hepatitis after hepatitis E virus infection in a patient with non-Hodgkin lymphoma taking rituximab. Ann Intern Med. 2009;150:430-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 178. | Bermúdez A, Marco F, Conde E, Mazo E, Recio M, Zubizarreta A. Fatal visceral varicella-zoster infection following rituximab and chemotherapy treatment in a patient with follicular lymphoma. Haematologica. 2000;85:894-895. [PubMed] |
| 179. | Del Prete CJ, Cohen NS. A case of rituximab-induced hepatitis. Cancer Biother Radiopharm. 2010;25:747-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 180. | Mössner E, Brünker P, Moser S, Püntener U, Schmidt C, Herter S, Grau R, Gerdes C, Nopora A, van Puijenbroek E. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115:4393-4402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 765] [Cited by in RCA: 709] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 181. | Dalle S, Reslan L, Besseyre de Horts T, Herveau S, Herting F, Plesa A, Friess T, Umana P, Klein C, Dumontet C. Preclinical studies on the mechanism of action and the anti-lymphoma activity of the novel anti-CD20 antibody GA101. Mol Cancer Ther. 2011;10:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 182. | Goldenberg DM, Rossi EA, Stein R, Cardillo TM, Czuczman MS, Hernandez-Ilizaliturri FJ, Hansen HJ, Chang CH. Properties and structure-function relationships of veltuzumab (hA20), a humanized anti-CD20 monoclonal antibody. Blood. 2009;113:1062-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |