Copyright
©The Author(s) 2017.
Figure 1 Arachidonic acid-induced human platelet aggregation and prostaglandin formation (Silver et al[3] 1973).
Arachidonate acid (AA), 0.5 mmol/L induces a normal platelet aggregation curve (A). AA induces secretion of large amounts of prostaglandins after platelet aggregation, but little or no prostaglandins secretion occur after platelet aggregation induced by thrombin, collagen and epinephrin (B). AA, thrombin, collagen, epinephrine and ADP induce aggregation and secretion of rather equal amounts radioactivity from PRP preincubated with 14C-serotonin or 14C-adenine (C). These findings implicate that spontaneous in vivo shear induced aggregation of sticky JAK2 mutated platelet in the endarteriolar circulation is associated with high prostaglandin levels as the cause of the inflammatory pain and signs (redness, warmth and congestion) of erythromelalgia in JAK2-mutated thrombocythemia (Michiels et al[53] in 1985 and Michiels[80] in 2017).
Figure 2 Dutch design by Michiels and Van Vliet on membrane phospholipid - arachidonic acid metabolism in platelets as compared to endothelial cells conceptualize in 1976, three years before the Moncada and Vane[13] publication in the NEJM.
Arachidonic acid (AA) is metabolized by lipoxygenase into (12-HPETE) and 12 HETE, and by platelet cyclooxygenase into prostagandin endoperoxides G2 and H2, which consist of PGE2, PG2alpha, PGD2, malondialdehyde (M.D.A.) and HHT. Prostaglandin endoperoxides in platelets are metabolized by thromboxane synthetase into thromboxane A2. Thromboxane A2 is potent inducer of platelet aggregation and smooth muscl cell contraction. AA induced prostaglandin endoperoxides in endothelial cells are metabolized by prostacyclin synthethase into prostacycline. Endothelial cell (EC) derived prostacyclin causes vasodilatation and prevents platelet aggregation and platelet derived thromboxane causes vasoconstriction and platelet aggregation. Prostacyclin is continuously produced by endothelial cells, which have a nucleus to synthesize cyclo-oxygenase and prostacyclin. The biological half life times of prostacycline and thromboxne A2 are short and broken down to the inactived metabolites 6-keto-PGF-1-alpha and thromboxane B2, which are sereceted by the kidneys into the urine (Figure 15). Ticlopedine and clopidogrel inhibit ADP induced platelet aggregation without affecting platelet cyclo-oxygenase (Figure 13). Upon platelet activation of constitutively activated JAK2-platelets by shear stress starts the membrane phospholipids → phopspholipase A2 → arachidonic acid (AA) → cyclooxygenase biochemical pathway induced prostaglandin endoperoxides G2 and H2 production by platelets are the cause of the inflammatory signs erythromelalgia (Figure 4) featured by fibromuscular intimal proliferation and occlusive platelet thrombi (Figures 9 and 10). Release of platelet derived growth factor accounts for the fibromuscular intimal proliferation (Figures 6, 9 and 10) followed by von Willebrand (VWF) rich occlusive platelet thrombi (Figure 15). As platelets do not have a nucleus, irreversible inhibition of platelet cyco-oxygenase (COX-1) persist for the rest of platelet life time in the circulation and cures erythromelagia and migraine-like cerebrovascular ischemic manifestations[37,52,53].
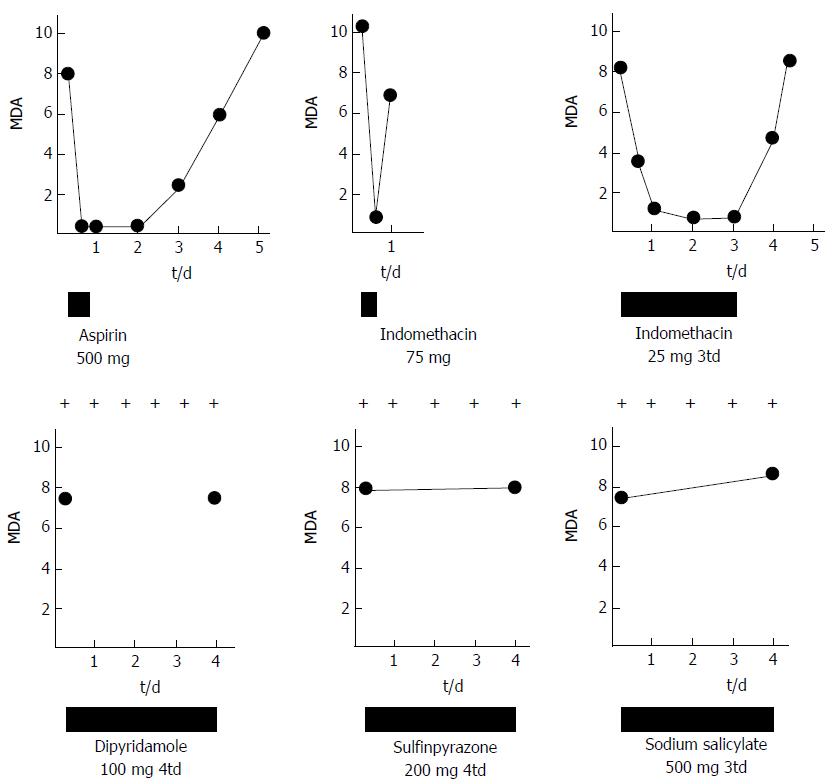
Figure 3 The effect of platelet aggregation inhibiting drugs on stimulated platelet aggregation, erythromelalgia and malondialdehyde concentration in N-ethylmaleimide stimulated platelet rich plasma.
The effect of aspirin (acetylsalicylic acid), indomethacin, dipyridamol, sulfinpyrazon and sodium salicylate on erythromelalgia and MDA production by arachidonic acid stimulated platelets in platelet-rich plasma of symptomatic thrombocythemia patients with ET or PV complicated by erythromelalgia. MDA: Malondialdehyde.
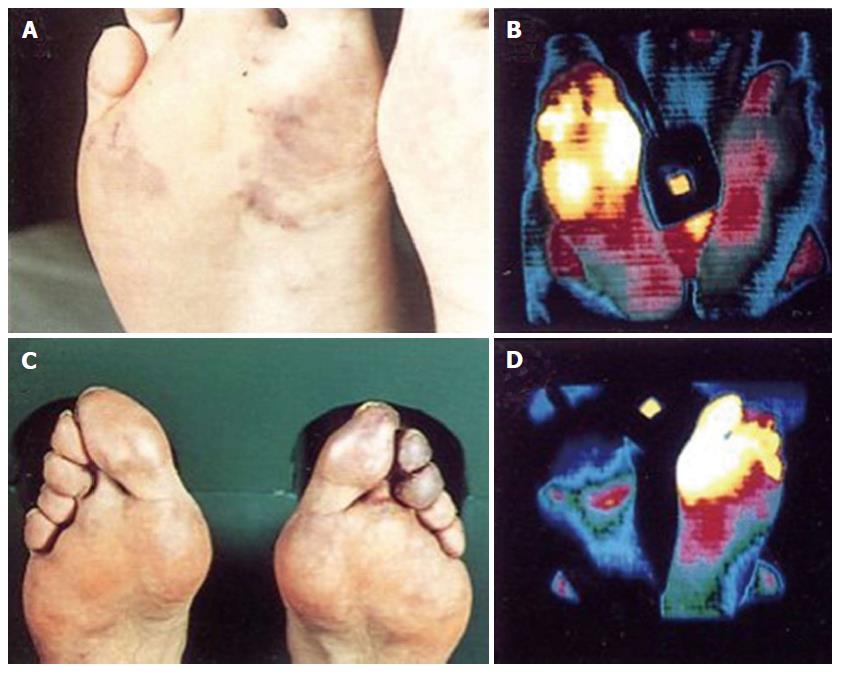
Figure 4 Isothermpgrams of two essential thrombocythemia patients with erythromelalgia in toes and fore foot sole.
Typical mottled red blue congestion and thermographic visualization of erythromelalgia in the fore foot and toes. Skin surface temterature: blue 24 °C-25 °C; green 26 °C-27 °C; purple 28 °C-29 °C; red 30 °C-31 °C; yellow 32 °C and white 33 °C. Complete correction of the upper leg thermograms after effective treatment with aspirin.
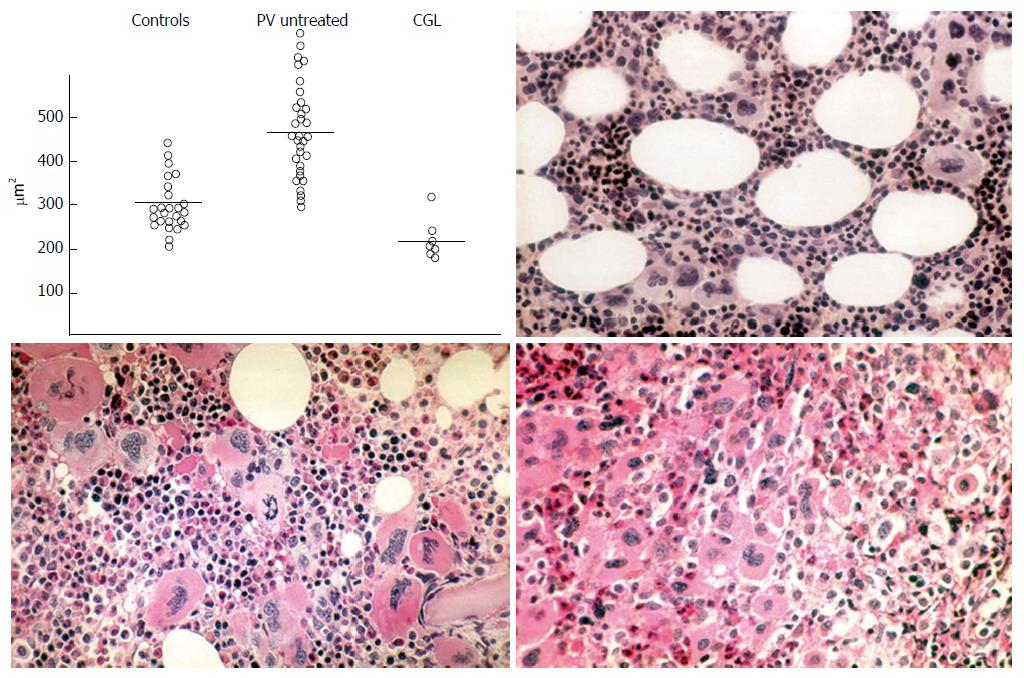
Figure 5 Planimetry of megakaryocyte sizes (μm2) from bone marrow smears in controls, polycythemia vera and chronic granulocytic leukemia upper left: Normal size megakaryocytes in controls; large megakaryocytes in untreated polycythemia vera and small sized megakaryocytes in chronic granulocytic leukemia (Frantzen et al[40]).
Demonstration by Michiels (1981) of a spectrum of clustered large megakaryocytes with hyperlobulated nuclei and a normocellular bone marrow in essential thrombocythemia (ET) vs increased bone marrow cellularity duet o increased erythropoiesis in ET and polycythemia vera (PV) vs increased trilinear eythrocythemic, megakaryocythemia and granulocythemic (EMG) proliferation in classical PV according to Dameshek[38] (1950) and Kurnicke et al[39].
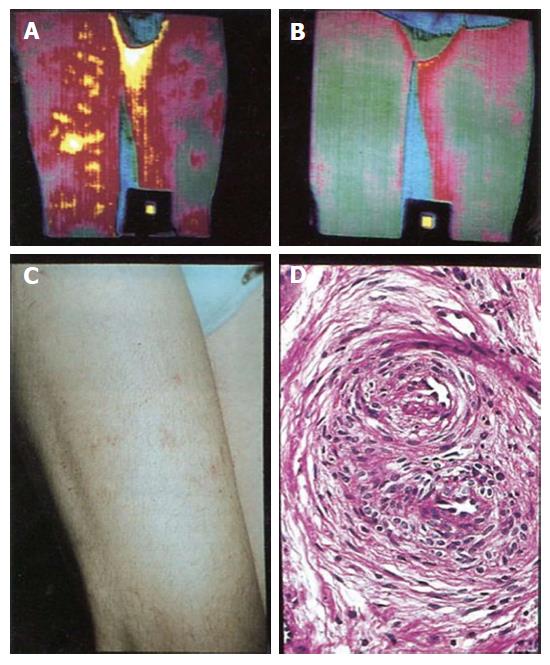
Figure 6 Isothermpgrams of upper legs showing “superficial thrombophlebitis” (A) in the right upper leg, which completely disappeared after treatment with aspin once daily (B) and superficial thrombophlebitis manifested as red painful indurated hot spots, erythromelalgia of the skin (C) in the upper leg caused by fibromuscular intimal proliferation (endarteritis oblitterans) as documented by histotolgy from skin punch biopsies (D) from the red spots.
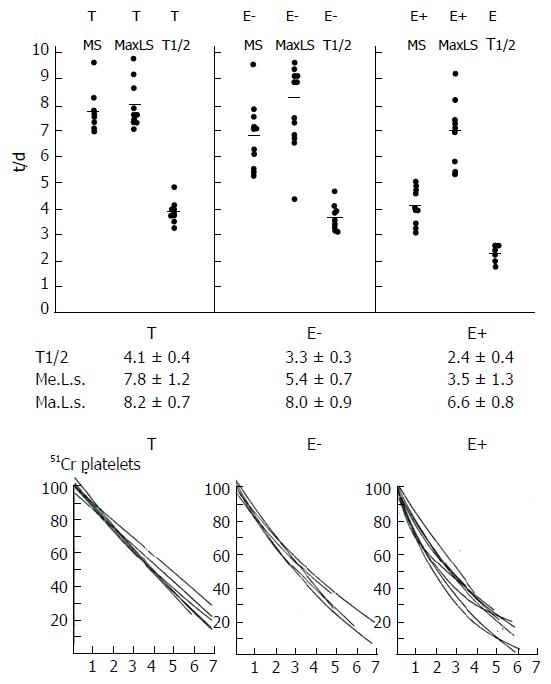
Figure 7 Platelet survival times and platelet disappearance curves according to Branehög et al[50] in 10 thrombocythemia patients complicated by erythromelalgi (E+), 10 asymptomatic thrombocythemia patients (E-), and 11 asymptomatic patients with thrombocytosis (T).
Curvilinear platelet survival curves in E+ indicates a consumptive diappearance of thrombocythemic platelets from the circulation: Slight curvilinear platelet survival curves in E- suggest slight, but insignificant random platelet consumption; and linear platelet survival curves in group T with reactive thrombocytosis indicate a non-random, age-related disappearance of platelets from the circulation. MS = mean survival. T1/2; MaLS = maximal life span according to Branehög et al[50].
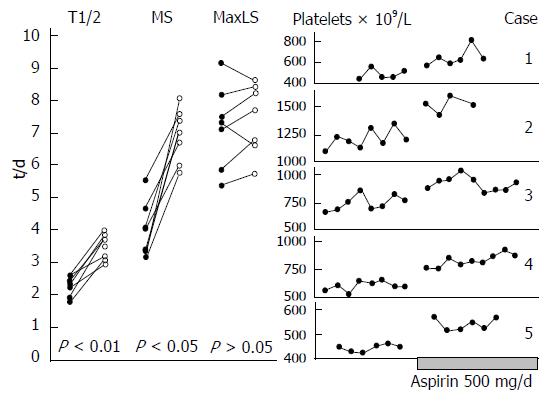
Figure 8 Platelet survival curves in seven E+ thrombocythemia patients before and after aspirin 500 mg/day, and peripheral blood platelet counts before and after maintenance aspirin treatment 250 mg/d.
MS: Mean survival; T1/2; MaLS: Maximal life span according to Branehög et al[50].
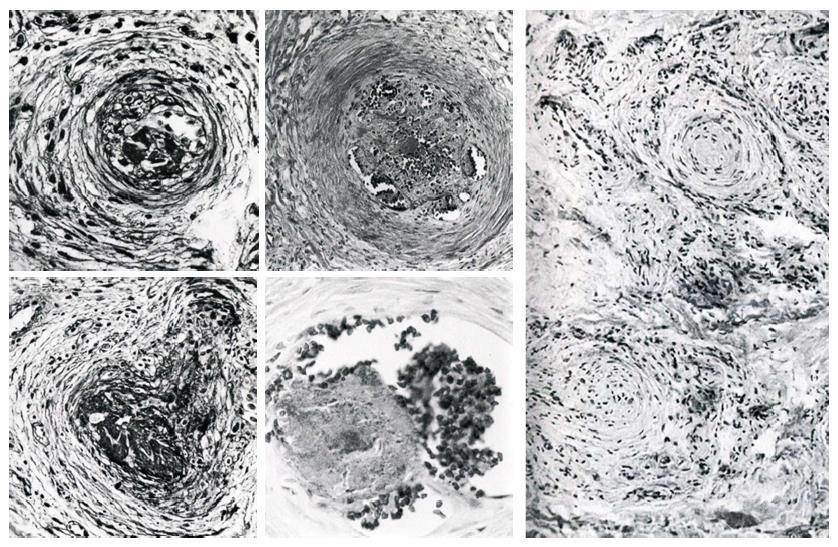
Figure 9 Arterioles with swelling of endothelial cells, proliferation of cells of the inner layer below the media and normal venules, capillaries and nerves (upper and left), and elastica von Gieson stain showing a normal membrana elestica interna (mei) in a normal arteriole (A).
Source Michiels 1981. The membrana elstica interna (mei) is splitted up and fragmented between the proliferating cells in arteriole B with intimal proliferation in in skin areas of very typical red congested erythromelalgia within one week after discontinuation of aspirin in two cases with essential thrombocythemia. Source Michiels 1981. Immunofluorescence of proliferating cells in the intima of affected arterioles shows on layer endothelial cells with antiserum against factor VIII and multilayer proliferation of smooth muscle cells with antiserum against smooth muscle cells indcative for fibromusclar intimal proliferation of affected arterioles in erythromelalgic thrombocythemia (upper). Partial and complete occlusion by a fresh thrombus in acrocyanotic erthomelalgia three weeks after discontinuation of aspirin (lower). Source Michiels 1981.
Figure 10 Thrombotic occlusion of arterioles on top of fibrouscular intimal proliferation.
Thromboangiitis oblitterans (left panel) and recanalisation of arterioles showing vessel wall fibosis of arterioles in two cases of erythromelalgia complicated by acrocyanosis and digital gangrene (middle panel). Source Michiels 1981. Oniony structure by vascular and perivascular fibrosis of occluded fibromusclar intimal proliferation in acrocyanotic digital ischemia of untreated endstage erythromelalgia that had transformed into aspirin resistent Raynaud phenomenon (right). Source Michiels 1981.
Figure 11 Results of treatment in 13 patients with primary or essential thrombocythemia and 13 patients with polycythemia vera and thrombocythemia.
Source Michiels et al[53], 1985. Busulfan induced remission of thrombocythemia (platelet counts < 350 × 109/L) lasted 2 to 9 years (long busulfan remitters) in essential thrombocythemia (ET) patients, which became asymptomatic with no recurrence of erythromelalgia after discontinuation of aspirin during thrombocythemia remission periods of 2 to 9 years (R). Erythromelalgia recurred in eight [5 ET, 3 polycythemia vera (PV)] of twelve (8ET, 4PV) at platelet counts between 400 to 550 × 109/L (P) after thrombocythemia remission periods of 2 to 8 years. Busulfan induced remissions of thrombocythemia in the majority of PV patients lasted several months to a few years (short buslfan remittors) indicating the need to treat with repeated courses of busulfan.
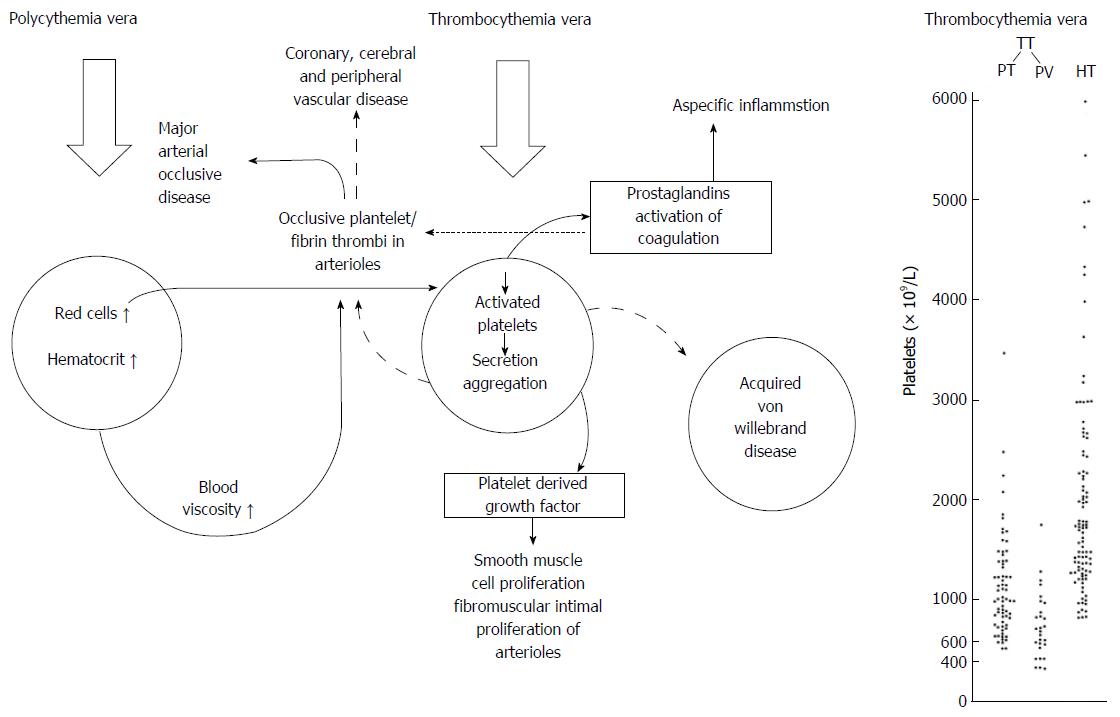
Figure 12 Pathophysiology of erythromelalgia as multicellular processes caused by platelet mediated microvascular erythromelalgic arteriolar inflammation and thrombosis in myeloproliferative thrombocythemia vera and major arterial thrombotic disease in polycythemia vera.
Shear induced production of prostaglandin endoperoxides from activated platelets in arterioles account for red warm congested swelling. Platelet derived growth fator (PDGF) released during platelet secretion can readily explain the fibromuscular intimal proliferation of areterioles first descibed by Michiels in 1981 and published by Michiels et al[52,53] in 1984 and 1985. Right: Platelet counts in 99 case histories of erythromelalgia thrombotic thrombocythemia (ETT) subdivided in ET (n = 69) and PV (n = 30) and in 100 case histories with hemorrhagic thrombocythemia (HT), Source Michiels 1981.
Figure 13 A schematic representation of platelet adhesion through von Willebrand factor and glycoprotein Ib disturbances in Bernard Soulier syndrome, von Willebrand disease and aggregation disturbances in Glanzmann’s thrombasthenia, and receptor defects of ADP (P2PY12, P2Y1), thrombin, thromboxane, platelet activating factor (PAF and collagen induced intracellular metabolites).
Source: Rao et al. Inherited defects in platelet signaling. Sem Thromb Hemostas 2004; 30: 525-535. Shear induced platelet activation of JAK2 hypersensitive (sticky) platelets (blue arrow) in arterioles starts with liberation of arachidonic acid from platelet membrane phopholipids by phopholipase A2 (PLA2) and metabolization of arachidonic acid by cyclooxygenase (CO) into prostaglandin endoperoxides PGG2/PGH2 and subsequent thromboxane A2 (TxA2) generation by thromboxane synthetase (TS). Spontaneous activation and aggregation of JAK2 sticky platelets (arrow) occur in the arteriolar peripheral and cerebral endarterial circulation with the production of large amount of prostaglandin endoperoxides as the cause of aspirin responsive inflammatory component of erythromelalgia followed by fibromuscular intimal proliferation and occlusion platelet thrombi in arterioles (Figures 9 and 10). Aspirin cures erythromelalgia in JAK2 thrombocythemia by irreversible inhibition of platelet cycolooxygenase (CO)[37]. In this process the activation of platelets by ADP (P2Y12), thrombin and collagen receptors are not involved in the etiology of erythromelalgia and cerebral vascular disturbances[52,53,79,80]. vWF: Von Willebrand factor; BSS: Bernard Soulier syndrome; vWD: Von Willebrand disease; Ca: Calcium; MLC: Myosin light chain; IP3: Inositoltriphosphate; PLC: Phospholipase C; PIP2: Phosphatidylinositol biphosphate; DG: Diacylglycerl; AC: Adenylcyclase; PKC: Protein kinase C.
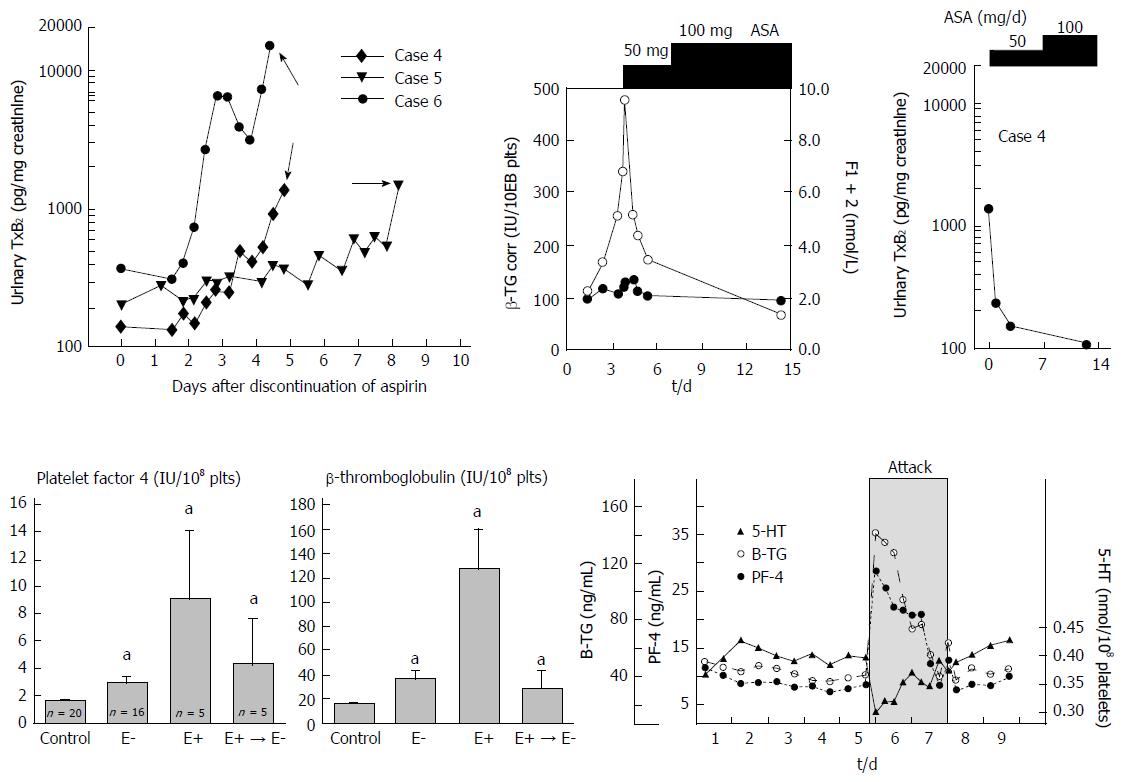
Figure 14 Vaso-active substances, prostaglandins endoperoxides and other factors released during JAK2 mutated platelet aggregation account for the inflammatory symptoms in JAK2-thrombocythemia of ET and PV patients.
Upper part: Simultaneous study of clinical signs and symptoms of erythromelalgia, platelet activation markers and increased urinary thromboxane B2 (TxB2) excretion (right) in three ET patients during attacks of erythromelalgia after discontinuation of aspirin. This was associated with large amounts of urinary thromboxane B2 (TxB2) excretion (right) and high levels of beta-thromboglobulin (middle) at time of aspirin responsive erythromelalgic symptoms in JAK2 thrombocythemia. Erythromelalgia was successfully treated with a platelet-specific aspirin regimen of 50 mg per day, which was associated with correction of beta-TG to normal (right) and correction of TxB2 urinary excretion to normal (right). Treatment with 100 mg aspirin per day did even further decrease platelet activation markers beta-TG and TxB2 urinary excretion reaching completely normal levels[80]. Lower part: The effects of intervention with aspirin on platelet factor 4 (PF4) and bete-thromboglobulin (beta-TG) in 20 controls, 16 cases of thrombocythemia without erythromelalgia (E-), in 5 cases of thrombocythemia complicated by erythromelalgia (E+) and no aspirin, and in 5 cases after curative treatment of erythromelalgia in thrombocythemia patients (E+ → E-left and middle)[66,67]. Decreased platelet 5-HT and increased beta-TG and PF-4 during a documented migraine attack (grey zone) demonstrating that in such patients migraine is a platelet disorder with documented in vivo platelet activation during the attack[53,62,64,67]. aP < 0.05 vs control.
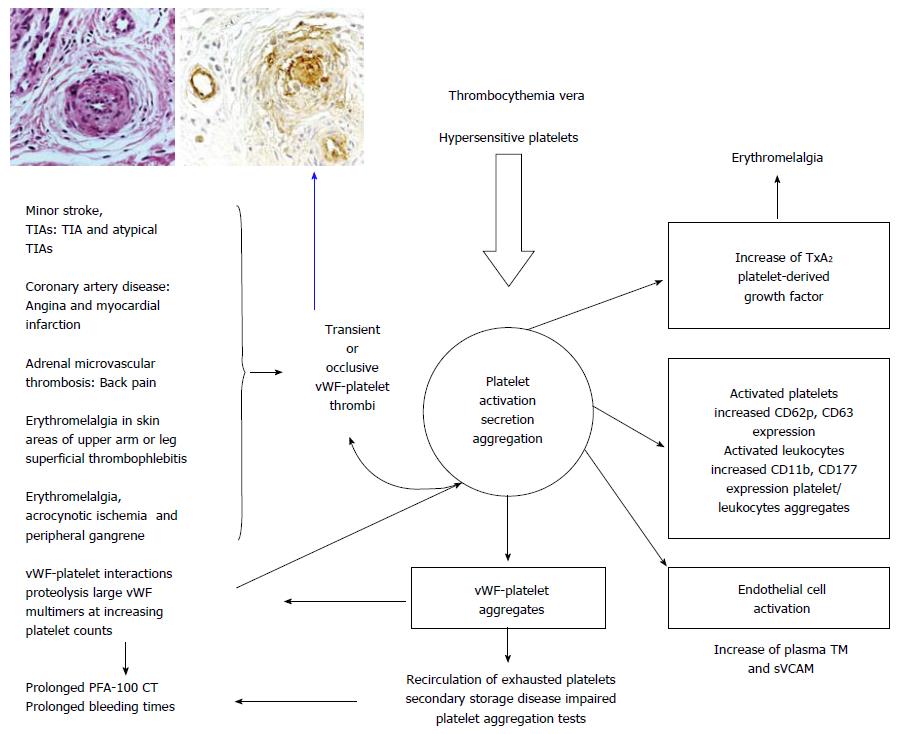
Figure 15 Shear induced platelet activation of constitutively JAK2V617F hypersensitive sticky platelet with increased CD62p and CD 63 expression) in thrombocythemra vera of JAK2V617F-positive ET and PV patients is the cause of a broad spectrum of platelet-mediated arteriolar inflammatory and thrombotic manifestations of erythromelalgia, digital ischemic complications, superficial thrombophlebitis, MIAs, TIAs, adrenal microvascular thrombosis and TIAs or even stroke and acute coronary syndrome in particular when thrombocythemia[79,80] is associated with increased activated leucocytes and erythrocyte count of polycythemia vera (increased cellular blood viscosity (Figure 12).
In this process of in vivo platelet activation and secretion, reversible VWF-platelet aggregates activate endothelial cells to secrete thrombomoduline (TM) and sVCAM[79,80], whereas secreted PDGM accounts for the fibromuscular intimal proliferation (inset left) followed by occlusion of arterioles by VWF-rich platelet thrombi (inset right). After reversible aggration the platelets recirculate as exhausted platelets with secondary storage pool deficiency and impaired platelet functional defects. The platelet - Von Willebrand factor (VWF) interactions leads to proteolysis of large vWF multimers at increasing platelet counts from below to above 1000 × 109/L (Figure 13 right and Figure 16 peak 1 and 4).
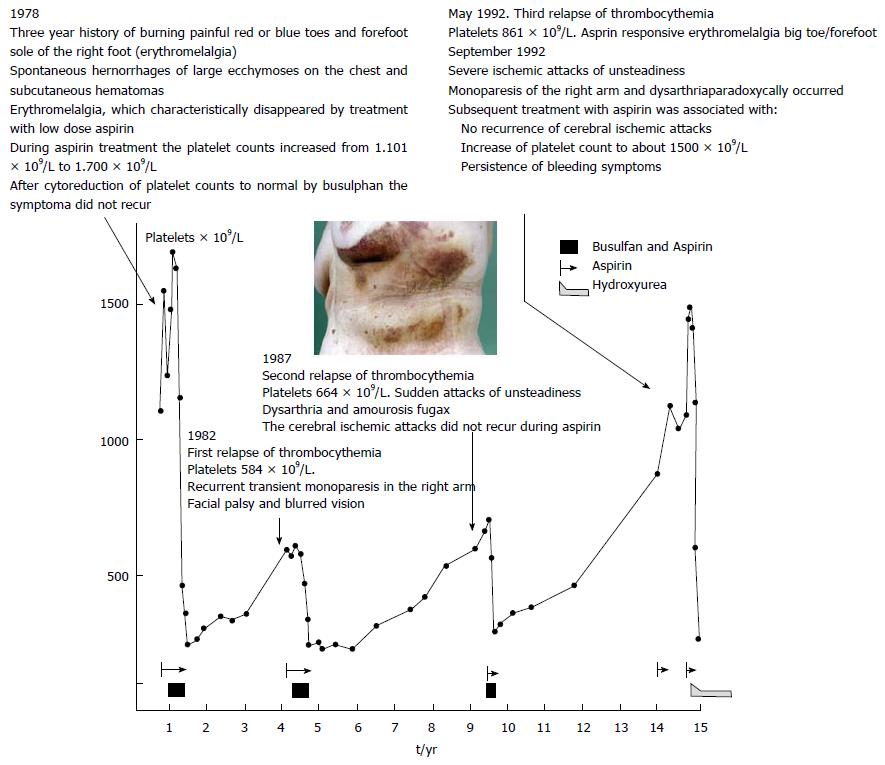
Figure 16 Longterm observations in a 72-year-old woman with a three years history of burning painful red or blue toes and forefoot sole of the right foot (erythromelalgia) presented in December 1978 with spontaneous hemorrhages of large ecchymoses on the chest and subcutaneous hematotomas.
Paradoxical occurrences of platelet mediated thrombosis and bleeding in this case with thrombocythemia (ET with features of PV in the bone marrow: Prodromal PV) 1978 peak 1 in the figure. The first episode of erythromelalgia for 3 years followed by simultaneous occurrence of thrombotic thrombocythemia and hemorrhagic thrombocythemia at platelet counts around 1100 × 109/L in 1982. Aspirin was effective at peak 1 for the relief of erythromelalgia, which was associated with a further increase of platelet count to about 1500 × 109/L. During periods of thrombocythemia at peak 1, 2, 3 and 4 busulphan induced complete remissions of thrombocythemia resulted in normal platelet counts below 400 × 109/L, which was associated with no recurrence of erythromelalgia when not on aspirin. At peak 2 and 3 in the figure, recurrence of a second and third episode of thrombotic thrombocythemia occurred at platelet counts between 600 and 800 × 109/L. In 1992 at peak 4 the patient suffered from an episode of thrombotic and hemorrhagic thrombocythemia at platelet counts of 1040 × 109/L. Again aspirin relieved the erythromelalgia, which was associated with a further increase of platelet count to to around 1500 × 109/L but the hemorrhagic manifestation persisted which associated with acquired von Willebrand factor deficiency type 2A (acquired von Willebrand syndrome: AVWS and disappeared after correction of platelet count to normal) (Figure 15).
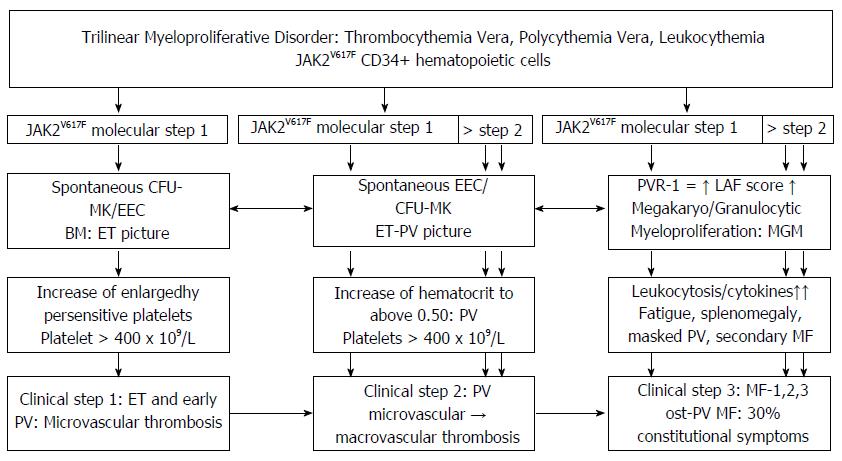
Figure 17 The molecular etiology of JAK2V617F heterozygous mutated essential thrombocythemia (essential thrombocythemia picture Step 1) and evolution into combined herozygous/homozygous or homozygous JAK2V617F mutated sequential stages of prodromal polycythemia vera classical PV (ET/PV pictures) and post-polycythemia vera myeloid metaplasia of bone marrow and spleen complicated by chronic idiopathic or secondary myelofibrosis.
Figure 18 Low dose aspirin 75 mg OD vs placebo in 796 patients with unstable angina or non-Q myocardial infarction in the presence of arterioclerotic vascular pathology was effective to reduce the probability of death or myocardial infarction during one year follow-up.
A: Aspirin 75 mg OD vs placebo in 796 patients with unstable angina or non-Q myocardial infraction (MI) reduced the probability of death or MI from about 20% to 10% during 1-year follow-up[82]; B: Aspirin/marcoumar vs aspirin/ticlopedin after percutaneous cutaneous intervention (PCI) reduced the combined events of cardiac death, MI, bypass or recurrent PCI from 6.2 to 1.6% after 1-mo follow-up[83]; C: Aspirin/placebo vs aspirin/clopidogrel in 2625 treated PCI patients reduced the composite of cardiovascular death, MI, or urgent revascularization from 6.5% to 4.5% in the PCI-CURE study[84]; D: The extended substudy of the PCI-CURE reduced the combined cardiovascular death and MI reduced from 12.6% to 8.8% after 1-year follow-up[84].
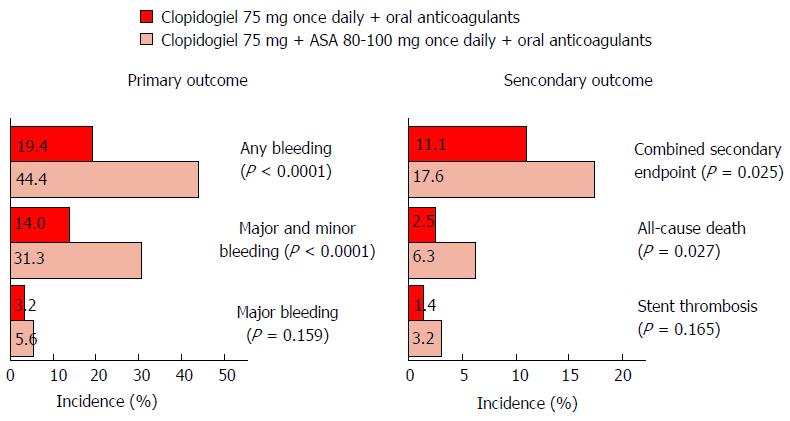
Figure 19 Dual clopidogrel and coumarin compared to triple clopidogrel coumarin and aspirin in 573 patients undergoing PCI receiving oral anticoagulation for another reason in the WOEST study was equal in terms of death, reoclusion and revascularization but superior for dual clopidogrel/coumarin in terms of any bleeding (44.
4% vs 19.4%), major and minor bleeding (31.3% vs 14%) and major bleeding (5.6% vs 3.2%)[85].
- Citation: Michiels JJ. Aspirin cures erythromelalgia and cerebrovascular disturbances in JAK2-thrombocythemia through platelet-cycloxygenase inhibition. World J Hematol 2017; 6(3): 32-54
- URL: https://www.wjgnet.com/2218-6204/full/v6/i3/32.htm
- DOI: https://dx.doi.org/10.5315/wjh.v6.i3.32