Peer-review started: October 19, 2020
First decision: December 1, 2020
Revised: December 10, 2020
Accepted: December 23, 2020
Article in press: December 23, 2020
Published online: January 25, 2021
Processing time: 90 Days and 16.5 Hours
There is indication that fecal microbiota transplant (FMT) has the potential to alter the course of chronic skin disease, but few studies have investigated this phenomenon beyond case reports. Research with larger sample sizes is needed to provide a more thorough assessment of possible associations and to establish a broader foundation upon which to base hypotheses.
To identify associations between FMT and skin conditions, particularly infectious and inflammatory etiologies, and the role of dermatology post-FMT.
We conducted a retrospective cohort study involving a chart review of all patients whom received FMT between January 2013 and December 2019 at a single academic medical center. Dermatologic follow-up was assessed for the two years after FMT or through March 2020 for more recent procedures. Dermatologic diagnoses and visits within the study time frame were recorded and assessed for trends. This study was exploratory in nature. Descriptive statistics were calculated, and the t-test, Pearson’s chi-squared test, and Fisher’s exact test were used to calculate P values.
Most patients were female (61.5%) and ethnically not Hispanic or Latino (93.6%). Median age was 38 (range, 17-90). In total, 109 patients who underwent 111 fecal microbiota transplant events were included. Twenty-six events (23.4%) involved a dermatology office visit post-procedure, and of these events, 20 out of the 26 (76.9%) had an infectious or inflammatory skin condition. The mean time to first visit was 10.0 (± 7.0) mo. The most common diagnoses were dermatophyte, wart(s), and dermatitis, though no specific diagnoses predominated in a way indicating FMT had a significant impact. More patients with a post-FMT skin disease diagnosis had a history of Crohn’s disease compared to those without (P = 0.022), but results could be affected by a small sample size.
Our study is limited by its retrospective nature, but the findings allow a glimpse at dermatologic conditions post-FMT. Few significant associations were found, but potential associations between FMT and skin disease should be further investigated, preferably in prospective studies, to identify how FMT might be of use for treating infectious and inflammatory skin diseases.
Core Tip: Recent evidence indicates fecal microbiota transplant could affect certain skin diseases and their treatment. Given that our study indicated no clear pattern of infectious or inflammatory skin disease after fecal microbiota transplant but also that dermatology follow-up was not common, future studies should either use retrospective data sources with more complete dermatology records for patients receiving fecal microbiota transplant or attempt large prospective studies.
- Citation: Snyder AM, Abbott J, Jensen MK, Secrest AM. Fecal microbiota transplant and dermatologic disorders: A retrospective cohort study assessing the gut microbiome’s role in skin disease. World J Dermatol 2021; 9(1): 1-10
- URL: https://www.wjgnet.com/2218-6190/full/v9/i1/1.htm
- DOI: https://dx.doi.org/10.5314/wjd.v9.i1.1
Chronic inflammatory diseases are increasingly being linked to the gut microbiome[1], but few publications have explored how the gut microbiome influences inflammatory and infectious skin conditions. The microbiome has long been considered a potential contributor to certain skin diseases, but research is still lacking to support associations between skin disease and treatments that target the microbiome, in particular the gut microbiome. Fecal microbiota transplant (FMT) is a procedure that introduces a healthy donor’s gut microbiome through feces to a patient with refractory or recalcitrant Clostridioides difficile (C. difficile) infection. Though FMT has primarily been used to treat C. difficile infections, this treatment has also been investigated in numerous studies for its effects on ulcerative colitis (UC) and Crohn’s disease[2], as well as more recent expansion to successfully treat other seemingly unrelated conditions like recurrent hepatic encephalopathy[3], milk allergy[4,5], and autism spectrum disorder[6].
The role of FMT in managing infectious and inflammatory conditions has now expanded into dermatology. Recent case reports provide evidence of temporal improvement in chronic inflammatory skin disease after FMT, including reports of two patients with alopecia universalis, who saw hair regrowth on multiple regions of the body after FMT[7]. Also, patients with psoriasis[8] and psoriatic arthritis[9] reportedly experienced improvement after FMT. Further, some mouse studies have even attempted to understand the role of FMT for management of melanoma[10,11]. However, larger studies on FMT and skin disease in humans are needed to better comprehend the extent of skin conditions which FMT could potentially influence. Here, we present a retrospective cohort study involving patients whom received FMT. We identify and describe dermatologic conditions post-FMT, specifically infectious and inflammatory etiologies, with the goal of better understanding the effects of FMT on skin disease.
We conducted a retrospective cohort study using electronic medical record (EMR) data from University of Utah Health. A total of 141 patients with FMT code ICD-10-CM Z94.89 on their records from January 2013 to December 2019 were identified. Patients were included for analysis if (1) their record included a clinician’s report on the FMT procedure(s) at University of Utah Health; (2) their record included visit information at University of Utah Health up through March 2020 that was not related to an FMT procedure or follow-up on C. difficile infection post-FMT; and (3) the patient had not died within eight weeks after FMT. Patients not meeting all three of these criteria were excluded.
Information for analysis was collected from the EMR on sex, ethnicity, age (calculated based on birth date and first FMT at our institution), fecal transplant history, history of gastrointestinal conditions and solid organ transplant[12] before FMT procedure, antibiotic use within eight weeks after FMT (excluding topical antibiotics), chronic inflammatory skin conditions pre-FMT and/or inflammatory or infectious skin conditions documented within six months prior to FMT, and subsequent dermatologic evaluation in our Department of Dermatology up to two years after FMT or through March 2020 for more recent FMT procedures. Antibiotic use (related to treating a C. difficile infection or not) within eight weeks after FMT was collected due to research that indicates FMT may be less effective if there is antibiotic exposure in this time frame[13]. Race calculations are not presented due to uncertainty about the accuracy of this information in the EMR; conversely, ethnicity information came from demographic information believed to be provided by the patients.
Information collected was based on FMT events, which could include more than one FMT procedure. If there were at least two years between two FMT procedures, the two procedures were counted as separate FMT events and had separate evaluations of antibiotic use, skin conditions prior to FMT, subsequent dermatologic evaluation, and FMT, gastrointestinal condition, and solid organ transplant histories. Procedures occurring within two years of each other were counted as the same FMT event, and information was collected for the most recent FMT procedure in an event. The variable of exception was age, which was calculated based on a patient’s first FMT at our institution.
We included two years of dermatology follow-up per FMT event to account for changes in skin conditions which might more directly be attributed to microbiota that persisted after FMT[6,14]. Information on skin conditions was obtained from dermatology office visits, and information on occurrence of dermatology office visits was recorded as well, excluding visits that were classified as being specifically for elective/cosmetic surgery. Where needed, laboratory reports were reviewed to help assess what a skin condition reported for a visit was.
This study was exploratory in nature, so extensive statistical analyses were not performed and some analyses were post hoc. A t-test was used for continuous variables, and Pearson’s chi-squared test or Fisher’s exact test were used for categorical variables as appropriate. Two-tailed tests were used where possible. The lead author of this study has had training and experience with biomedical statistics, and she determined and conducted the statistical analyses. Microsoft Excel 2016 (Microsoft, Redmond, WA, United States) and STATA version 16 software (StataCorp LLC, College Station, TX, United States) were used for calculations.
Exempt status was obtained from the University of Utah Institutional Review Board (#76927).
Thirty-two patients (22.7%) out of the 141 patients identified were excluded based on our inclusion criteria. Out of the remaining 109 patients, 25 (22.9%) had a dermatology office visit post-FMT, and 19 (17.4%) had a skin condition diagnosis at one or more of these visits. Over one-third had additional gastrointestinal conditions beyond C. difficile. Nineteen patients (17.4%) had a history of Crohn’s disease, and 21 (19.3%) had a history of UC (Table 1). Other gastrointestinal conditions beyond C. difficile, Crohn’s disease, and UC that were observed include serrated polyposis syndrome, microscopic colitis, alcohol-related cirrhosis, primary sclerosing cholangitis (in addition to Crohn’s disease or UC), and small bowel obstruction with toxic megacolon. Eight patients (7.3%) had a documented chronic inflammatory skin condition and/or an inflammatory or infectious skin condition within six months prior to FMT, including acne (n = 3), antiphospholipid antibody syndrome with lupus, diffuse cutaneous systemic sclerosis, perianal strep, small plaque parapsoriasis, urticarial vasculitis, varicella zoster virus, and wart(s). Most patients were female (61.5%) and identified as ethnically not Hispanic or Latino (93.6%). The median age was 38, and the age range was 17 to 90 years. Solid organ transplant was seen in 5 patients (4.6%), and all solid organ transplants were performed at least 10 mo before FMT. Antibiotic use was reported in almost a quarter of patients. The distribution of these variables among patients whom had a noted skin condition at a post-FMT dermatology office visit vs those whom did not is also shown in Table 1; most of the demographic characteristics were relatively similar between these groups, except that proportionally more patients with noted skin conditions had a history of Crohn’s disease (P = 0.022). Most patients with a post-FMT diagnosis of wart(s) had a history of Crohn’s disease as did both patients diagnosed with tinea versicolor post-FMT.
| Total (n = 109) | Post-FMT skin condition noted (n = 19) | No post-FMT skin condition noted (n = 90) | P values | |
| Sex, n (%) | 0.72 | |||
| Male | 42 (38.5) | 8 (42.1) | 34 (37.8) | |
| Female | 67 (61.5) | 11 (57.9) | 56 (62.2) | |
| Ethnicity, n (%) | 0.72 | |||
| Hispanic or Latino | 5 (4.6) | 0 (0.0) | 5 (5.6) | |
| Not Hispanic or Latino | 102 (93.6) | 19 (100.0) | 83 (92.2) | |
| Undisclosed or unknown | 2 (1.8) | 0 (0.0) | 2 (2.2) | |
| Age | 0.96 | |||
| Mean (± SD) | 43.6 (±18.9) | 43.4 (±18.5) | 43.7 (±19.1) | |
| Median (range) | 38 (17-90) | 39 (21-90) | 38 (17-87) | |
| History of Crohn's disease, n (%)a,b | 0.022d | |||
| Yes | 19 (17.4) | 7 (36.8) | 12 (13.3) | |
| No | 90 (82.6) | 12 (63.2) | 78 (86.7) | |
| History of ulcerative colitis, n (%)a | 0.76 | |||
| Yes | 21 (19.3) | 4 (21.1) | 17 (18.9) | |
| No | 88 (80.7) | 15 (78.9) | 73 (81.1) | |
| History of solid organ transplant, n (%)a | 0.21 | |||
| Yes | 5 (4.6) | 2 (10.5) | 3 (3.3) | |
| No | 104 (95.4) | 17 (89.5) | 87 (96.7) | |
| Antibiotic use within eight weeks post-FMT, n (%) | 0.77 | |||
| Yes | 25 (22.9) | 5 (26.3) | 20 (22.2) | |
| No | 84 (77.1) | 14 (73.7) | 70 (77.8) | |
| Skin conditions pre-FMT, n (%) | 0.63 | |||
| Yes | 8 (7.3) | 2 (10.5) | 6 (6.7) | |
| No | 101 (92.7) | 17 (89.5) | 84 (93.3) | |
| Number of FMT procedures per patient, n (%)c | 0.047d | |||
| One | 82 (75.2) | 15 (78.9) | 67 (74.4) | |
| Two | 24 (22.0) | 2 (10.5) | 22 (24.4) | |
| Three | 3 (2.8) | 2 (10.5) | 1 (1.1) |
Two patients had more than one FMT event, leading to a total of 111 FMT events. Of these events, a strong majority (96.4%) were administered via colonoscopy, and other methods of administration included ileoscopy, pouchoscopy, and sigmoidoscopy.
Twenty-six FMT events (23.4%) had dermatology office visits post-FMT. Twenty of these events (76.9%) had a noted infectious or inflammatory skin condition at any of these visits, and 19 events (73.1%) had a noted condition at the first dermatology visit post-FMT.
A total of 53 dermatology office visits were noted (Table 2). First visits after the most recent FMT occurred within an average of 10.0 (± 7.0) mo post-FMT. There were nine first visits within six months after FMT, and of these visits, five (55.6%) included at least one diagnosis of the following: acne, intertrigo, rosacea, telogen effluvium, tinea versicolor, and urticaria.
| Visit | Number of events | mean ± SD months to visit | Median months to visit | Minimum months to visit | Maximum months to visit |
| 1st | 26 | 10.0 ± 7.0 | 9.5 | 0 | 22 |
| 2nd | 10 | 11.5 ± 6.4 | 11 | 5 | 23 |
| 3rd | 7 | 12.0 ± 5.0 | 13 | 6 | 20 |
| 4th | 4 | 12.5 ± 4.4 | 14.5 | 6 | 15 |
| 5th | 3 | 16.3 ± 1.5 | 16 | 15 | 18 |
| 6th | 1 | 16.0 ± --- | 16 | 16 | 16 |
| 7th | 1 | 17.0 ± --- | 17 | 17 | 17 |
| 8th | 1 | 18.0 ± --- | 18 | 18 | 18 |
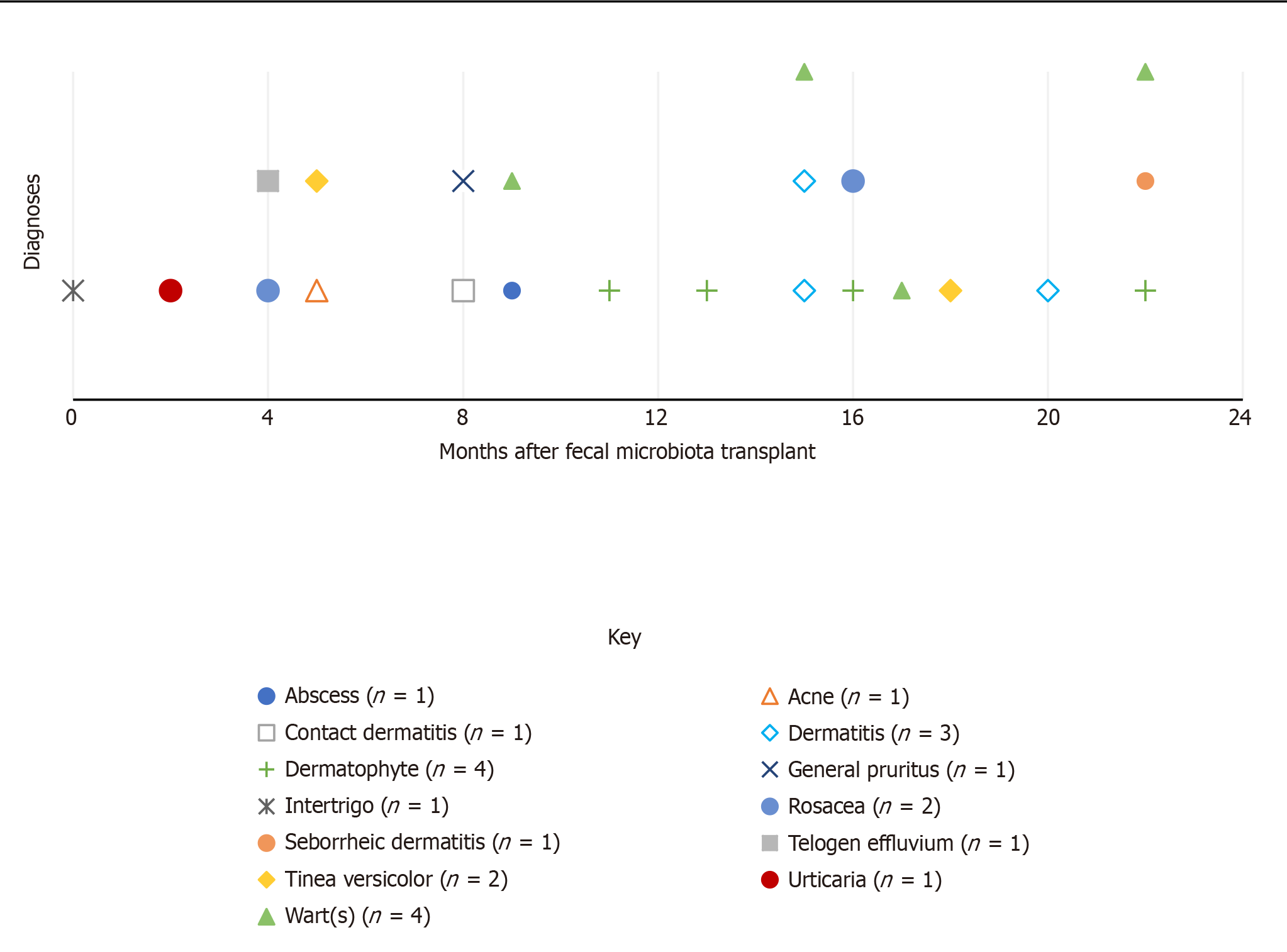
Figure 1 illustrates the distribution of new skin conditions over the months post-FMT at any dermatology office visit. Diagnoses were included in this figure if they were not noted as a pre-FMT condition and they were not subsequent diagnoses of the same condition if the patient was seen more than once for a particular condition post-FMT [overall, this excluded diagnoses of diffuse cutaneous systemic sclerosis, rosacea, and wart(s)]. Dermatophyte, wart(s), and dermatitis were the most common conditions to appear, and all occurred after the first eight months post-FMT. Overall, it seems there was not a clear pattern to the distribution of new diagnoses after FMT.
We present a retrospective review of FMTs performed at our institution to elucidate potential associations with dermatologic conditions and the role of dermatologic evaluation. Through our study, we were able to reveal that infectious and inflammatory etiologies were relatively infrequently documented post-FMT. Additionally, these patients did not appear to have predilection for any particular dermatologic etiologies. The results of this study present information to formulate new ideas on future research, but there are limitations to what conclusions can be drawn.
Previous research indicates there is a variety of skin conditions that appear to be potentially influenced by the gut microbiome. In addition to alopecia[7] and psoriasis[8,9], changes in acne[15] and eczema[4] in FMT recipients have been noted, though these particular studies provide minimal evidence. Acne patients do appear to have differences in gut microbial composition compared to non-acne patients, and these differences may be affected by environment-related factors like diet and stress[16]. A gut-brain-skin axis hypothesis has been presented to explain connections between factors previously thought to be unrelated, and improvements in acne due to probiotic use provide further clues about the impact of gut bacteria[16]. Atopic dermatitis patients also appear to have differences in bacterial composition as well as subsequent immune system-related gene expression compared to controls[17,18]. This and other evidence indicates that the immune system may be impacted by the gut microbiome. For example, the gut microbiome has been investigated for its role in cancer immunotherapy[19] and appears to be involved in antitumor immunity and inhibition of tumor growth for melanoma[20]. Studies on fecal transfer from human melanoma patients to mice have suggested that the gut microbiome is involved in immune responses and may impact tumor growth during immunotherapy[10,11].
Manipulating the mouse gut microbiome with antibiotics has also been shown to affect melanoma tumor growth given how the immune system was impacted[11,21]. It is possible for antibiotics to impact immune system responses via effects on the gut microbiome, and this can subsequently impact colonization resistance that would otherwise protect the body against certain infectious diseases, such as C. difficile–the bacterial species that is the primary reason for administering FMT[22]. Some autoimmune disorders have been associated with antibiotics and the gut microbiome[23]. However, it is still unclear how much antibiotics play a part in these conditions when considering other exposures, including medical interventions (such as delivery methods during the birth process), lifestyle, diet, and certain infectious diseases which have also been explored regarding their impact on the gut microbiome and subsequent impact on autoimmune disease[23].
Given this evidence, it is plausible that effects on the immune system, whether due to FMT or other means of altering the gut microbiome, could impact infectious and inflammatory skin conditions. Interestingly, there was a wide variety of infectious and inflammatory skin conditions noted in our study, though many of these were unique to individual patients. Thus, our results do not suggest that FMT leads to flaring of specific acute or chronic dermatologic conditions, but given the limited evidence, we cannot identify clear cause-and-effect relationships between skin diseases and FMT.
Our study was limited by its retrospective nature. Most patients who received FMT at University of Utah Health did not have a follow-up dermatology office visit on record, and we cannot be certain that patients did not visit dermatologists at other healthcare institutions. Of note, some FMT procedures were fairly recent as of the time this report was written, and so this factor could have contributed to the lack of dermatology visits for some patients. There was no clear indication of FMT-induced changes in skin conditions for most patients, but for the same reasons we cannot assume there were associations, we cannot assume that there was no FMT-induced change.
Most diagnoses were made after the first six months post-FMT, which makes it difficult to determine whether the noted dermatologic conditions were influenced by FMT or other factors, such as antibiotics. In one study, most microbial species recovered within 1.5 mo after an antibiotic cocktail of meropenem, gentamicin, and vancomycin, though certain species did not recover even after 180 d[24]. Another study found that microbial compositions remained changed up to four months after one course of clindamycin and up to 12 mo after one course of ciprofloxacin[25]. Our study could assess treatments prescribed at our institution but cannot confirm through retrospective analysis that some patients did not receive antibiotics elsewhere if not reported in our EMR. We did find that use of antibiotics within eight weeks post-FMT was not common for our overall sample and did not differ significantly between those with or without a reported post-FMT skin condition. Thus, using only the records in our system, it would seem that antibiotics did not play a significant role in disease development. It is still reasonable to assume that any benefits from FMT could later have been offset by exposure to other factors in the months and years when post-FMT dermatology visits were conducted, and in our study, we limited the time frame of dermatology follow-up to two years after FMT to account for these other factors, given evidence that microbial changes from FMT may last up to two years[6,14]. However, this time frame could also be a limitation.
Of further note, we did not conduct analyses on diet. Previous research indicates that the gut microbiome can be influenced by different aspects of diet[26-29], including use of probiotics[30], and so dietary factors would be important to assess where possible. However, using the EMR alone does not provide much detail to assess diet and dietary changes, and with the amount of time that passed before most of the dermatology office visits, we cannot confirm that dietary changes would not have impacted outcomes post-FMT. Further study involving dietary interventions or questionnaires would be needed to address how diet might be associated with the gut microbiome and subsequent skin disease.
Another significant limitation is that we were not able to find enough information on stool donors to determine how all donors were screened or whether donors had any of the noted skin conditions and could have transferred a disease-inducing microbial composition to recipients. Generally, stool donors might be screened for gastrointestinal diseases, certain pathogens, etc. related to overall health or gastroenterology concerns, but there is not much emphasis on screening for a broad range of dermatologic conditions[31]. Overall, our study simply does not have enough data to be certain these other factors did not impact disease development or even the lack thereof post-FMT.
Potential associations between FMT and skin disease should be investigated to further elucidate the association between skin disease and the gut microbiome and to further understand how FMT might be of use for treating infectious and inflammatory skin conditions. Though dermatologic assessment might not seem important for treatment of a C. difficile infection, FMT can affect more than just gastrointestinal disease. Better documentation of skin conditions before and after FMT are needed to perform more effective retrospective studies on this subject. Where possible, prospective studies may be necessary to ascertain better evidence of cause-and-effect relationships between FMT and skin disease as the complexity of the gut microbiome and environmental factors that influence it makes it difficult to find enough information retrospectively. Finally, clinicians administering FMT should be aware of the potential for dermatologic changes in their patients after FMT, and we encourage them to consider referring their FMT patients to a dermatologist for addressing dermatologic concerns.
This retrospective cohort study was completed to evaluate the impact of fecal microbiota transplant (FMT) on skin disease, a subject not currently well-studied.
FMT has grown in popularity as a possible treatment for diseases beyond recurrent Clostridioides difficile infections, and there is growing evidence that FMT could be a treatment for skin disease. Overall, the goal was to assess potential patterns of skin disease that could be notable for future research on FMT.
To determine the impact of FMT on the development of skin disease post-FMT and identify pitfalls in dermatologic care that could impact future studies on this relatively underexplored subject.
A retrospective chart review was conducted on all patients whom received FMT between January 2013 to December 2019 at a single academic medical center. Dermatologic follow-up was assessed for the two years after FMT or through March 2020 for more recent procedures. Dermatology visits and inflammatory and infectious dermatologic diagnoses were recorded.
The most common diagnoses were dermatophyte, wart(s), and dermatitis. Mean time to first dermatology visit was 10.0 (± 7.0) mo. Overall, no apparent FMT-related trends in skin disease were observed. Little information on the condition of most patients’ skin pre- and post-FMT was captured in the electronic medical record. Thus, more information is needed on dermatology visits and particularly visits within the first few months post-FMT.
Due to the extended interval between FMT and dermatology visits, it was difficult to assess whether reported diseases were affected by or resulted from FMT. While FMT could potentially have clinically significant effects on certain skin diseases, this study was limited by its retrospective nature and could not find clear patterns of post-FMT skin disease. It was concluded that prospective studies may be the best avenue for further assessing the relationship between FMT and skin disease.
Future research will need to address temporality of dermatologic visits after FMT to provide a better indication of the effect on skin disease. Ideally, dermatologic follow-up should occur within two months post-FMT. Given limitations of the electronic medical record, prospective studies will need to be conducted to assess future relationships between FMT and skin disease.
Manuscript source: Unsolicited manuscript
Specialty type: Dermatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li H S-Editor: Zhang L L-Editor: A P-Editor: Wang LYT
| 1. | Bernstein CN, Forbes JD. Gut Microbiome in Inflammatory Bowel Disease and Other Chronic Immune-Mediated Inflammatory Diseases. Inflamm Intest Dis. 2017;2:116-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 2. | Paramsothy S, Paramsothy R, Rubin DT, Kamm MA, Kaakoush NO, Mitchell HM, Castaño-Rodríguez N. Faecal Microbiota Transplantation for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J Crohns Colitis. 2017;11:1180-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 322] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 3. | Bajaj JS, Fagan A, Gavis EA, Kassam Z, Sikaroodi M, Gillevet PM. Long-term Outcomes of Fecal Microbiota Transplantation in Patients With Cirrhosis. Gastroenterology 2019; 156: 1921-1923. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 4. | Liu SX, Li YH, Dai WK, Li XS, Qiu CZ, Ruan ML, Zou B, Dong C, Liu YH, He JY, Huang ZH, Shu SN. Fecal microbiota transplantation induces remission of infantile allergic colitis through gut microbiota re-establishment. World J Gastroenterol. 2017;23:8570-8581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (4)] |
| 5. | Feehley T, Plunkett CH, Bao R, Choi Hong SM, Culleen E, Belda-Ferre P, Campbell E, Aitoro R, Nocerino R, Paparo L, Andrade J, Antonopoulos DA, Berni Canani R, Nagler CR. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat Med. 2019;25:448-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 312] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 6. | Kang DW, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S, Caporaso JG, Krajmalnik-Brown R. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci Rep. 2019;9:5821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 421] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 7. | Rebello D, Wang E, Yen E, Lio PA, Kelly CR. Hair Growth in Two Alopecia Patients after Fecal Microbiota Transplant. ACG Case Rep J. 2017;4:e107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Zamudio-Tiburcio A, Bermúdez-Ruiz H, Reyes-López PA. Psoriasis is candidate for intestinal microbiota transplantation? EC Microbiol. 2019;15:455-460. |
| 9. | Selvanderan SP, Goldblatt F, Nguyen NQ, Costello SP. Faecal microbiota transplantation for Clostridium difficile infection resulting in a decrease in psoriatic arthritis disease activity. Clin Exp Rheumatol. 2019;37:514-515. [PubMed] |
| 10. | Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2999] [Cited by in RCA: 3293] [Article Influence: 470.4] [Reference Citation Analysis (0)] |
| 11. | Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Bérard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1834] [Cited by in RCA: 2537] [Article Influence: 253.7] [Reference Citation Analysis (0)] |
| 12. | Naldi L, Venturuzzo A, Invernizzi P. Dermatological Complications After Solid Organ Transplantation. Clin Rev Allergy Immunol. 2018;54:185-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Allegretti JR, Kao D, Sitko J, Fischer M, Kassam Z. Early Antibiotic Use After Fecal Microbiota Transplantation Increases Risk of Treatment Failure. Clin Infect Dis. 2018;66:134-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Kumar R, Yi N, Zhi D, Eipers P, Goldsmith KT, Dixon P, Crossman DK, Crowley MR, Lefkowitz EJ, Rodriguez JM, Morrow CD. Identification of donor microbe species that colonize and persist long term in the recipient after fecal transplant for recurrent Clostridium difficile. NPJ Biofilms Microbiomes. 2017;3:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Borody TJ, Paramsothy S, Agrawal G. Fecal microbiota transplantation: indications, methods, evidence, and future directions. Curr Gastroenterol Rep. 2013;15:337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 16. | Lee YB, Byun EJ, Kim HS. Potential Role of the Microbiome in Acne: A Comprehensive Review. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 17. | Park YM, Lee SY, Kang MJ, Kim BS, Lee MJ, Jung SS, Yoon JS, Cho HJ, Lee E, Yang SI, Seo JH, Kim HB, Suh DI, Shin YH, Kim KW, Ahn K, Hong SJ. Imbalance of Gut Streptococcus, Clostridium, and Akkermansia Determines the Natural Course of Atopic Dermatitis in Infant. Allergy Asthma Immunol Res. 2020;12:322-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 18. | Lee MJ, Kang MJ, Lee SY, Lee E, Kim K, Won S, Suh DI, Kim KW, Sheen YH, Ahn K, Kim BS, Hong SJ. Perturbations of gut microbiome genes in infants with atopic dermatitis according to feeding type. J Allergy Clin Immunol. 2018;141:1310-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 19. | Li W, Deng Y, Chu Q, Zhang P. Gut microbiome and cancer immunotherapy. Cancer Lett. 2019;447:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 20. | Li Y, Tinoco R, Elmén L, Segota I, Xian Y, Fujita Y, Sahu A, Zarecki R, Marie K, Feng Y, Khateb A, Frederick DT, Ashkenazi SK, Kim H, Perez EG, Day CP, Segura Muñoz RS, Schmaltz R, Yooseph S, Tam MA, Zhang T, Avitan-Hersh E, Tzur L, Roizman S, Boyango I, Bar-Sela G, Orian A, Kaufman RJ, Bosenberg M, Goding CR, Baaten B, Levesque MP, Dummer R, Brown K, Merlino G, Ruppin E, Flaherty K, Ramer-Tait A, Long T, Peterson SN, Bradley LM, Ronai ZA. Gut microbiota dependent anti-tumor immunity restricts melanoma growth in Rnf5-/- mice. Nat Commun. 2019;10:1492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 21. | Sethi V, Kurtom S, Tarique M, Lavania S, Malchiodi Z, Hellmund L, Zhang L, Sharma U, Giri B, Garg B, Ferrantella A, Vickers SM, Banerjee S, Dawra R, Roy S, Ramakrishnan S, Saluja A, Dudeja V. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology 2018; 155: 33-37. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 298] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 22. | Becattini S, Taur Y, Pamer EG. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol Med. 2016;22:458-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 580] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 23. | De Luca F, Shoenfeld Y. The microbiome in autoimmune diseases. Clin Exp Immunol. 2019;195:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 320] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 24. | Palleja A, Mikkelsen KH, Forslund SK, Kashani A, Allin KH, Nielsen T, Hansen TH, Liang S, Feng Q, Zhang C, Pyl PT, Coelho LP, Yang H, Wang J, Typas A, Nielsen MF, Nielsen HB, Bork P, Wang J, Vilsbøll T, Hansen T, Knop FK, Arumugam M, Pedersen O. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat Microbiol. 2018;3:1255-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 505] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 25. | Zaura E, Brandt BW, Teixeira de Mattos MJ, Buijs MJ, Caspers MP, Rashid MU, Weintraub A, Nord CE, Savell A, Hu Y, Coates AR, Hubank M, Spratt DA, Wilson M, Keijser BJ, Crielaard W. Same Exposure but Two Radically Different Responses to Antibiotics: Resilience of the Salivary Microbiome vs Long-Term Microbial Shifts in Feces. mBio. 2015;6:e01693-e01615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 319] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 26. | McDonald D, Hyde E, Debelius JW, Morton JT, Gonzalez A, Ackermann G, Aksenov AA, Behsaz B, Brennan C, Chen Y, DeRight Goldasich L, Dorrestein PC, Dunn RR, Fahimipour AK, Gaffney J, Gilbert JA, Gogul G, Green JL, Hugenholtz P, Humphrey G, Huttenhower C, Jackson MA, Janssen S, Jeste DV, Jiang L, Kelley ST, Knights D, Kosciolek T, Ladau J, Leach J, Marotz C, Meleshko D, Melnik AV, Metcalf JL, Mohimani H, Montassier E, Navas-Molina J, Nguyen TT, Peddada S, Pevzner P, Pollard KS, Rahnavard G, Robbins-Pianka A, Sangwan N, Shorenstein J, Smarr L, Song SJ, Spector T, Swafford AD, Thackray VG, Thompson LR, Tripathi A, Vázquez-Baeza Y, Vrbanac A, Wischmeyer P, Wolfe E, Zhu Q; American Gut Consortium; Knight R. American Gut: an Open Platform for Citizen Science Microbiome Research. mSystems. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 559] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 27. | Hehemann JH, Correc G, Barbeyron T, Helbert W, Czjzek M, Michel G. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature. 2010;464:908-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 736] [Article Influence: 49.1] [Reference Citation Analysis (1)] |
| 28. | Hehemann JH, Kelly AG, Pudlo NA, Martens EC, Boraston AB. Bacteria of the human gut microbiome catabolize red seaweed glycans with carbohydrate-active enzyme updates from extrinsic microbes. Proc Natl Acad Sci. 2012;109:19786-19791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 29. | Wan Y, Wang F, Yuan J, Li J, Jiang D, Zhang J, Li H, Wang R, Tang J, Huang T, Zheng J, Sinclair AJ, Mann J, Li D. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut. 2019;68:1417-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 418] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 30. | Saxelin M, Lassig A, Karjalainen H, Tynkkynen S, Surakka A, Vapaatalo H, Järvenpää S, Korpela R, Mutanen M, Hatakka K. Persistence of probiotic strains in the gastrointestinal tract when administered as capsules, yoghurt, or cheese. Int J Food Microbiol. 2010;144:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 31. | Kim KO, Gluck M. Fecal Microbiota Transplantation: An Update on Clinical Practice. Clin Endosc. 2019;52:137-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (1)] |