Published online Jan 17, 2023. doi: 10.5315/wjh.v10.i2.15
Peer-review started: August 2, 2022
First decision: October 21, 2022
Revised: November 3, 2022
Accepted: November 23, 2022
Article in press: November 23, 2022
Published online: January 17, 2023
Processing time: 167 Days and 20.4 Hours
Hematopoietic stem cell transplantation (HSCT) becomes a standard form of cellular therapy for patients with malignant diseases. HSCT is the first-choice of immunotherapy, although HSCT can be associated with many complications such as graft-versus-host disease (GVHD) which is a major cause of morbidity and mortality after allogeneic HSCT. It has been shown that certain gut microbiota could exert protective and/or regenerative immunomodulatory effects by the production of short-chain fatty acids (SCFAs) such as butyrate in the experimental models of GVHD after allogeneic HSCT. Loss of gut commensal bacteria which can produce SCFAs may worsen dysbiosis, increasing the risk of GVHD. Expression of G-protein coupled receptors such as GPR41 seems to be upre-gulated in the presence of commensal bacteria, which might be associated with the biology of regulatory T cells (Tregs). Treg cells are a suppressive subset of CD4 positive T lymphocytes implicated in the prevention of GVHD after allogeneic HSCT. Here, we discuss the current findings of the relationship between the modification of gut microbiota and the GVHD-related immunity, which suggested that tactics with certain probiotics for the beneficial symbiosis in gut-immune axis might lead to the elevation of safety in the allogeneic HSCT.
Core Tip: Potential efficacy of probiotics for the treatment of graft vs host disease after hematopoietic stem cell transplantation has been shown here.
- Citation: Yoshikawa S, Taniguchi K, Sawamura H, Ikeda Y, Tsuji A, Matsuda S. Advantageous tactics with certain probiotics for the treatment of graft-versus-host-disease after hematopoietic stem cell transplantation. World J Hematol 2023; 10(2): 15-24
- URL: https://www.wjgnet.com/2218-6204/full/v10/i2/15.htm
- DOI: https://dx.doi.org/10.5315/wjh.v10.i2.15
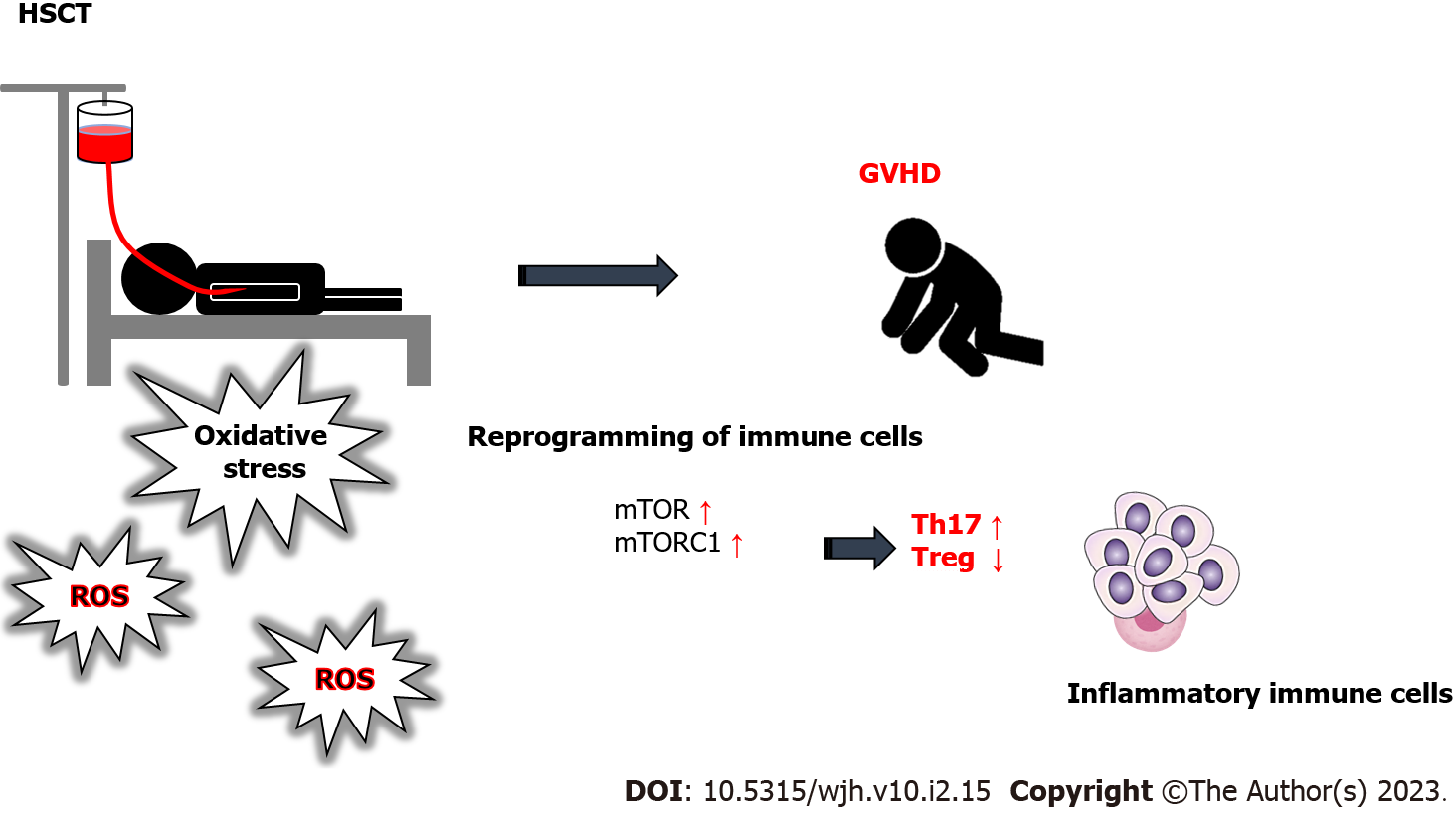
Hematopoietic stem cell transplantation (HSCT) is a broadly accomplished curative therapy for several hematological diseases, which is achieved by circulatory infusion of hematopoietic stem cells to the patients from human leukocyte antigen (HLA)-matched allogeneic donor or from the autologous patient themselves[1] (Figure 1). However, the HSCT techniques are restricted by potentially life-threatening complications, and one of the most serious complications is graft vs host disease (GVHD)[2], which is a pathogenic condition that arises when immune cells of the graft might systematically distinguish the host as foreign enemy, and affect the recipient’s tissues/organs in the body induced by the influx of donor-derived effector T cells into peripheral tissues[3] (Figure 1). The pathophysiology of GVHD may include donor T cells and/or inflammatory cytokine-mediated injury to patient’s tissues as a result of transplant and/or conditioning regimen. Immune reactions underlying the GVHD may also include greater proliferation and/or migration of active immune cells to the target tissue or organ. Several organs could be targeted by the GVHD. Therefore, the patient should be prepared with rigorous chemotherapy and/or radiotherapy to reduce immunological resistance in addition to extinguish residual malignant cells before HSCT.
Remarkably, several risk factors in gut might play important roles in the initiation of GVHD[4]. Gut microbiota has been hypothesized to have a role in GVHD onset[5]. In addition, the path of gut microbiota to the recovery following HSCT might be related to the risk of developing GVHD[6]. It has been suggested that potential modifiable targets of gut microbiota could reduce the risk of GVHD[4,7]. For example, a prolonged gut microbiota-dysbiosis following HSCT has in turn been demonstrated to increase a risk of innate immune system activation and/or systemic infections, causing the development of GVHD[8]. Equally, the prompt recovery of gut microbiota may protect the host against the onset of GVHD by the keen preservation of immune homeostasis[9]. The gut flora can make the difference when it comes to allogenic HSCT[10]. As prevention and/or treatment of the GVHD are the imperative issue for improving the efficacy of HSCT, it is significant that homeostasis of gut microbiota could possibly reduce the risk of GVHD.
GVHD is generally characterized by cytokine production, proliferation, and migration of reactive T cells of donor. Therefore, oxidative stress is frequently elevated in the tissues/organs of recipients with allogeneic HSCT, which may further contribute to the progression of the GVHD[11]. Patients with allogeneic HSCT may have various risk factors for developing GVHD such as acute kidney injury[12]. In particular, GVHD is a chief risk factor for the development of renal failure and/or acute kidney injury in HSCT recipients[13]. The other risk factors are sepsis, and nephrotoxic medications including amphotericin B and/or cyclosporine[13]. A reprogramming of immune cells might be a feature of GVHD, which is connected with the differentiation of CD4+ cells to the pathogenic type 1 T helper (Th1) and type 17 T helper (Th17) cells as well as the insufficiency of the immune-suppressive regulatory T cells (Tregs)[14] (Figure 1). In addition, the reprogramming of cellular metabolism is also a feature of GVHD, showing that mTOR inhibition may reduce the GVHD and increase the potency of peripheral Tregs as well as induction of Tregs from CD4 positive T cells[14,15]. The immuno-metabolic effects might be aimed at metabolic management of GVHD. In fact, it has been shown that the metabolic reprogramming might represent a promising strategy for the therapeutic target of GVHD[16,17]. Following the HSCT, donor T cells are stimulated by the antigens of mismatched recipient cells to undergo glycolytic metabolic reprogramming and form allogeneic effector T cells[18].
Metabolic cellular regulation is important for immune-regulation, and cytokine production and/or metabolic condition of HSCT recipients have been revealed as a risk of GVHD development[19]. Previous studies have suggested that differences in metabolism of immune cells are significantly associated with the pathology of GVHD[20]. T cells could undergo distinct metabolic reprogramming in response to allogenic antigens, suggesting that the reactive T cells might depend on glycolysis to meet ATP demands[21]. It is indispensable to evaluate the metabolic reprogramming of T cells in order to appropriately respond to the allogenic antigens after HSCT[21]. On the other hand, it has been shown that Tregs could suppress the reactive T cells responsible for the GVHD and/or allogenic graft rejection[22]. Therefore, the immune-suppressive donor Tregs could considerably prevent GVHD and/or graft rejection[23]. In fact, adaptive transfer of human Tregs has demonstrated significant efficacy in preventing GVHD after allogeneic HSCT[24].
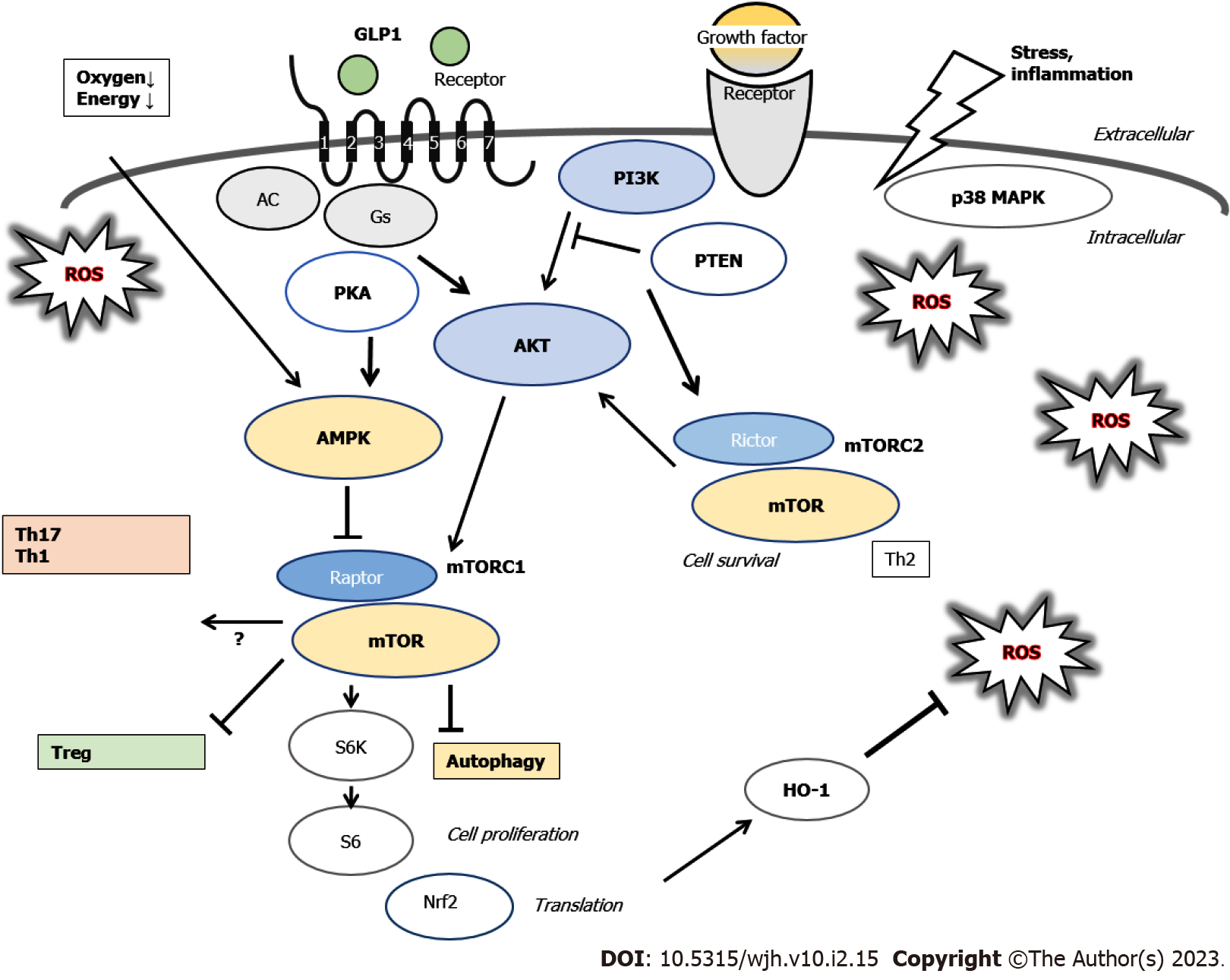
Blockade of the nutrient sensor mechanistic target of rapamycin (mTOR) using its antagonist, such as rapamycin, is an additional key aspect of metabolic reprogramming in GVHD[25]. Mechanistic target of rapamycin complex 1 (mTORC1) is sensitive to rapamycin inhibition, while mTORC2 is not[25]. Hyper-activation of mTORC1-signaling might be necessary for the pathogenesis of inflammatory bowel disease which is multi-factorial chronic intestinal inflammation driven by pathogenic T cells[26]. Tregs are tightly controlled by the activation of nutrient-fueled mTORC1[27]. Inhibition of mTORC1 may protect the bioactivity and/or homeostasis of human Tregs from apoptosis[28]. Cytokine situation towards Th17 over Treg immunity could be found through impaired autophagy by decreasing mTORC1[29]. It has been shown that the mTORC1 could drive the proinflammatory expansion of Th1, Th17, and double-negative T cells, and might inhibit the development of Tregs[30] (Figure 2).
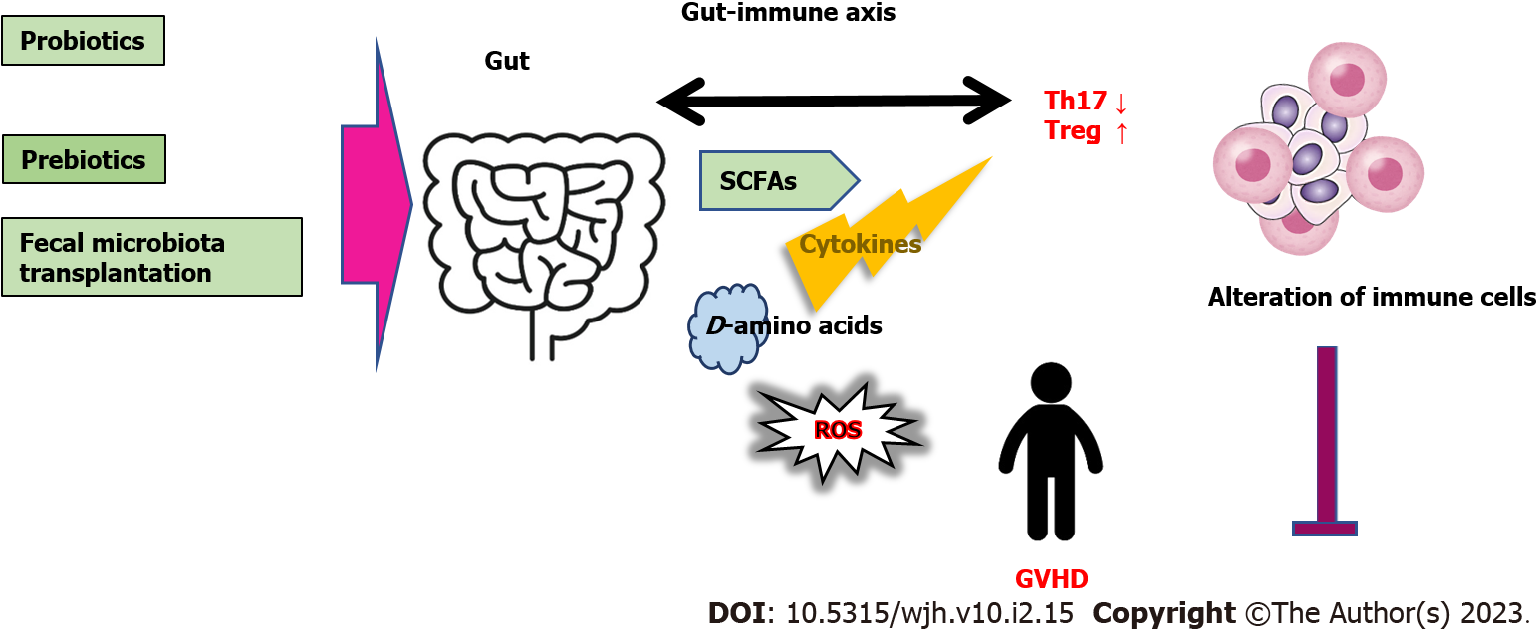
Gut microbiota might be associated with the development of GVHD, in which loss of diversity in the microbiota could be a risk factor for the GVHD[31,32]. Gut microbiota might be perturbed due to basic HSCT condition, various infections, and/or use of antibiotics, resulting in increased inflammatory factors influencing the Treg/Th17 balance, thus promoting the development of GVHD. It is noteworthy that these inflammatory factors are associated with the Treg/Th17 balance. Therefore, gut microbiota with inflammatory diseases could affect GVHD and/or the balance of Treg/Th17[33,34]. In general, diet has an influence on the construction of gut microbiota. In particular, the activities of gut microbiota may rely on a well-adjusted production of short-chain fatty acids (SCFAs) such as acetate, propionate and/or butyrate, which are mainly the products of gut fermentation of non-digestible polysaccharides such as cellulose and/or resistant starch in vegetables[35]. SCFAs have been recognized as mediators of immune responses, including pathways of cytokine production, which is important to minimize the risk of GVHD[36]. It has been shown that SCFAs are effective antimicrobial and/or anti-inflammatory compounds supporting the epithelial barrier for metabolic homeostasis in the host[37]. Therefore, some microbial metabolites including the SCFAs might protect against the GVHD by adjusting immune-reactions[38]. Remarkably, increased production of microbiota-derived SCFAs could improve the Treg/Th17 balance[39]. In addition, elevated production of SCFAs may lead to the enhanced Treg generation and the suppressed Th17 development[40]. Moreover, SCFAs may up-regulate the production of anti-inflammatory cytokines resulting in the induction of Tregs[41]. Therefore, loss of beneficial gut commensals that can produce SCFAs might affect the severity of GVHD. Clinical-scale production of human Tregs might be complex and difficult in another way. It has been shown that certain microbiota strains with the high production of butyrate could decrease GVHD[36,42]. Interestingly, the butyrate, isovaleric acid, and/or branched-chain fatty acids could activate mTORC1 in hepatocytes, suggesting that a diet could potentiate the mTORC1 via the alterations in gut microbiota[43]. In addition, it has been indicated that GPR41, a G protein-coupled receptor for SCFAs including butyrate, could evoke the mTORC1 phosphorylation[44]. On the contrary, the specific modification of microbial metabolites could have another effect on the condition of GVHD, suggesting that an unrecognized role of microbial metabolites has beneficial effects on GVHD[36].
Gut microbiota possess the great capability to produce D-amino acids which are applied as nutrients to keep bacterial growth and to control spore sprouting[45]. In general, various bacterial species could produce racemases that convert L-amino acids to D-amino acids[46]. Accordingly, higher D-amino acids levels have been linked to the mass of gut microbiota[47]. Interestingly, D-serine suppressed the proliferation of activated CD4 positive T cells and limited their ability to differentiate to Th1 and/or Th17 cells[48]. Consequently, D-amino acids could be effective in preventing GVHD[49]. Similarly, it is well known that disruption of gut microbiota-diversity could exacerbate GVHD[50]. Probiotics and/or prebiotics could also activate the growth of several microorganisms in the gut for health profits of the host[51]. Remarkably, a low diversity of microorganisms might diminish the favorable effect of prebiotics[52]. Therefore, elucidation of the structures, functions, and/or activities of gut microbiota in the host might contribute to the safety of various treatment in HSCT. Undoubtedly, intervention tactics including prebiotics, probiotics, and/or fecal microbiota transplantation (FMT), directing the gut microbiota could become potential new treatment options for the GVHD (Figure 3).
Gut microbiota consists of a numerous multispecies community that may establish symbiosis with the host, which are defined as constructively functional microorganisms with a health benefit on the host[53]. The gut microbiota are influenced by many factors such as diet, use of antibiotics, and/or geographic environment[54]. Changes in the microbiota are known to be affected by those dietary and/or environmental factors, which have been revealed to initiate redox signaling within the gut mucosa cells[55]. Mechanisms of redox signaling might lead to an inflammatory incident. Thus, the gut microbiota may be also arrested in a sophisticated balance at this viewpoint. Consequently, gut microbiota, reactive oxygen species (ROS), oxidative stress, inflammation, and several inflammatory diseases might be closely associated with each other. In fact, perturbations in the microbial balance may be associated with the initiation of inflammatory bowel diseases[56]. The ROS are defined as oxygen hugging energetic molecules capable of reacting with various organic molecules in a cell, which are also derived from inflammatory reactions[57]. Generally, living cells reluctantly release ROS for essential ATP synthesis, which may cause DNA damage in cells[58,59]. Some important physiological roles of ROS include the regulation of enzymes involved in autophagy, DNA synthesis, and/or DNA repair[60]. Certain degrees of ROS could change the signaling pathways to control mRNA and/or protein expression, which governs the cell destiny either survival or death[58,61]. Therefore, gut microbiota might also govern the cell destiny in some cases[62]. Understanding redox regulation of physiological processes seems to be important for developing therapeutic approach with gut microbiota. In addition, patients who undergo HSCT are often suffering from nutritional deficiencies with weight loss possibly due to treatment side effects[63]. Accordingly, nutritional support after the HSCT for the beneficial gut microbiota has become a strategic aspect to be considered.
Probiotics, a procedure for the manipulation of gut microbiota, are active microorganisms that have been deliberated to nutritionally contribute to the host health with adequate amounts of administration. Most probiotics are firstly isolated from healthy human individuals and estimated to be safe. It has been revealed that probiotics could reduce inflammation and/or oxidative stress[64]. For example, probiotics with certain bacteria such as Akkermansia and Lactobacillus could alleviate systemic metabolism in inflammatory diseases[65]. Therefore, probiotics with administration of Lactobacillus plantarum is reasonable to minimize the risk of developing a GVHD in patients undergoing HSCT[66]. Probiotics could inspire the gut immune system. For example, the immune-stimulatory effects of several Lactobacillus species have been discovered. In addition, Lactobacillus gasseri and Lactobacillus johnsonii could alter the enteric cytokine production by activating dendritic cells[67]. Probiotics could be utilized in treating various diseases. Gut microbiota could induce the maturation of dendritic cells and/or the differentiation of naive T cells into several lymphocyte subsets including Th17 and/or Treg cells[68]. Th17 cells are particularly affected by the abundance of specific commensal bacteria[69]. In the homeostasis of gut microbiota, non-pathogenic bacteria may play a significant role in the stability of adaptive immunity by the regulation of Treg cells[70]. Diet-induced shifts in microbiota composition might have insightful effects on the host immunity especially on T cells[71]. Curiously, high salt intake with diet could drive autoimmunity by inducing Th17 cells[72]. As mentioned above, production of D-amino acids might be correlated with a relative profusion of bacterial species with specific racemases in the gut microbiota[73]. Higher levels of D-amino acids with the higher mass of gut microbiota may suggest that increased abundance of such bacteria is associated with a stressed gut environment for the recovery[47,74]. In addition, different bacterial species may produce distinct profiles of D-amino acids[75]. Successful alteration in the composition of gut microbiota might be considered as an innovative therapeutic tactics[76]. D-amino acids and ROS-biosynthesis in the gut could be relevant to the improvement and pathogenesis of the GVHD, respectively[77].
There might be intricate innate or adaptive immune mechanisms in the mucosa of gastrointestinal tract. Both innate and adaptive immune mechanisms are integrated with the active phase of gut pathologies[78]. In addition, the immune system might link the gut microbiota even to the progress of neuropsychiatric disorders such as depressive behaviors[79]. Specific gut microbiota metabolites could alter the Treg/Th17 ratio[80]. In addition, the imbalance of the Treg/Th17 ratio has been implicated in the development of chronic stresses[81]. There might be a probable crosstalk between adaptive immune system and the gut microbiota raising a gut-immune axis (Figure 3).
FMT is known for its outstanding efficacy in recurrent Clostridioides difficile infection with more than 90% rate of cure[82], which may aim to restore gut homeostasis by transferring gut bacteria and microbes from healthy individuals’ stool[83]. FMT in patients with GVHD has also been associated with favorable clinical outcomes[84]. The treatment seems to be generally safe, but serious adverse events such as pneumonia and/or sudden death have been reported[85]. At present, the long-term safety remains unclear[86,87]. As a matter of fact, the detailed effect of modifications in gut microbiota on the disease processes of GVHD in HSCT has not been well understood. However, it has been revealed that protecting the intestinal microenvironment could be a novel strategy to manage GVHD[88]. Even if it remains unclear whether the effect of modifications of microbiota are indirect to the severity of GVHD or not, they may be advantageous for the prevention and/or treatment of GVHD in HSCT recipients. Immunologically, it has been shown that a disturbed association between intestine-epithelial cells and the gut microbiota could cause pathogenic responses to the host[89]. For example, a high-calorie diet can potentially rearrange the gut microbiota, which could disturb the balance of Th17/Treg cells, interrupt the immunological homeostasis, then exacerbate an inflammatory damages[90]. Several human studies have revealed that loss of diversity of gut microbiota following the allogeneic HSCT may be linked to the considerable gut injury[91]. In addition, prolonged antibiotic use may also decrease the diversity, which might increase the risk of GVHD[92]. These findings may indicate potential changeable targets to decrease the risk of GVHD and/or to increase the safety survival rate after allogeneic HSCT. The allogeneic HSCT is a solid potential therapeutic option for patients with a diversity of malignancies. Therefore, further in-depth studies will be mandatory to define the immune responses controlled by gut microbiota involved in the development of inflammatory severe responses after allogeneic HSCT. A deeper investigation into internal molecular mechanisms and/or immune-neurological pathways should be carried out in the future.
Probiotics and/or fecal microbiota transplantation might have a potential efficacy for the treatment of GVHD after hematopoietic stem cell transplantation.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chan WYK, China; Wang WJ, China; Zhou S, China S-Editor: Liu JH L-Editor: Ma JY - MedE P-Editor: Liu JH
| 1. | Orrantia A, Terrén I, Astarloa-Pando G, Zenarruzabeitia O, Borrego F. Human NK Cells in Autologous Hematopoietic Stem Cell Transplantation for Cancer Treatment. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Neves JF, Marques A, Valente R, Barata D. Nonlethal, attenuated, transfusion-associated graft-versus-host disease in an immunocompromised child: case report and review of the literature. Transfusion. 2010;50:2484-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Santos E Sousa P, Ciré S, Conlan T, Jardine L, Tkacz C, Ferrer IR, Lomas C, Ward S, West H, Dertschnig S, Blobner S, Means TK, Henderson S, Kaplan DH, Collin M, Plagnol V, Bennett CL, Chakraverty R. Peripheral tissues reprogram CD8+ T cells for pathogenicity during graft-versus-host disease. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Kumari R, Palaniyandi S, Hildebrandt GC. Microbiome: An Emerging New Frontier in Graft-Versus-Host Disease. Dig Dis Sci. 2019;64:669-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Biagi E, Zama D, Nastasi C, Consolandi C, Fiori J, Rampelli S, Turroni S, Centanni M, Severgnini M, Peano C, de Bellis G, Basaglia G, Gotti R, Masetti R, Pession A, Brigidi P, Candela M. Gut microbiota trajectory in pediatric patients undergoing hematopoietic SCT. Bone Marrow Transplant. 2015;50:992-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, No D, Gobourne A, Viale A, Dahi PB, Ponce DM, Barker JN, Giralt S, van den Brink M, Pamer EG. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 674] [Article Influence: 61.3] [Reference Citation Analysis (2)] |
| 7. | Zou YT, Zhou J, Zhu JH, Wu CY, Shen H, Zhang W, Zhou SS, Xu JD, Mao Q, Zhang YQ, Long F, Li SL. Gut Microbiota Mediates the Protective Effects of Traditional Chinese Medicine Formula Qiong-Yu-Gao against Cisplatin-Induced Acute Kidney Injury. Microbiol Spectr. 2022;10:e0075922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 8. | Koyama M, Mukhopadhyay P, Schuster IS, Henden AS, Hülsdünker J, Varelias A, Vetizou M, Kuns RD, Robb RJ, Zhang P, Blazar BR, Thomas R, Begun J, Waddell N, Trinchieri G, Zeiser R, Clouston AD, Degli-Esposti MA, Hill GR. MHC Class II Antigen Presentation by the Intestinal Epithelium Initiates Graft-versus-Host Disease and Is Influenced by the Microbiota. Immunity. 2019;51:885-898.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 180] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 9. | Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3394] [Cited by in RCA: 3546] [Article Influence: 177.3] [Reference Citation Analysis (5)] |
| 10. | Parco S, Benericetti G, Vascotto F, Palmisciano G. Microbiome and diversity indices during blood stem cells transplantation - new perspectives? Cent Eur J Public Health. 2019;27:335-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Sofi MH, Wu Y, Schutt SD, Dai M, Daenthanasanmak A, Heinrichs Voss J, Nguyen H, Bastian D, Iamsawat S, Selvam SP, Liu C, Maulik N, Ogretmen B, Jin J, Mehrotra S, Yu XZ. Thioredoxin-1 confines T cell alloresponse and pathogenicity in graft-versus-host disease. J Clin Invest. 2019;129:2760-2774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Wanchoo R, Stotter BR, Bayer RL, Jhaveri KD. Acute kidney injury in hematopoietic stem cell transplantation. Curr Opin Crit Care. 2019;25:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Krishnappa V, Gupta M, Manu G, Kwatra S, Owusu OT, Raina R. Acute Kidney Injury in Hematopoietic Stem Cell Transplantation: A Review. Int J Nephrol. 2016;2016:5163789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Mhandire K, Saggu K, Buxbaum NP. Immunometabolic Therapeutic Targets of Graft-versus-Host Disease (GvHD). Metabolites. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Pidala J, Walton K, Elmariah H, Kim J, Mishra A, Bejanyan N, Nishihori T, Khimani F, Perez L, Faramand RG, Davila ML, Nieder ML, Sagatys EM, Holtan SG, Lawrence NJ, Lawrence HR, Blazar BR, Anasetti C, Sebti SM, Betts BC. Pacritinib Combined with Sirolimus and Low-Dose Tacrolimus for GVHD Prevention after Allogeneic Hematopoietic Cell Transplantation: Preclinical and Phase I Trial Results. Clin Cancer Res. 2021;27:2712-2722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Tijaro-Ovalle NM, Karantanos T, Wang HT, Boussiotis VA. Metabolic Targets for Improvement of Allogeneic Hematopoietic Stem Cell Transplantation and Graft-vs.-Host Disease. Front Immunol. 2019;10:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Bantug GR, Galluzzi L, Kroemer G, Hess C. The spectrum of T cell metabolism in health and disease. Nat Rev Immunol. 2018;18:19-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 322] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 18. | Patsoukis N, Bardhan K, Weaver J, Herbel C, Seth P, Li L, Boussiotis VA. The role of metabolic reprogramming in T cell fate and function. Curr Trends Immunol. 2016;17:1-12. [PubMed] |
| 19. | Swimm A, Giver CR, DeFilipp Z, Rangaraju S, Sharma A, Ulezko Antonova A, Sonowal R, Capaldo C, Powell D, Qayed M, Kalman D, Waller EK. Indoles derived from intestinal microbiota act via type I interferon signaling to limit graft-versus-host disease. Blood. 2018;132:2506-2519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 20. | Kalaeva E, Kalaev V, Efimova K, Chernitskiy A, Safonov V. Protein metabolic changes and nucleolus organizer regions activity in the lymphocytes of neonatal calves during the development of respiratory diseases. Vet World. 2019;12:1657-1667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Nguyen HD, Chatterjee S, Haarberg KM, Wu Y, Bastian D, Heinrichs J, Fu J, Daenthanasanmak A, Schutt S, Shrestha S, Liu C, Wang H, Chi H, Mehrotra S, Yu XZ. Metabolic reprogramming of alloantigen-activated T cells after hematopoietic cell transplantation. J Clin Invest. 2016;126:1337-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 22. | Martin-Moreno PL, Tripathi S, Chandraker A. Regulatory T Cells and Kidney Transplantation. Clin J Am Soc Nephrol. 2018;13:1760-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Walton K, Fernandez MR, Sagatys EM, Reff J, Kim J, Lee MC, Kiluk JV, Hui JYC, McKenna D Jr, Hupp M, Forster C, Linden MA, Lawrence NJ, Lawrence HR, Pidala J, Pavletic SZ, Blazar BR, Sebti SM, Cleveland JL, Anasetti C, Betts BC. Metabolic reprogramming augments potency of human pSTAT3-inhibited iTregs to suppress alloreactivity. JCI Insight. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, Curtsinger J, Verneris MR, MacMillan ML, Levine BL, Riley JL, June CH, Le C, Weisdorf DJ, McGlave PB, Blazar BR, Wagner JE. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood. 2016;127:1044-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 321] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 25. | Salmond RJ. mTOR Regulation of Glycolytic Metabolism in T Cells. Front Cell Dev Biol. 2018;6:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 26. | Chi X, Jin W, Bai X, Zhao X, Shao J, Li J, Sun Q, Su B, Wang X, Yang XO, Dong C. RORα is critical for mTORC1 activity in T cell-mediated colitis. Cell Rep. 2021;36:109682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Xiang H, Tao Y, Jiang Z, Huang X, Wang H, Cao W, Li J, Ding R, Shen M, Feng R, Li L, Guan C, Liu J, Ni J, Chen L, Wang Z, Ye Y, Zhong Q, Zou Q, Wu X. Vps33B controls Treg cell suppressive function through inhibiting lysosomal nutrient sensing complex-mediated mTORC1 activation. Cell Rep. 2022;39:110943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 28. | Eskandari SK, Allos H, Al Dulaijan BS, Melhem G, Sulkaj I, Alhaddad JB, Saad AJ, Deban C, Chu P, Choi JY, Kollar B, Pomahac B, Riella LV, Berger SP, Sanders JSF, Lieberman J, Li L, Azzi JR. mTORC1 Inhibition Protects Human Regulatory T Cells From Granzyme-B-Induced Apoptosis. Front Immunol. 2022;13:899975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Wang WJ, Zhang H, Chen ZQ, Zhang W, Liu XM, Fang JY, Liu FJ, Kwak-Kim J. Endometrial TGF-β, IL-10, IL-17 and autophagy are dysregulated in women with recurrent implantation failure with chronic endometritis. Reprod Biol Endocrinol. 2019;17:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 30. | Perl A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat Rev Rheumatol. 2016;12:169-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 31. | Shallis RM, Terry CM, Lim SH. Changes in intestinal microbiota and their effects on allogeneic stem cell transplantation. Am J Hematol. 2018;93:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Shono Y, van den Brink MRM. Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat Rev Cancer. 2018;18:283-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 219] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 33. | Han L, Jin H, Zhou L, Zhang X, Fan Z, Dai M, Lin Q, Huang F, Xuan L, Zhang H, Liu Q. Intestinal Microbiota at Engraftment Influence Acute Graft-Versus-Host Disease via the Treg/Th17 Balance in Allo-HSCT Recipients. Front Immunol. 2018;9:669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 34. | Riwes M, Reddy P. Microbial metabolites and graft versus host disease. Am J Transplant. 2018;18:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1428] [Cited by in RCA: 2249] [Article Influence: 281.1] [Reference Citation Analysis (1)] |
| 36. | Mathewson ND, Jenq R, Mathew AV, Koenigsknecht M, Hanash A, Toubai T, Oravecz-Wilson K, Wu SR, Sun Y, Rossi C, Fujiwara H, Byun J, Shono Y, Lindemans C, Calafiore M, Schmidt TM, Honda K, Young VB, Pennathur S, van den Brink M, Reddy P. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol. 2016;17:505-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 506] [Cited by in RCA: 505] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 37. | Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2779] [Cited by in RCA: 3085] [Article Influence: 237.3] [Reference Citation Analysis (0)] |
| 38. | Riwes M, Reddy P. Short chain fatty acids: Postbiotics/metabolites and graft versus host disease colitis. Semin Hematol. 2020;57:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Shi X, Huang H, Zhou M, Liu Y, Wu H, Dai M. Paeonol Attenuated Vascular Fibrosis Through Regulating Treg/Th17 Balance in a Gut Microbiota-Dependent Manner. Front Pharmacol. 2021;12:765482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 40. | Zhang W, Cheng C, Han Q, Chen Y, Guo J, Wu Q, Zhu B, Shan J, Shi L. Flos Abelmoschus manihot extract attenuates DSS-induced colitis by regulating gut microbiota and Th17/Treg balance. Biomed Pharmacother. 2019;117:109162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 41. | Asarat M, Apostolopoulos V, Vasiljevic T, Donkor O. Short-Chain Fatty Acids Regulate Cytokines and Th17/Treg Cells in Human Peripheral Blood Mononuclear Cells in vitro. Immunol Invest. 2016;45:205-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 149] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 42. | Docampo MD, da Silva MB, Lazrak A, Nichols KB, Lieberman SR, Slingerland AE, Armijo GK, Shono Y, Nguyen C, Monette S, Dwomoh E, Lee N, Geary CD, Perobelli SM, Smith M, Markey KA, Vardhana SA, Kousa AI, Zamir E, Greenfield I, Sun JC, Cross JR, Peled JU, Jenq RR, Stein-Thoeringer CK, van den Brink MRM. Alloreactive T cells deficient of the short-chain fatty acid receptor GPR109A induce less graft-versus-host disease. Blood. 2022;139:2392-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 43. | Choi BS, Daniel N, Houde VP, Ouellette A, Marcotte B, Varin TV, Vors C, Feutry P, Ilkayeva O, Ståhlman M, St-Pierre P, Bäckhed F, Tremblay A, White PJ, Marette A. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 44. | Mikami D, Kobayashi M, Uwada J, Yazawa T, Kamiyama K, Nishimori K, Nishikawa Y, Nishikawa S, Yokoi S, Taniguchi T, Iwano M. AR420626, a selective agonist of GPR41/FFA3, suppresses growth of hepatocellular carcinoma cells by inducing apoptosis via HDAC inhibition. Ther Adv Med Oncol. 2020;12:1758835920913432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Cava F, Lam H, de Pedro MA, Waldor MK. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol Life Sci. 2011;68:817-831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 46. | Radkov AD, Moe LA. Bacterial synthesis of D-amino acids. Appl Microbiol Biotechnol. 2014;98:5363-5374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 47. | Ketting D, Wadman SK, Spaapen LJ, Van der Meer SB, Duran M. Gas chromatography method for the separation of amino acids enantiomers in plasma and urine. Application in a case of short bowel syndrome. Clin Chim Acta. 1991;204:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Asakawa T, Onizawa M, Saito C, Hikichi R, Yamada D, Minamidate A, Mochimaru T, Asahara SI, Kido Y, Oshima S, Nagaishi T, Tsuchiya K, Ohira H, Okamoto R, Watanabe M. Oral administration of D-serine prevents the onset and progression of colitis in mice. J Gastroenterol. 2021;56:732-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Aharoni R, Schlegel PG, Teitelbaum D, Roikhel-Karpov O, Chen Y, Arnon R, Sela M, Chao NJ. Studies on the mechanism and specificity of the effect of the synthetic random copolymer GLAT on graft-versus-host disease. Immunol Lett. 1997;58:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Ma T, Chen Y, Li LJ, Zhang LS. Opportunities and Challenges for Gut Microbiota in Acute Leukemia. Front Oncol. 2021;11:692951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Chenhuichen C, Cabello-Olmo M, Barajas M, Izquierdo M, Ramírez-Vélez R, Zambom-Ferraresi F, Martínez-Velilla N. Impact of probiotics and prebiotics in the modulation of the major events of the aging process: A systematic review of randomized controlled trials. Exp Gerontol. 2022;164:111809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 52. | Yoshifuji K, Inamoto K, Kiridoshi Y, Takeshita K, Sasajima S, Shiraishi Y, Yamashita Y, Nisaka Y, Ogura Y, Takeuchi R, Toya T, Igarashi A, Najima Y, Doki N, Kobayashi T, Ohashi K, Suda W, Atarashi K, Shiota A, Hattori M, Honda K, Kakihana K. Prebiotics protect against acute graft-versus-host disease and preserve the gut microbiota in stem cell transplantation. Blood Adv. 2020;4:4607-4617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 53. | Hacıoglu S, Kunduhoglu B. Probiotic Characteristics of Lactobacillus brevis KT38-3 Isolated from an Artisanal Tulum Cheese. Food Sci Anim Resour. 2021;41:967-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 54. | Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, Inoue H, Tameda M, Shiraki K, Ito M, Takei Y, Takase K. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015;15:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 394] [Cited by in RCA: 412] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 55. | Alam A, Leoni G, Quiros M, Wu H, Desai C, Nishio H, Jones RM, Nusrat A, Neish AS. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat Microbiol. 2016;1:15021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 56. | Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 406] [Article Influence: 29.0] [Reference Citation Analysis (1)] |
| 57. | Wu S, Liu X, Cheng L, Wang D, Qin G, Zhang X, Zhen Y, Wang T, Sun Z. Protective Mechanism of Leucine and Isoleucine against H2O2-Induced Oxidative Damage in Bovine Mammary Epithelial Cells. Oxid Med Cell Longev. 2022;2022:4013575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 58. | Ikeda Y, Nagase N, Tsuji A, Taniguchi K, Kitagishi Y, Matsuda S. Comprehension of the Relationship between Autophagy and Reactive Oxygen Species for Superior Cancer Therapy with Histone Deacetylase Inhibitors. Oxygen. 2021;1:22-31. [DOI] [Full Text] |
| 59. | Ikeda Y, Taniguchi K, Nagase N, Tsuji A, Kitagishi Y, Matsuda S. Reactive oxygen species may influence on the crossroads of stemness, senescence, and carcinogenesis in a cell via the roles of APRO family proteins. Explor Med. 2021;2:443-454. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Tsitsipatis D, Martindale JL, Ubaida-Mohien C, Lyashkov A, Yanai H, Kashyap A, Shin CH, Herman AB, Ji E, Yang JH, Munk R, Dunn C, Lukyanenko Y, Yang X, Chia CW, Karikkineth AC, Zukley L, D'Agostino J, Kaileh M, Cui CY, Beerman I, Ferrucci L, Gorospe M. Proteomes of primary skin fibroblasts from healthy individuals reveal altered cell responses across the life span. Aging Cell. 2022;21:e13609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 61. | Arfin S, Jha NK, Jha SK, Kesari KK, Ruokolainen J, Roychoudhury S, Rathi B, Kumar D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 346] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 62. | Hu S, Kuwabara R, de Haan BJ, Smink AM, de Vos P. Acetate and Butyrate Improve β-cell Metabolism and Mitochondrial Respiration under Oxidative Stress. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 63. | Walrath M, Bacon C, Foley S, Fung HC. Gastrointestinal side effects and adequacy of enteral intake in hematopoietic stem cell transplant patients. Nutr Clin Pract. 2015;30:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | Dai Y, Quan J, Xiong L, Luo Y, Yi B. Probiotics improve renal function, glucose, lipids, inflammation and oxidative stress in diabetic kidney disease: a systematic review and meta-analysis. Ren Fail. 2022;44:862-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 65. | Han C, Jiang YH, Li W, Liu Y. Astragalus membranaceus and Salvia miltiorrhiza ameliorates cyclosporin A-induced chronic nephrotoxicity through the "gut-kidney axis". J Ethnopharmacol. 2021;269:113768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 66. | Ladas EJ, Bhatia M, Chen L, Sandler E, Petrovic A, Berman DM, Hamblin F, Gates M, Hawks R, Sung L, Nieder M. The safety and feasibility of probiotics in children and adolescents undergoing hematopoietic cell transplantation. Bone Marrow Transplant. 2016;51:262-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 67. | Mohamadzadeh M, Pfeiler EA, Brown JB, Zadeh M, Gramarossa M, Managlia E, Bere P, Sarraj B, Khan MW, Pakanati KC, Ansari MJ, O'Flaherty S, Barrett T, Klaenhammer TR. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4623-4630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 68. | Lee BJ, Bak YT. Irritable bowel syndrome, gut microbiota and probiotics. J Neurogastroenterol Motil. 2011;17:252-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 69. | Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3529] [Cited by in RCA: 3525] [Article Influence: 220.3] [Reference Citation Analysis (0)] |
| 70. | Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 1020] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 71. | Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 1270] [Article Influence: 141.1] [Reference Citation Analysis (0)] |
| 72. | Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mähler A, Balogh A, Markó L, Vvedenskaya O, Kleiner FH, Tsvetkov D, Klug L, Costea PI, Sunagawa S, Maier L, Rakova N, Schatz V, Neubert P, Frätzer C, Krannich A, Gollasch M, Grohme DA, Côrte-Real BF, Gerlach RG, Basic M, Typas A, Wu C, Titze JM, Jantsch J, Boschmann M, Dechend R, Kleinewietfeld M, Kempa S, Bork P, Linker RA, Alm EJ, Müller DN. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 853] [Cited by in RCA: 932] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 73. | Gilmore MS, Skaugen M, Nes I. Enterococcus faecalis cytolysin and lactocin S of Lactobacillus sake. Antonie Van Leeuwenhoek. 1996;69:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 74. | Karpe AV, Hutton ML, Mileto SJ, James ML, Evans C, Shah RM, Ghodke AB, Hillyer KE, Metcalfe SS, Liu JW, Walsh T, Lyras D, Palombo EA, Beale DJ. Cryptosporidiosis Modulates the Gut Microbiome and Metabolism in a Murine Infection Model. Metabolites. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 75. | Lam H, Oh DC, Cava F, Takacs CN, Clardy J, de Pedro MA, Waldor MK. D-amino acids govern stationary phase cell wall remodeling in bacteria. Science. 2009;325:1552-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 436] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 76. | Snelson M, Clarke RE, Nguyen TV, Penfold SA, Forbes JM, Tan SM, Coughlan MT. Long Term High Protein Diet Feeding Alters the Microbiome and Increases Intestinal Permeability, Systemic Inflammation and Kidney Injury in Mice. Mol Nutr Food Res. 2021;65:e2000851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 77. | Nagase N, Ikeda Y, Tsuji A, Kitagishi Y, Matsuda S. Efficacy of probiotics on the modulation of gut microbiota in the treatment of diabetic nephropathy. World J Diabetes. 2022;13:150-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 78. | Tavakoli P, Vollmer-Conna U, Hadzi-Pavlovic D, Grimm MC. A Review of Inflammatory Bowel Disease: A Model of Microbial, Immune and Neuropsychological Integration. Public Health Rev. 2021;42:1603990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 79. | Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1039] [Cited by in RCA: 1355] [Article Influence: 150.6] [Reference Citation Analysis (0)] |
| 80. | Britton GJ, Contijoch EJ, Mogno I, Vennaro OH, Llewellyn SR, Ng R, Li Z, Mortha A, Merad M, Das A, Gevers D, McGovern DPB, Singh N, Braun J, Jacobs JP, Clemente JC, Grinspan A, Sands BE, Colombel JF, Dubinsky MC, Faith JJ. Microbiotas from Humans with Inflammatory Bowel Disease Alter the Balance of Gut Th17 and RORγt+ Regulatory T Cells and Exacerbate Colitis in Mice. Immunity. 2019;50:212-224.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 384] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 81. | Hong M, Zheng J, Ding ZY, Chen JH, Yu L, Niu Y, Hua YQ, Wang LL. Imbalance between Th17 and Treg cells may play an important role in the development of chronic unpredictable mild stress-induced depression in mice. Neuroimmunomodulation. 2013;20:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 82. | Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, Gallagher G, Kerr H, Kuruvilla J, Lee SJ, Moore J, Nevill T, Popradi G, Roy J, Schultz KR, Szwajcer D, Toze C, Foley R; Canadian Blood and Marrow Transplant Group. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 288] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 83. | Shouval R, Geva M, Nagler A, Youngster I. Fecal Microbiota Transplantation for Treatment of Acute Graft-versus-Host Disease. Clin Hematol Int. 2019;1:28-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 84. | Zhao Y, Li X, Zhou Y, Gao J, Jiao Y, Zhu B, Wu D, Qi X. Safety and Efficacy of Fecal Microbiota Transplantation for Grade IV Steroid Refractory GI-GvHD Patients: Interim Results From FMT2017002 Trial. Front Immunol. 2021;12:678476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 85. | Iqbal U, Anwar H, Karim MA. Safety and efficacy of encapsulated fecal microbiota transplantation for recurrent Clostridium difficile infection: a systematic review. Eur J Gastroenterol Hepatol. 2018;30:730-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 86. | Rapoport EA, Baig M, Puli SR. Adverse events in fecal microbiota transplantation: a systematic review and meta-analysis. Ann Gastroenterol. 2022;35:150-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 87. | Michailidis L, Currier AC, Le M, Flomenhoft DR. Adverse events of fecal microbiota transplantation: a meta-analysis of high-quality studies. Ann Gastroenterol. 2021;34:802-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 88. | Zhou Z, Shang T, Li X, Zhu H, Qi YB, Zhao X, Chen X, Shi ZX, Pan G, Wang YF, Fan G, Gao X, Zhu Y, Feng Y. Protecting Intestinal Microenvironment Alleviates Acute Graft-Versus-Host Disease. Front Physiol. 2020;11:608279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 89. | Zeiser R, Warnatz K, Rosshart S, Sagar, Tanriver Y. GVHD, IBD, and primary immunodeficiencies: The gut as a target of immunopathology resulting from impaired immunity. Eur J Immunol. 2022;52:1406-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 90. | Liu H, Bai C, Xian F, Liu S, Long C, Hu L, Liu T, Gu X. A high-calorie diet aggravates LPS-induced pneumonia by disturbing the gut microbiota and Th17/Treg balance. J Leukoc Biol. 2022;112:127-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 91. | Weber D, Hiergeist A, Weber M, Dettmer K, Wolff D, Hahn J, Herr W, Gessner A, Holler E. Detrimental Effect of Broad-spectrum Antibiotics on Intestinal Microbiome Diversity in Patients After Allogeneic Stem Cell Transplantation: Lack of Commensal Sparing Antibiotics. Clin Infect Dis. 2019;68:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 92. | Romick-Rosendale LE, Haslam DB, Lane A, Denson L, Lake K, Wilkey A, Watanabe M, Bauer S, Litts B, Luebbering N, Dandoy CE, Davies SM. Antibiotic Exposure and Reduced Short Chain Fatty Acid Production after Hematopoietic Stem Cell Transplant. Biol Blood Marrow Transplant. 2018;24:2418-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |