Revised: March 29, 2013
Accepted: April 10, 2013
Published online: May 6, 2013
Processing time: 217 Days and 4.1 Hours
New assays for serum immunoglobulin (Ig) free and heavy chain quantification were developed for routine clinical practice. Serum free light chain (sFLC) assay was shown to improve detection, management and prognostication in all plasma cell dyscrasias. More precisely, sFLC measurements proved to be prognostic for the progression of monoclonal gammopathy of undetermined significance and smoldering multiple myeloma (MM), became markers of response and survival in amyloid light-chain amyloidosis and contributed to accurate follow-up of patients with light chain and non secretory MM. In addition, sFLC and they ratio (sFLCR) were shown useful for the prognosis and monitoring of intact Ig myeloma; their evaluation was incorporated in the new uniform response criteria. sFLC or sFLCR were also observed abnormal in B-cell non-Hodgkin lymphoma/chronic lymphocytic leukemia (CLL). Moreover, increased sFLC levels, summated sFLC or abnormal sFLCR predict shorter overall survival in early-stage CLL while increased sFLC constituted an independent, adverse prognostic factor for event-free and overall survival in diffuse large B-cell lymphoma and Waldenstrom’s macroglobulinemia. Clinical applications of heavy Ig chain separately (HLC) measurements are more recent and mainly concern MM in which HLC deriving ratios correlated with parameters of disease activity and constituted an adverse survival marker.
Core tip: Recently manufactured assays allow the quantification of serum immunoglobulin (Ig) free light chain (sFLC) or of κ or λ restricted heavy Ig chain separately (HLC). These measurements, or the calculation of their corresponding ratios, were shown useful for routine clinical practice in Hematology. sFLC measurements added important prognostic information for monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma (MM), amyloid light-chain (AL) amyloidosis, Waldenstrom’s macroglobulinemia and chronic lymphocytic leukemia while they contributed to accurate follow-up of MGUS, MM and AL amyloidosis patients. HLC measurements are more recent and mainly concern MM in which they constituted a prognostic marker.
- Citation: Kyrtsonis MC, Maltezas D, Koulieris E, Tzenou T, Harding SJ. Contribution of new immunoglobulin-derived biomarkers in plasma cell dyscrasias and lymphoproliferative disorders. World J Hematol 2013; 2(2): 6-12
- URL: https://www.wjgnet.com/2218-6204/full/v2/i2/6.htm
- DOI: https://dx.doi.org/10.5315/wjh.v2.i2.6
During the past decade, new assays for serum immunoglobulin (Ig) free and, more recently, heavy chain quantification were developed for routine practice[1].
Igs are produced during B lymphocyte development where they are initially expressed on the surface of the cell. Production of Igs continues throughout B-cell development and terminal differentiation into plasma cells where it is greatest; lymphoplasmocytes and plasma cells normally secrete Ig.
Igs are symmetrical and are made up of mirror imaged identical light and heavy chains. There are five classes of heavy chain, γ, α, μ, δ and ε with two classes of light chain κ and λ; with approximately × 2 greater κ production compared to λ. B-cells and plasma cells produce an excess of serum free light chains (sFLC) during normal Ig synthesis that enter the blood and the extravascular compartment. This production is rapidly cleared (2-6 h) and metabolized by the kidney although trace quantities (1-10 mg/L) can be found in the urine. In patients with plasma cell dyscrasias (PCD) and B-cell lymphoproliferative disorders (LPD) homogeneous FLC are produced by the malignant clone. sFLC are important biomarkers and may be present in serum in very large excess. Their quantification was not possible before the development of immunoassays utilizing specific polyclonal sheep antisera against κ and λ epitopes that are not visible when the FLC are bound to their heavy chain partners[2].
These assays have revolutionized the ability to detect and quantify sFLC with a sensitivity of less than 0.5 mg/L. Furthermore, the calculation of a κ/λ ratio (sFLCR), which incorporates both monoclonal Ig production and polyclonal Ig suppression, offers additional prognostic information[3].
Monoclonal Ig heavy chain is routinely detected by serum protein electrophoresis (SPEP), identified by immunofixation and quantified by SPEP-densitometry or nephelometry. Guidelines recommend SPEP to monitor monoclonal Ig concentrations as markers of response and relapse. However, SPEP quantification can be inaccurate at low concentrations (< 3 g/L) and can be difficult in patients where the monoclonal Ig co-migrates with other proteins, commonly seen in IgA and IgM isotypes. In such instances guidelines recommend the use of total Ig nephelometric assays, which do not distinguish between the monoclonal and polyclonal Ig’s and will be insensitive as the Ig concentration approaches the normal range. Furthermore, SPEP linearity at high concentrations and the variable catabolism of monoclonal IgG can make assessment of the serum load inaccurate. Production of immunoassays targeting the unique junctional epitope between the light chain and heavy chain constant region of Ig enables separate quantification for the different heavy Ig classes bounded to their respective light chain (HLC) i.e., IgGκ, IgGλ, IgAκ, IgAλ, IgMκ and IgMλ. Measuring the molecules in pairs then produces a ratio of the involved/uninvolved-polyclonal Igs (HLCR)[1-4].
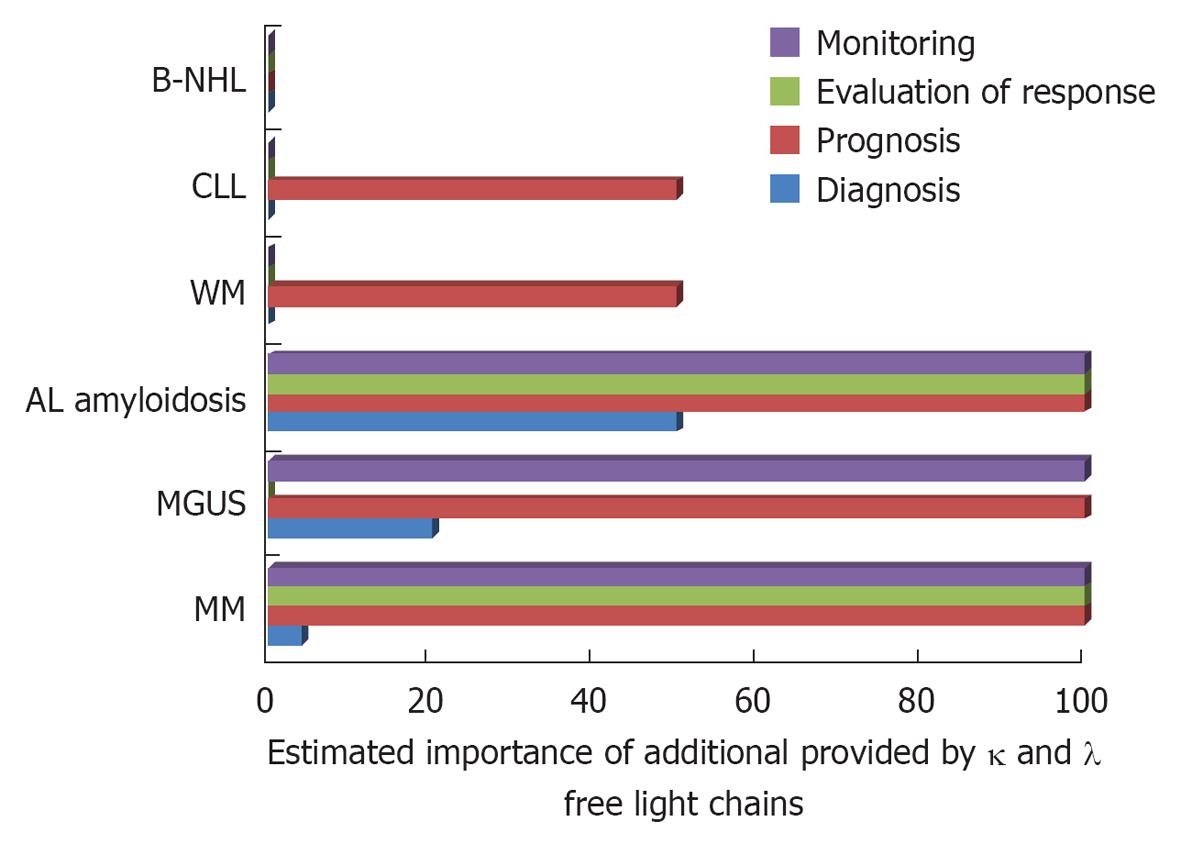
The diagnostic and prognostic utility of these tests in the management of PCD and B-cell LPD is established by now is some disease entities and under evaluation in others[5]. Herein, we will describe their main contributions in these disorders that, with regard to sFLC measurements, are depicted in Figure 1.
Monoclonal gammopathy of undetermined significance (MGUS) is a preneoplastic condition, defined as serum monoclonal protein < 30 g/L, < 10% clonal plasma cells in the bone marrow (BM) and no evidence of end organ damage. sFLCR is abnormal in approximately 1/3 of MGUS patients. It was shown that abnormal sFLCR constitutes an independent factor for disease progression[6]. Using three risk factors, abnormal sFLC, presence of non-IgG MGUS and monoclonal protein ≥ 15 g/L, a powerful stratification model to predict disease evolution to multiple myeloma (MM) was produced[7].
Preliminary HLC results in MGUS patients suggest that isotype specific HLC-IgG pair suppression is an indicator of susceptibility to evolve to myeloma; however the same was not observed for HLC-IgA[8].
MM is characterized by BM plasma cell infiltration and the presence of serum/urine monoclonal Ig. Clinical manifestations vary widely. The disease may be indolent or extremely severe and accompanied by significant morbidity. Survival ranges from a few months to more than a decade.
Serum FLC quantification is useful for diagnosis, response evaluation, monitoring and prognostic purposes.
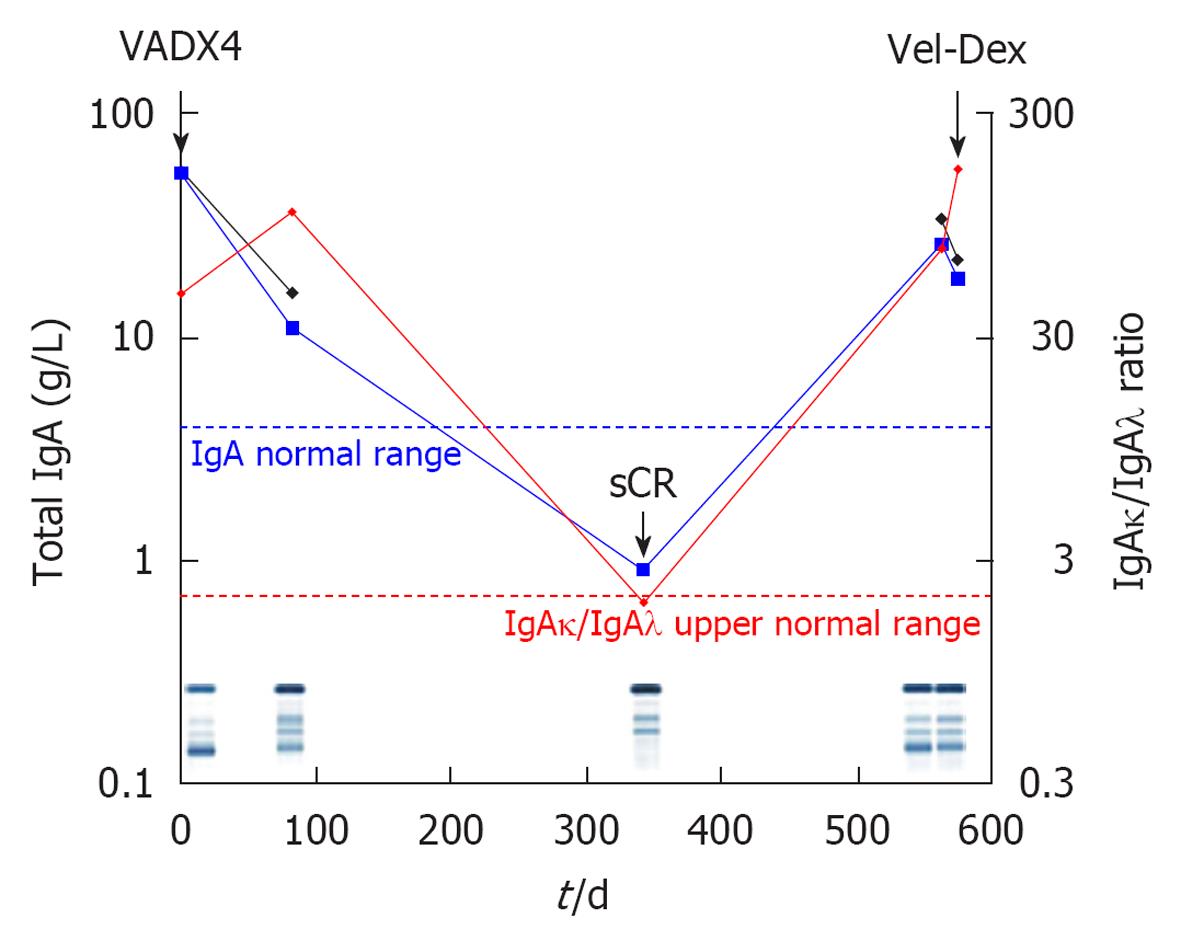
Serum FLC measurements are more accurate than quantification of urine proteinuria in light chain myeloma (LCM) while in the majority of non secretory MM, low levels of abnormal serum FLCs are actually found (oligosecretory disease). In both aforementioned MM subgroups, sFLC fluctuations can be used for disease monitoring[9]. Figure 2 shows the clinical course of an LCM patient along with sFLC fluctuations; it is interesting to observe that this patient would have been characterized “non-secretory” because she has no serum monoclonality and never presented albuminurea or positive urine immunofixation in spite of increased sFLC and it would have been impossible to monitor her disease without sFLC measurements.
In an attempt to improve criteria of response to treatment[10], sFLCR was incorporated to the MM uniform response criteria[11] and its normalization along with immunohistological confirmation of clonal disease absence, defined a deeper response, the stringent complete remission (sCR). A better evaluation of the depth of response is important as the quality of response influences treatment free and overall survival after treatment[12]. However, the impact of sCR compared to CR and very good partial remission in terms of progression free and overall survival has not been fully proven yet.
With regard to prognosis, sFLC and sFLCR were shown predictive of outcome in all MM subcategories. Patients with smoldering myeloma and abnormal sFLCR (< 0.125 or > 8) were shown to have an increased progression risk[13]. An adverse outcome was observed in patients with overt MM and sFLCR > median or < 0.03 or > 32[14,15] while the combination of sFLCR and other markers of disease activity (LDH, β2-microglobulin, genetic abnormalities) provided powerful prognostic models[16]. There have also been proposals of sFLCR incorporation in to the International Score System[15,17], that remain to be validated in larger patient cohorts and in the new agents era.
sFLC measurements during follow-up of patients are useful, not only for the evaluation of response as already mentioned, but also because, with the improvement of treatment modalities resulting in prolonged survival, unusual relapses may be observed. Light chain escape is a transformation that may occur, characterized by a shift in secretion from intact Ig to LC only in a subset of patients[18,19].
The rationale for HLC measurements in MM is quite obvious. Serum monoclonal Ig quantification is part of MM diagnostic criteria and it is also used for monitoring response and relapse. However, MM aggressiveness was not found related to the amount of Ig secretion[20] as detected by classical densitometry or nephelometry total Ig quantification although Ig amount was a risk factor of Durie and Salmon’s staging system[21]. It is indeed attractive to study whether the specified and precise quantification of the monoclonal component renders it a prognostic indicator.
New assays (Hevylite) that allow the separate quantification of the involved IgGκ, IgGλ, IgAκ and IgAλ, along with their deriving ratios: IgGκ/IgGλ, IgGλ/IgGκ, IgAκ/IgAλ and IgAλ/IgAκ[1,22], were recently introduced; their utility was shown by several groups. It was preliminary reported that increased HLC-IgG and -IgA ratios were predictive of a shorter progression-free survival[23] and overall survival[24,25].
Two recent studies showed the first that HLCR correlated with parameters of disease activity and tumor burden including anemia, sFLCR, increased β2-microglobulin and marrow infiltration of more than 50%, while HLCR values above median constituted an independent predictor of adverse survival[26]. In addition, the same study found that the depression of uninvolved polyclonal Igs and increased HLCR were related to a shorter time to treatment[26]. The second study evaluated MM patients in complete remission after autologous stem-cell transplantation and showed that an increased κ/λ ratio of the uninvolved isotype was associated with a longer progression-free and overall survival[27].
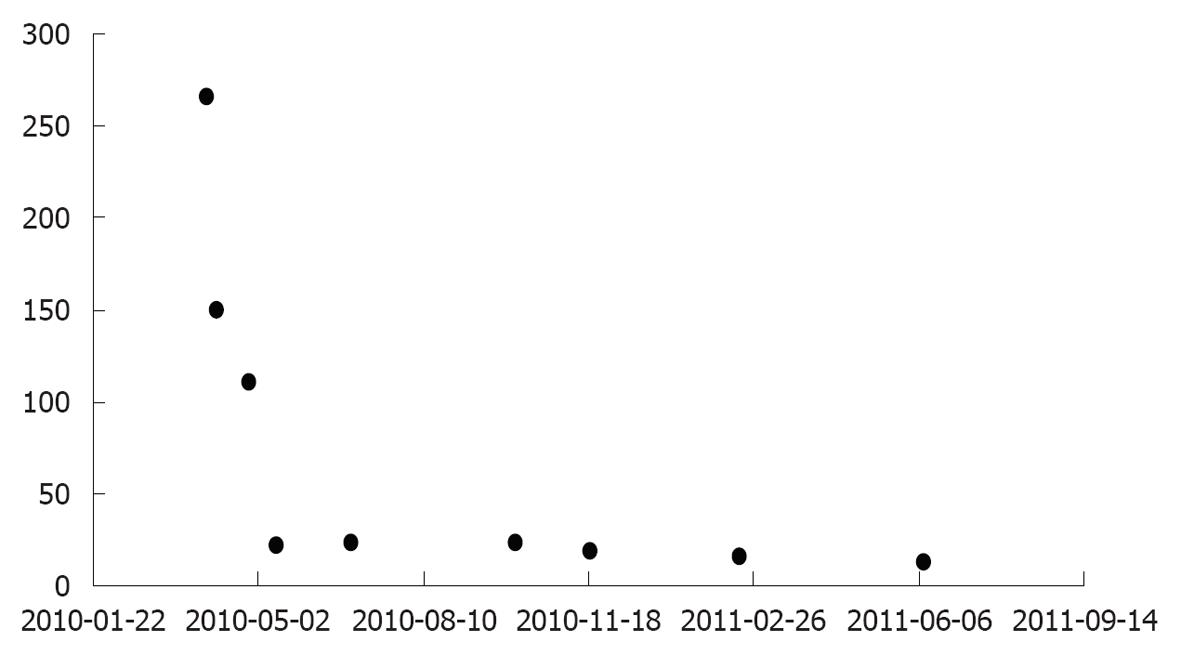
Serum HLC measurements may also offer additional information during follow-up, compared to classical total Ig quantification (Figure 3).
Systemic amyloid light-chain (AL) amyloidosis is due to the deposition of misfolded monoclonal light chains or their fragments in tissues or organs, leading to their dysfunction[28]. The diagnosis is frequently difficult to make and in the absence of characteristic amyloidosis signs or of serum intact Ig paraprotein, and physicians should be sensitized to AL amyloidosis eventuality in order to detect it. In such a context sFLC measurements are useful and will be found increased in up to 94%-98% of patients, even in the absence of any Ig monoclonal peak on serum electrophoresis or immunoelectrophoresis. Indeed, diagnosis should be subsequently biopsy proven.
In addition, sFLC serum concentrations allow easy monitoring of response to treatment[29]. Figure 4 shows serum FLC fluctuations in response to treatment in a patient with AL amyloidosis presenting BM, stomach and renal involvement. The case is however extreme because usually sFLC values are much lower in this disease.
sFLC levels at diagnosis constitute an adverse marker of survival in AL amyloidosis[30]. The addition of cardiac biomarkers (cardiac troponin T and N-terminal pro–B-type natriuretic peptide) to sFLC levels was highly predictive of patients’ survival[31] and a new prognostic staging system was built[32].
Preliminary data on HLC measurements in AL amyloidosis appear promising. In a subset of AL amyloidosis patients with no detectable serum or urinary monoclonal bands and a normal sFLC ratio, the HLC ratio was abnormal in 19% of cases, identifying 2 IgAκ, 3 IgAλ, and 4 IgGκ clones[33].
Focal infiltration by monoclonal plasma cells in the absence of systemic disease is observed when solitary plasmacytomas are formed. They represent 3%-5% of PCD; they may arise from bone or be extramedullary, extra osseous. Bone solitary plasmacytomas present an increased tendency to evolve to MM; sFLC measurements help monitoring these patients and increased sFLCR was shown to constitute an independent risk prognostic factor of evolution[34,35].
Waldenstroms’ macroglobulinemia (WM) is a lymphoplasmacytic lymphoma characterized by the presence of a serum IgM monoclonal component. The disease is rare and presents a wide range of clinical manifestations including fatigue, hyperviscosity symptoms, lymphadenopathy, organomegaly, peripheral neuropathy and other. Asymptomatic patients do not require treatment and usually enjoy a prolonged survival, while patients with aggressive symptomatic disease should be immediately treated with chemotherapy.
There are so far only preliminary results on the contribution of sFLC and HLC levels in WM patients at diagnosis. It was shown that sFLC may be increased and, in such case, correlate with markers of disease activity, such as increased β2M, anemia[36] and low serum albumin levels. Patients with elevated sFLC presented shorter time to treatment[37] and adverse outcome[38]. Increased HLC-IgM were also found correlated with markers of disease activity such as BM infiltration of more than 50% and low serum albumin levels while high HLCR correlated with shorter time to treatment[39].
Chronic lymphocytic leukemia (CLL) is the most common type of leukemia in the Western world and presents a variable outcome. More than two third of the patients are asymptomatic at the time of diagnosis and may not require treatment for months and even years. Currently available disease prognostic markers, including Rai and Binet staging systems and underlying molecular alterations do not apply perfectly for asymptomatic patients and other prognostic tools are under investigation. For the majority of CLL patients, life expectancy largely depends on time to first treatment[40].
It was shown that increased sFLC are observed in almost half of CLL cases, and that sFLCR abnormalities are present in a significant proportion of patients and identify those at risk of progressive disease[41,42].
More recently, increased polyclonal sFLC were also found to constitute an adverse marker for time to first treatment in CLL[43]. This finding was confirmed by Morabito et al[44] that evaluated the sum of absolute κ and λ sFLC and found that the prognostic impact of sFLC (κ + λ) value above 60.6 mg/mL was superior compared to FLCR; thus, a model, based on four variables, namely sFLC (κ + λ) more than 60.6 g/L, Binet staging, ZAP-70, and cytogenetics was built and separated 4 patients’ groups with different time to treatment.
sFLC may potentially be increased in any B-cell lymphomas (B-NHL). In a series of 208 patients with various B-NHL, sFLC were found increased in 13% (26/202)[45].
Increased sFLC has been shown associated with adverse outcome in patients with diffuse large B-cell lymphoma[46] and predictive of increased risk of non-Hodgkin’s lymphoma development in human immunodeficiency virus infected patients[47]. The contribution of sFLC measurements is currently being investigated in other B-cell NHL also[48].
In all malignant B-cell disorders and especially in true PCD, FLC measurements help patients’ accurate diagnosis, prognosis and monitoring. Ongoing studies on the contribution of the more recent HLC assays, show that they will most probably acquire the same importance.
P- Reviewer De Re V S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Bradwell AR. Serum Free Light Chain Analysis (Plus Hevylite). 6th ed. Birmingham: The Binding Site Ltd 2010; 18-58, 301-318. |
| 2. | Bradwell AR, Carr-Smith HD, Mead GP, Tang LX, Showell PJ, Drayson MT, Drew R. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001;47:673-680. [PubMed] |
| 3. | Bradwell AR, Harding SJ, Fourrier NJ, Wallis GL, Drayson MT, Carr-Smith HD, Mead GP. Assessment of monoclonal gammopathies by nephelometric measurement of individual immunoglobulin kappa/lambda ratios. Clin Chem. 2009;55:1646-1655. [PubMed] |
| 4. | Keren DF. Heavy/Light-chain analysis of monoclonal gammopathies. Clin Chem. 2009;55:1606-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Harding SJ, Mead GP, Bradwell AR, Berard AM. Serum free light chain immunoassay as an adjunct to serum protein electrophoresis and immunofixation electrophoresis in the detection of multiple myeloma and other B-cell malignancies. Clin Chem Lab Med. 2009;47:302-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Rajkumar SV, Kyle RA, Therneau TM, Melton LJ, Bradwell AR, Clark RJ, Larson DR, Plevak MF, Dispenzieri A, Katzmann JA. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106:812-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 468] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 7. | Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, Kröger N, Einsele H, Vesole DH, Dimopoulos M. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 667] [Cited by in RCA: 582] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 8. | Katzmann J, Clark R, Dispenzieri A, Kyle R, Landgren O, Bradwell A, Rajkumar SV. Isotype-Specific Heavy/Light Chain (HLC) Suppression as a Predictor of Myeloma Development in Monoclonal Gammopathy of Undetermined Significance (MGUS). Blood (ASH Annual Meeting Abstracts). 2009;114:1788. |
| 9. | Drayson M, Tang LX, Drew R, Mead GP, Carr-Smith H, Bradwell AR. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood. 2001;97:2900-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 263] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Bladé J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G, Gertz M, Giralt S, Jagannath S, Vesole D. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1212] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 11. | Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1926] [Cited by in RCA: 2102] [Article Influence: 110.6] [Reference Citation Analysis (0)] |
| 12. | Gay F, Larocca A, Wijermans P, Cavallo F, Rossi D, Schaafsma R, Genuardi M, Romano A, Liberati AM, Siniscalchi A. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: analysis of 1175 patients. Blood. 2011;117:3025-3031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 13. | Kyle RA, Remstein ED, Therneau TM, Dispenzieri A, Kurtin PJ, Hodnefield JM, Larson DR, Plevak MF, Jelinek DF, Fonseca R. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356:2582-2590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 626] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 14. | Kyrtsonis MC, Vassilakopoulos TP, Kafasi N, Sachanas S, Tzenou T, Papadogiannis A, Galanis Z, Kalpadakis C, Dimou M, Kyriakou E. Prognostic value of serum free light chain ratio at diagnosis in multiple myeloma. Br J Haematol. 2007;137:240-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Snozek CL, Katzmann JA, Kyle RA, Dispenzieri A, Larson DR, Therneau TM, Melton LJ, Kumar S, Greipp PR, Clark RJ. Prognostic value of the serum free light chain ratio in newly diagnosed myeloma: proposed incorporation into the international staging system. Leukemia. 2008;22:1933-1937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Kumar S, Zhang L, Dispenzieri A, Van Wier S, Katzmann JA, Snyder M, Blood E, DeGoey R, Henderson K, Kyle RA. Relationship between elevated immunoglobulin free light chain and the presence of IgH translocations in multiple myeloma. Leukemia. 2010;24:1498-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Kyrtsonis MC, Vassilakopoulos TP, Kafasi N, Maltezas D, Anagnostopoulos A, Terpos E, Elefterakis-Papaiakovou E, Pouli A, Repousis P, Delimpasi S. The addition of sFLCR improves ISS prognostication in multiple myeloma. Blood (ASH Annual Meeting Abstracts). 2007;110:Abstr 1490. |
| 18. | Kühnemund A, Liebisch P, Bauchmüller K, zur Hausen A, Veelken H, Wäsch R, Engelhardt M. ‘Light-chain escape-multiple myeloma’-an escape phenomenon from plateau phase: report of the largest patient series using LC-monitoring. J Cancer Res Clin Oncol. 2009;135:477-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Koulieris E, Bartzis V, Tzenou T, Kafasi N, Efthymiou A, Mpitsanis K, Dimou M, Maltezas D, Panayiotidis P, Kyrtsonis MC. Free light chain clonal escape reflects relapse in intact immunoglobulin multiple myeloma (MM). Haematologica. 2011;96:S150. |
| 20. | Kyrtsonis MC, Maltezas D, Koulieris E, Bitsani K, Pessach I, Efthymiou A, Bartzis V, Tzenou T, Panayiotidis P. The contribution of prognostic factors to the better management of multiple myeloma patients. Multiple Myeloma - An Overview. Rijeka: InTech 2012; 145-174. [DOI] [Full Text] |
| 21. | Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842-854. [PubMed] |
| 22. | Katzmann JA, Stankowski-Drengler TJ, Kyle RA, Karen SL, Snyder MR, Lust JA, Dispenzieri A. Specificity of serum and urine protein electrophoresis for the diagnosis of monoclonal gammopathies. Clin Chem. 2010;56:1899-1900. [PubMed] |
| 23. | AvetLoiseau H, Harousseau JL, Moreau P, Mathiot C, Facon T, Attal M, Bradwell A, Harding S. Heavy/light chain specific immunoglobulin ratios at presentation are prognostic for progression free survival in the IFM 2005-01 myeloma trial. Blood (ASH Annual Meeting Abstracts). 2009;14:1818. |
| 24. | Koulieris E, Kyrtsonis MC, Kafassi N, Maltezas D, Bartzis V, Tzenou T, Dimou M, Georgiou G, Mirbahai L, Panayiotidis P. Heavy Chain Ratio (HLCR) IgG/IgG or IgA/IgA: Experience and Clinical Implications In Multiple Myeloma at Diagnosis and During Disease Course. Blood (ASH Annual Meeting Abstracts). 2010;116:Abstract 5019. |
| 25. | Ludwig H, Faint J, Zojer N, Bradwell AR, Young P, Milosavljevic D, Hübl W, Harding S. Serum heavy/light chain and free light chain measurements provide prognostic information, allow creation of a prognostic model and identify clonal changes (clonal tiding) through the course of multiple myeloma (MM). Blood (ASH Annual Meeting Abstracts). 2011;118:2883. |
| 26. | Koulieris E, Panayiotidis P, Harding SJ, Kafasi N, Maltezas D, Bartzis V, Tzenou T, Dimou M, Georgiou G, Mirbahai L. Ratio of involved/uninvolved immunoglobulin quantification by Hevylite™ assay: clinical and prognostic impact in multiple myeloma. Exp Hematol Oncol. 2012;1:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Tovar N, Fernández de Larrea C, Elena M, Cibeira MT, Aróstegui JI, Rosiñol L, Filella X, Yagüe J, Bladé J. Prognostic impact of serum immunoglobulin heavy/light chain ratio in patients with multiple myeloma in complete remission after autologous stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1076-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Gertz MA. How to manage primary amyloidosis. Leukemia. 2012;26:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Lachmann HJ, Gallimore R, Gillmore JD, Carr-Smith HD, Bradwell AR, Pepys MB, Hawkins PN. Outcome in systemic AL amyloidosis in relation to changes in concentration of circulating free immunoglobulin light chains following chemotherapy. Br J Haematol. 2003;122:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 279] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 30. | Dispenzieri A, Lacy MQ, Katzmann JA, Rajkumar SV, Abraham RS, Hayman SR, Kumar SK, Clark R, Kyle RA, Litzow MR. Absolute values of immunoglobulin free light chains are prognostic in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2006;107:3378-3383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 31. | Palladini G, Foli A, Milani P, Russo P, Albertini R, Lavatelli F, Obici L, Perlini S, Moratti R, Merlini G. Best use of cardiac biomarkers in patients with AL amyloidosis and renal failure. Am J Hematol. 2012;87:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, Laumann K, Zeldenrust SR, Leung N, Dingli D. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 820] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 33. | Wechalekar AD, Harding S, Lachmann HJ, Gillmore JD, Wassef NL, Thomas M, Gibbs SDJ, Sattianayagam P, Whelan CJ, Bradwell AR. Serum immunoglobulin heavy/light chain ratios (HevyLite) in patients with systemic AL amyloidosis. Amyloid. 2010;17:188-189. |
| 34. | Leleu X, Moreau AS, Hennache B, Dupire S, Faucompret JL, Facon T, Bradwell A, Reid S, Mead G. Serum free light chain immunoassays for monitoring solitary bone plasmacytoma. Haematologica. 2005;90:110. |
| 35. | Dingli D, Kyle RA, Rajkumar SV, Nowakowski GS, Larson DR, Bida JP, Gertz MA, Therneau TM, Melton LJ, Dispenzieri A. Immunoglobulin free light chains and solitary plasmacytoma of bone. Blood. 2006;108:1979-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Leleu X, Koulieris E, Maltezas D, Itzykson R, Xie W, Manier S, Dulery R, Boyle E, Gauthier J, Poulain S. Novel M-component based biomarkers in Waldenström’s macroglobulinemia. Clin Lymphoma Myeloma Leuk. 2011;11:164-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Itzykson R, Le Garff-Tavernier M, Katsahian S, Diemert MC, Musset L, Leblond V. Serum-free light chain elevation is associated with a shorter time to treatment in Waldenstrom’s macroglobulinemia. Haematologica. 2008;93:793-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Maltezas D, Tzenou T, Kafassi N, Koulieris E, Bartzis V, Dimou M, Efthymiou A, Georgiou G, Vassilakopoulos TP, Angelopoulou MK. Clinical Impact of Increased Serum Free Light Chains (sFLCs) or their Ratio (FLCR) in WM at diagnosis and during disease course. Proceedings of the 6th International Workshop on Waldenstrom’s macroglobulinemia; 2010 Oct 6-10; Venice; Italy. . |
| 39. | Kyrtsonis MC, Koulieris E, Maltezas D, Tzenou T, Harding S, Kastritis E, Kafasi N, Bartzis V, Eythymiou A, Bitsanis K. Prognostic Contribution of the New Immunoglobulin (Ig) Biomarkers (Freelite™ and Hevylite™) in Waldenstrom’s Macroglobulinemia (WM). AJMMS. 2012;2:136-143. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating MJ, Montserrat E, Rai KR. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446-5456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2442] [Cited by in RCA: 2470] [Article Influence: 145.3] [Reference Citation Analysis (0)] |
| 41. | Pratt G, Harding S, Holder R, Fegan C, Pepper C, Oscier D, Gardiner A, Bradwell AR, Mead G. Abnormal serum free light chain ratios are associated with poor survival and may reflect biological subgroups in patients with chronic lymphocytic leukaemia. Br J Haematol. 2009;144:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Yegin ZA, Ozkurt ZN, Yağci M. Free light chain: a novel predictor of adverse outcome in chronic lymphocytic leukemia. Eur J Haematol. 2010;84:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Maurer MJ, Cerhan JR, Katzmann JA, Link BK, Allmer C, Zent CS, Call TG, Rabe KG, Hanson CA, Kay NE. Monoclonal and polyclonal serum free light chains and clinical outcome in chronic lymphocytic leukemia. Blood. 2011;118:2821-2826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Morabito F, De Filippi R, Laurenti L, Zirlik K, Recchia AG, Gentile M, Morelli E, Vigna E, Gigliotti V, Calemma R. The cumulative amount of serum-free light chain is a strong prognosticator in chronic lymphocytic leukemia. Blood. 2011;118:6353-6361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Martin W, Abraham R, Shanafelt T, Clark RJ, Bone N, Geyer SM, Katzmann JA, Bradwell A, Kay NE, Witzig TE. Serum-free light chain-a new biomarker for patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Transl Res. 2007;149:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Maurer MJ, Micallef IN, Cerhan JR, Katzmann JA, Link BK, Colgan JP, Habermann TM, Inwards DJ, Markovic SN, Ansell SM. Elevated serum free light chains are associated with event-free and overall survival in two independent cohorts of patients with diffuse large B-cell lymphoma. J Clin Oncol. 2011;29:1620-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Landgren O, Goedert JJ, Rabkin CS, Wilson WH, Dunleavy K, Kyle RA, Katzmann JA, Rajkumar SV, Engels EA. Circulating serum free light chains as predictive markers of AIDS-related lymphoma. J Clin Oncol. 2010;28:773-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Charafeddine KM, Jabbour MN, Kadi RH, Daher RT. Extended use of serum free light chain as a biomarker in lymphoproliferative disorders: a comprehensive review. Am J Clin Pathol. 2012;137:890-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |