Peer-review started: January 29, 2020
First decision: May 5, 2020
Revised: May 29, 2020
Accepted: June 20, 2020
Article in press: June 20, 2020
Published online: August 25, 2020
Processing time: 193 Days and 10.4 Hours
Rhinocerebral mucormycosis (RCM) is a rare fatal fungal infection which is on the increase among immunocompromised hosts such as patients who have had hematological cancers, or have received immunosuppressive drugs, corticosteroids, or other T cell suppressing agents.
We report a case of RCM caused by Rhizopus oryzae, one of the most common opportunistic pathogens, in a patient suffering from a fourth relapse of acute myeloid leukemia. The patient developed RCM after he had received long-term antibiotic agents and corticosteroids. The pathogen was isolated three times from nasal secretions collected from the deep parts of the nasal cavity and was identified by morphology and internal transcribed spacer sequencing. Blood infection was excluded by droplet digital polymerase chain reaction and blood culture. The patient was empirically treated with caspofungin and voriconazole for several days while the lesions continued to progress. The patient was given amphotericin B in combination with caspofungin after RCM was suspected, and the lesions improved over the course of treatment, which lasted several days. However, the patient eventually died of the primary disease.
This case indicates that immunosuppressive drugs, including corticosteroids and antimetabolites in hematological tumor, do increase the risk of infections of this type. Early diagnosis, prompt and frequent surgical debridement, and treatment with amphotericin B without delay are all essential in combatting RCM.
Core tip:Rhizopus oryzae, a common but also useful environmental fungus, is usually employed in the brewing industry. Cases of rhinocerebral mucormycosis in humans are relatively rare. The case we report confirmed the pathogenic fungi through repeated molecular identification and advanced droplet digital polymerase chain reaction technology. We also discuss the patient’s laboratory test results and the early inefficacy of azole antifungal drugs. The high-risk factors and effective treatment for Rhizopus oryzae in such patients are also discussed.
- Citation: Feng YH, Guo WW, Wang YR, Shi WX, Liu C, Li DM, Qiu Y, Shi DM. Rhinocerebral mucormycosis caused by Rhizopus oryzae in a patient with acute myeloid leukemia: A case report. World J Dermatol 2020; 8(1): 1-9
- URL: https://www.wjgnet.com/2218-6190/full/v8/i1/1.htm
- DOI: https://dx.doi.org/10.5314/wjd.v8.i1.1
The fungal infection rhinocerebral mucormycosis (RCM) – the most common manifestation of mucormycosis – is usually fatal. The principal pathogenic genera in this family are Rhizopus, Mucor, and Basidia. Rhizopus oryzae (R. oryzae), one member of Rhizopus, is routinely found in soil, decaying vegetable matter, and other organic matter.
R. oryzae is an opportunistic pathogen in patients suffering from various immunocompromised conditions, including poorly controlled diabetes, kidney failure, organ transplantation, as well as the common outcomes of chemotherapy and immunosuppressive drug treatment[1]. According to the Centers for Disease Control and Prevention, five Americans died of RCM in 2001. The incidence of mucormycosis in general is difficult to estimate because early case reports lack etiological evidence at the molecular level. However, a search of domestic and foreign literature using "Rhizopus oryzae" and "mucormycosis" as keywords indicates that the incidence of mucormycosis caused by R. oryzae has increased markedly.
Here, we present a case of RCM in a 16-year-old patient with acute myeloid leukemia (AML) in China. The diagnosis was confirmed by typical clinical manifestations and repeated mycological identification of R. oryzae from nasal secretions. The patient was empirically treated with a combination of voriconazole and caspofungin during etiological examination and was switched to amphotericin B after mycological confirmation. Although the lesions improved after the treatment with amphotericin B, the patient unfortunately died of the primary disease 3 wk later.
At day -45, a 16-year-old male patient was treated with fludarabine, and a high dose of cytarabine and granulocyte colony stimulating factor after a fourth relapse of AML. However, severe myelosuppression developed during this treatment, which led to a high fever and positive detection of a multiple-drug resistant Escherichia coli (E. coli) in his blood culture. The bacterial infection was alleviated through a 9-d treatment with meropenem and vancomycin. Shortly after controlling the bacterial infection (at day -15), the patient developed redness and swelling of the left side of the nose without obvious cause. At day -14, the anterior endoscopic examination revealed that the left nasal cavity was full of gray and white, jelly-like secretions. No immediate special treatment was given, and the lesion became further aggravated over 3 d. A black crust overlaid on the left turbinate was also seen under rhinoscopy. At day -10, a topical voriconazole rinse at a concentration of (5 mg/mL) was used for external drainage in the nasal cavity along with a ketoconazole ointment (1%). However, the lesion continued to enlarge, and symptoms did not subside.
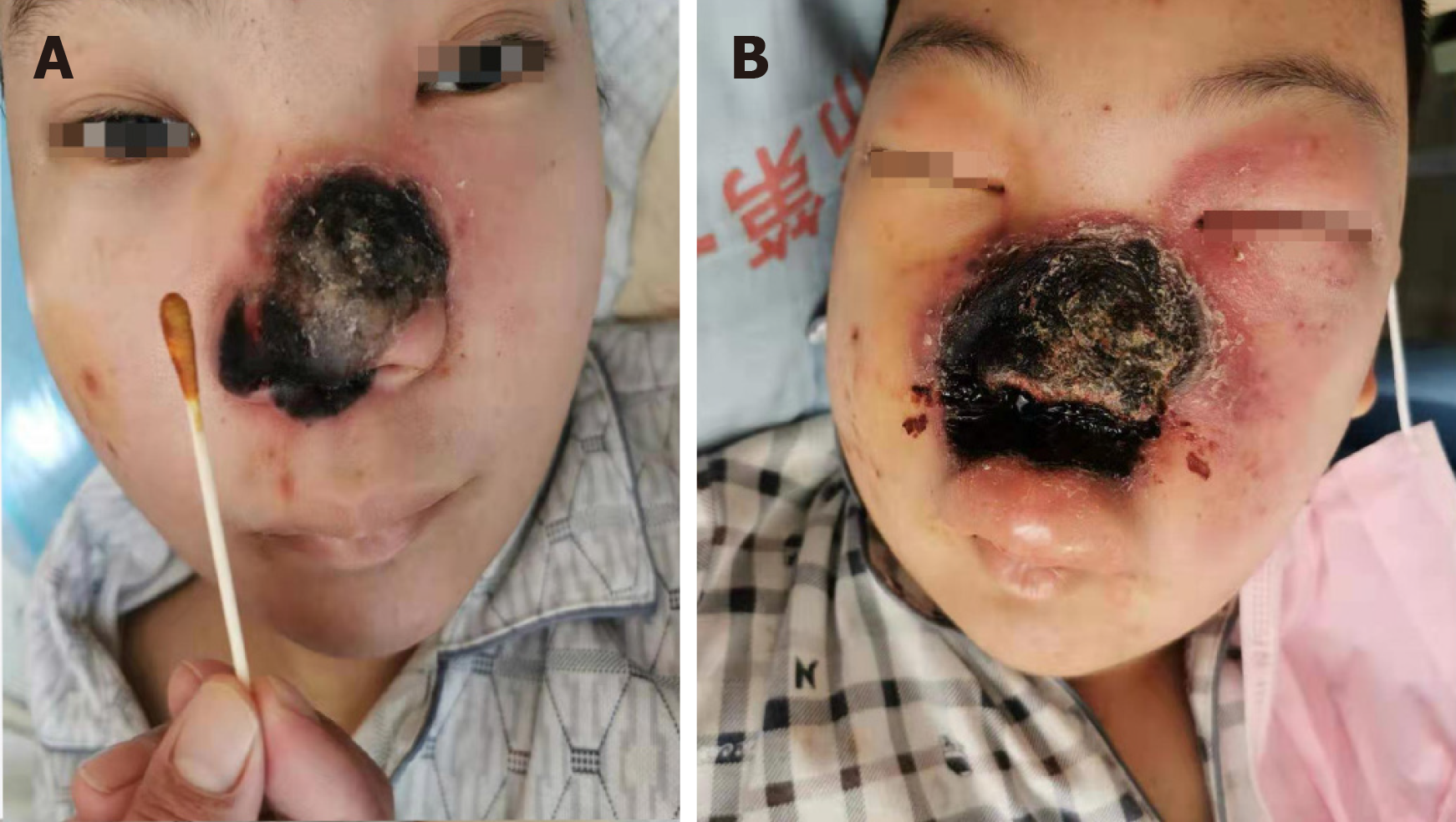
At day 0, the Medical Fungus Laboratory of the First People's Hospital of Jining was called in for a first consultation. The examination found that the lesion area was about half the size of the patient's palm at that time. The black scab surface was dry with active bleeding and swelling on the central area of the face (Figure 1). The left nasal cavity of the patient was filled with brown-red viscous liquid, and the left naris was almost occluded.
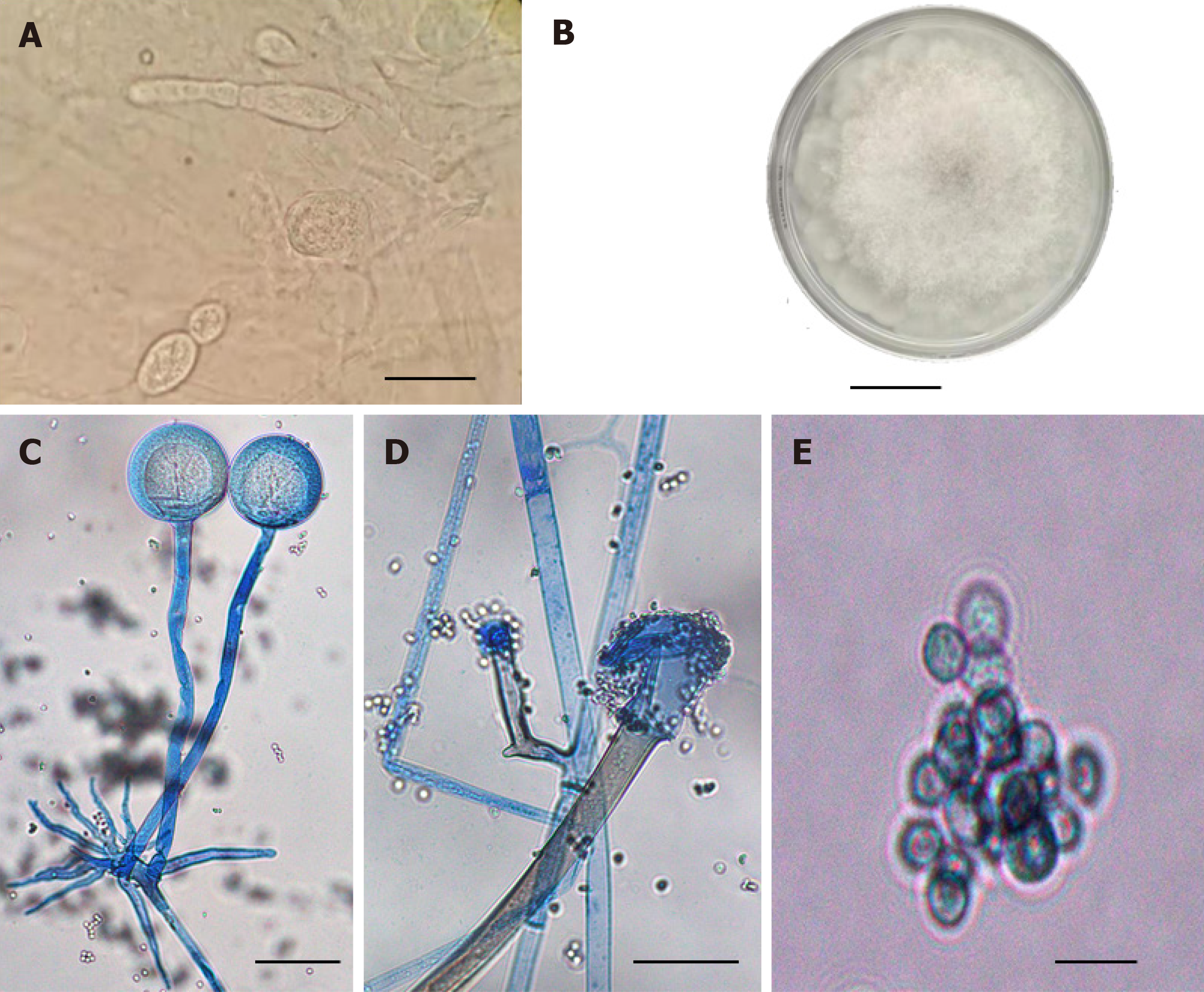
At day 0, after cleaning a few times with aseptic disposable swabs, smear samples were collected from the secretions outside and inside the nasal cavity. Under a microscope, thick and undivided hyphae, spherical sporangia-like structures, and spores were seen that were similar to the morphological characteristics of Mucorales (Figure 2B).
At day 0, the secretion samples from deep tissues were cultured at 33 °C on SDA and PDA media with chloramphenicol and actinomycin, respectively. The fungal isolate grew fast in both media and grayish-white filaments completely filled the 10 cm petri dish in 3 d (Figure 2A), forming typical non-septate or sparsely septate hyphae, spore-filled sporangia, sporangiophores, and rhizoids (Figure 2C-E). The unambiguous rhizoids (Figure 2C) are root-like structures arising from stolon hyphae opposite to sporangia.
At day +3, the identity of the isolated agent was further confirmed by sequencing of the ITS1/ITS4 region of rRNA. When compared with reference sequences in GenBank, our target sequence obtained a 100% coverage and 98.9% homology with R. oryzae No. F-22, and a 99% coverage and 99.05% homology with R. oryzae No. su-b3. Our sequencing data can be accessed in GenBank with registration number MN8419196.
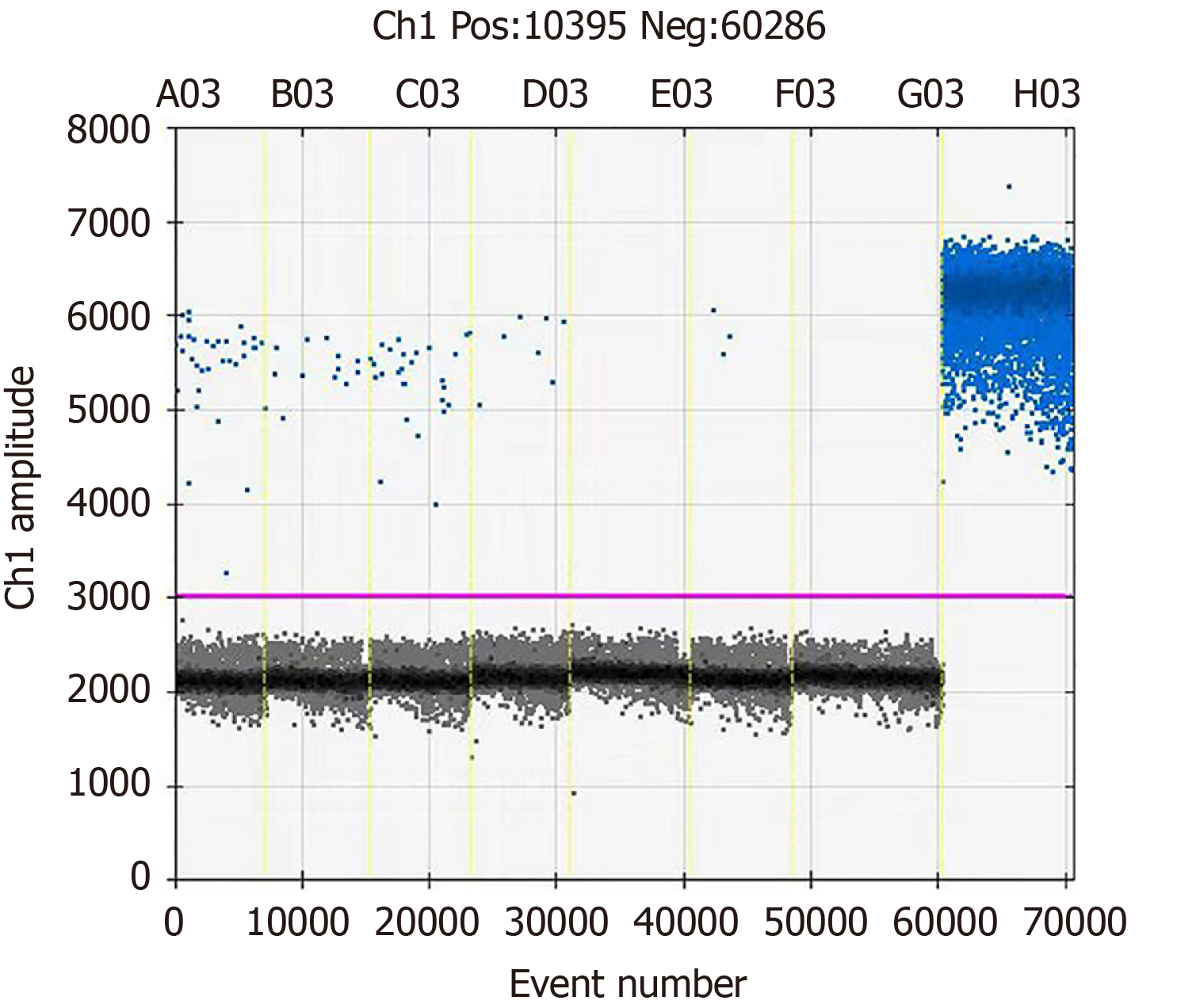
At day +2, to determine the possibility of fungemia, a venous blood sample was also collected, and fungal genomic DNA was detected following the whole blood DNA extraction and the droplet quantitative polymerase chain reaction (PCR) technology. The primers and corresponding fluorescent probes for fungal identification based on the 18S sequence are: Forward primer: 5'-TTGGTGGAGTGATTTGTCTGCT-3’; reverse primer: 5'-TCTAAGGGCATCACAGACCTG-3'; probe: FAM-TTAACCTACTAAATAGTGCTGCTAGC-BHQ1. However, the PCR result was negative (Figure 3).
At day +6, R. oryzae was detected a second time in culture in a second nasal sample set.
At day -10, after the first suspicion of fungal infection, a culture of extranasal secretion identified Aspergillus fumigatus, a serological test for Aspergillus was positive, and fungal D-glucan was 72.17 pg/mL. Intravenous injection of 50 mg caspofungin once a day was added to the regimen for a presumed Aspergillus infection, and platelet count was extremely low (1 × 109 platelet per liter).
Craniocerebral computed tomography (CT) examination was performed when the patient first developed symptoms, but no obvious abnormality on brain CT was found in the early stages of infection. Since platelet count was extremely low (1 × 109 platelet per liter), the collapsed soft tissue on the nose began to exhibit profuse bleeding when touched. Due to this infection and the primary disease, the patient was extremely weak. Since the hospital facilities were limited, a bedside CT examination was not possible. In addition, for purely economic reasons, patients with long-term illnesses and a poor prognosis for the primary disease are often exempted from more invasive tests (and expense) if the patient and the family agree. So further biopsy and/or histopathological examination and brain magnetic resonance imaging were not performed.
Based on the clinical findings above, along with pathological findings, the patient was diagnosed with RCM.
At day 0, amphotericin B at a dosage of 25 mg/d was initiated under the suspicion of RCM and increased to 30 mg/d 3 d later, which did not prevent the necrotic progression as the lesion became enlarged by another 33% and sharp pain began which required sedatives to relieve. The central area of the patient's face became swollen, spreading to the orbit and upper lip (Figure 1A). At day +6, however, the consistency of the results from the two separate samplings and clinical manifestation all supported RCM in this patient. Systemic amphotericin B was then increased to 35 mg per day and cold wet compress with amphotericin B was topically applied twice a day. After this high dosage of amphotericin B for 5 d, the affected area ceased to expand and the swelling on the periorbital and upper lip of the patient was also reduced.
Unfortunately, the patient died of circulatory failure due to the primary disease at day +21.
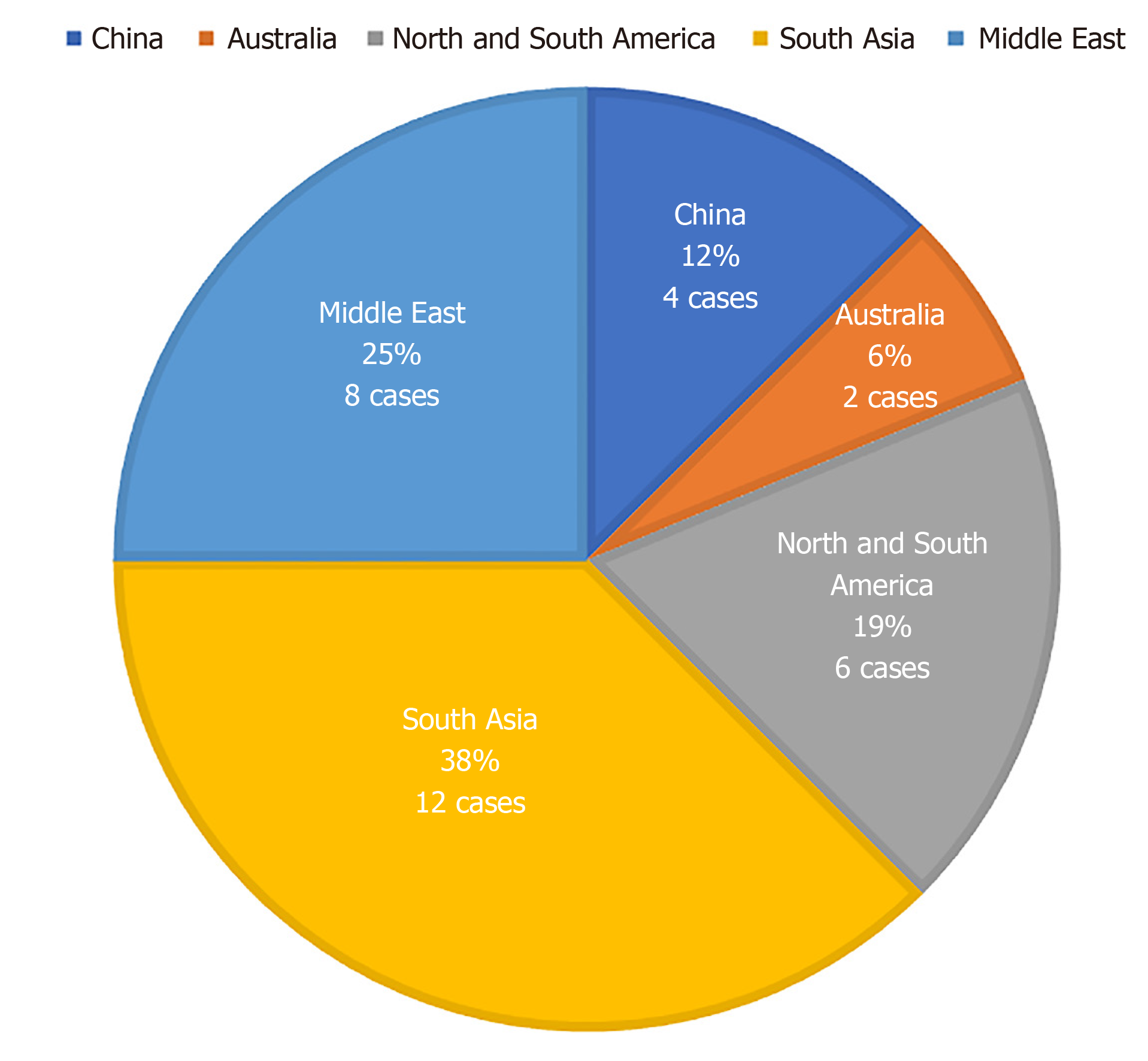
Mucormycosis is an opportunistic and highly invasive fungal infection. The mortality rate is as high as 50%-85% and rises to 100% in the disseminated type when untreated[2-4]. It tends to occur in patients with hematological malignancies and in patients undergoing both hematopoietic cell transplantation and solid organ transplantation[5,6]. Cutaneous, pulmonary, rhinofacial, and disseminated mucormycosis are common clinical types. In a study of patients with hematological malignancies, mucormycosis in the lung and orbital sinuses appeared in 64% and 24% of the cases, respectively, while brain involvement and disseminated infection appeared in only 19% and 8% of cases, respectively[7,8]. There are few reports of human infections due to R. oryzae. The regional distribution of cases reported in the last 5 years (Figure 4) shows that the Middle East and South Asia are two areas with a high incidence of R. oryzae-related infections. By searching the relevant case reports at home and abroad with the keywords “Rhinocerebral mucormycosis” and ”Rhizopus oryzae“, we found 14 cases of R. oryzae infection confirmed by molecular identification and effective treatment[9-17] (Table 1).
| Case | Clinical presentation | Clinical symptoms | Underlying disease | Treatment |
| 1 | Rhinocerebral mucormycosis | Headache, nosebleed | Hypertension and renal insufficiency | Posaconazole[9] |
| 2 | Rhinocerebral mucormycosis | Nasal soft tissue necrosis | Ulcerative colitis and immunosuppressive therapy | Liposomal amphotericin B[10] |
| 3 | Rhinocerebral mucormycosis | Sinusitis | Leukemia and iatrogenic diabetes | Amphotericin B[11] |
| 4 | Rhinocerebral mucormycosis | Sinusitis | Leukemia and iatrogenic diabetes | Surgical operation and amphotericin B[11] |
| 5 | Rhinocerebral mucormycosis | Nasal soft tissue necrosis | Chemotherapy of lymphoma | Posaconazole[11] |
| 6 | Rhinocerebral mucormycosis | Orbital swelling | Chemotherapy of lymphoma | Posaconazole[11] |
| 7 | Rhinocerebral mucormycosis | Nasal soft tissue necrosis | Leukemia | Liposomal amphotericin B[12] |
| 8 | Rhinocerebral mucormycosis | Headache and nosebleed | Diabetes | Amphotericin B[13] |
| 9 | Rhinocerebral mucormycosis | Nasal soft tissue necrosis | Diabetes | Posaconazole[14] |
| 10 | Rhinocerebral mucormycosis | Turbinate and orbital swelling | Leukemia | Liposomal amphotericin B[14] |
| 11 | Rhinocerebral mucormycosis | Turbinate and orbital swelling | Diabetes | Liposomal amphotericin B and posaconazole[15] |
| 12 | Rhinocerebral mucormycosis | Sinusitis and turbinate swelling | Chemotherapy of malignant tumor and diabetes | Liposomal amphotericin B and posaconazole[15] |
| 13 | Rhinocerebral mucormycosis | Nasal swelling and turbinate necrosis | Diabetes | Liposomal amphotericin B[16] |
| 14 | Rhinocerebral mucormycosis | Nosebleed, facial swelling | Lymphoma | Amphotericin B[17] |
Among these 14 cases, 5 had nasal and peripheral soft tissue necrosis, 6 had soft tissue swelling, 2 had sinusitis, and 1 had headache and nosebleed as initial manifestations. The underlying and possibly aggravating conditions of the patients were also reviewed, and we found 4 cases involving diabetes alone and 5 cases with malignant tumors such as leukemia or lymphoma, of which 3 were receiving chemotherapy at the time of infection. There were also 3 cases of diabetes mellitus complicated with leukemia or other malignant tumors, 1 case of hyperlipidemia and renal insufficiency, and 1 case of immunosuppressive therapy for ulcerative colitis. The above data show that diabetes, leukemia, and malignant tumors are the most common risk factors for RCM induced by R. oryzae. At the same time, swelling and necrosis of the nose and surrounding soft tissue was the most common initial manifestation. Therefore, patients with the above risk factors should be screened immediately once the corresponding clinical symptoms appear, and it is necessary to rule out Mucor fungal infections such as R. oryzae. The treatment regimens of the 14 cases were reviewed. Eight cases were treated with amphotericin B or liposome amphotericin B, 4 treated with posaconazole alone, and 2 with a combination therapy. This shows that amphotericin B and liposome amphotericin B are the most commonly used and effective drugs in the treatment of RCM caused by R. oryzae, but their nephrotoxicity cannot be ignored. Therefore, posaconazole and other drugs may have higher application value in infected patients with renal insufficiency. However, incomplete knowledge of this disease and a lack of diligence in tracing the root cause pathogen may mislead clinicians as to the low incidence of this pathogen in China. We hope that this case report will strengthen the awareness of such diseases.
Vascular invasion that causes necrosis of the infected tissue is one of the most frustrating features of this disease[18]. Rhinocerebral infection is usually induced by fungal spores in the air that spread to the orbital or intracranial structures through direct invasion or through blood vessels. Clinically, this case caused by R. oryzae had all the typical clinical manifestations of RCM. At first, it exhibited an erythematous and painful nodule in the nose and the surrounding soft tissue, which often leads to a history of broad-spectrum antibiotic treatment and then rapidly deteriorates to the formation of a black scab on top of the lesion and abundant purulent dark-red secretions in the nasal cavity. The above clinical symptoms are consistent with the most common initial symptoms in the case review. Prominent symptoms of vascular invasion and extranasal expansion[19] were also presented in our patient, including fever, facial edema, ophthalmoplegia, exophthalmos (proptosis of the eyes), nervous system defects, and complete blindness. Besides the leukopenia in this case, other risk factors may promote mucormycosis development. First, glucocorticoids had long been used in this patient for leukemia treatment, which led to relatively high levels of blood sugar. Second, the failure of hematopoietic function due to bone marrow suppression caused an accumulation of free iron molecules in the body, which was demonstrated by blood tests. The promoted growth of R. oryzae by high iron and low pH in the blood of patients with hyperglycemia and acidosis has been reported elsewhere[20,21]. High concentrations of glucose also increased the expression of GRP78 in endothelial cells, which assisted in pathogen attachment and invasion via the blood vessels[21]. Third, the unconfirmed Aspergillus and confirmed R. oryzae were secondary to a broad-spectrum antibiotic treatment to counteract a multiple-drug resistant form of E. coli. Although the use of broad-spectrum antibiotics has not always been clearly recorded in the case review, based on the clinical characteristics of our patient, such risk factors should excite greater concern. At present, the generally accepted standard of diagnosis of RCM is etiology or histopathology[22,23]. However, the ordinary histopathology could not be carried out in this patient due to the low coagulation function of the blood. The broad and non-septate hyphae, characteristic round or nearly round sporangia, and rhizoid structures shown in smear or/and culture samples collected at multiple times, all strongly suggested mucormycosis. The molecular identification confirmed the causative agent as R. oryzae.
The relevant imaging examination gave no specific clues at the onset of disease in our case. Even through imaging examination for diagnosis of RCM can be helpful, such an imaging examination often lacks typical features at the early stages of the disease. Typically, a large amount of low-density inflammatory exudates could be seen in the paranasal sinuses that can break through the periorbital bone wall, leading to corresponding bone destruction in the examination.
Test for fungal pathogens in venous blood samples of the patient was negative using droplet digital PCR technology in this study. The sensitivity of this PCR method has been noted in the early diagnosis of some systemic fungal infections[24], and our result did exclude a disseminated mucormycosis, and it is possible that the formation of micro thrombus in the local nasal area may have hindered the entry of the fungal cells into the peripheral circulation. For RCM caused by R. oryzae, the presence of fungal infection may be confirmed earlier by droplet digital PCR of the secretion from the infection site; particularly in the case of culture of slow-growing fungi, this technique has irreplaceable advantages.
Comprehensive treatment can significantly reduce the mortality of this disease. According to the prospective analysis, the effective treatment would include early detection, timely treatment, active resection, intravenous injection of amphotericin B, and improvement of underlying conditions for mucormycosis rhinocephalus[25]. Even through the primary ischemic necrosis may lead to fatal bacterial infection and early surgical treatment is often very necessary, surgical treatment was obviously not an option in our patient due to his poor coagulation function. We chose intravenous injection and topical application of amphotericin B for treatment, which in fact slowed down the deterioration of the skin although the patient could not survive his primary condition. Polyene amphotericin B alone[26], posaconazole and micafungin alone or in combination have all been recommended with good effects on mucormycosis caused by Rhizopus infection[9,27]. However, one study on R. oryzae has identified mutations in the CYP51A gene and other related genes, which may increase the natural resistance of R. oryzae to azole drugs[28] and may explain the transient Aspergillus isolation in our case. The R. oryzae in this patient was solely cultured positively one week post voriconazole and caspofungin treatment. There is a possibility that voriconazole combined with caspofungin was insufficient for this patient with mixed Aspergillus and Rhizopus infection. We infer that our patient may have mutations in CYP51A. The final mucormycosis in our patient was thus derived from an ineffective initial treatment after the Aspergillus was well controlled. Therefore, we should be more cautious in the treatment of R. oryzae with posaconazole and micafungin. In view of this, one should attach great importance to the etiological diagnosis of RCM in immunodeficient groups, because such patients may have multiple fungal infections, and a clear etiological diagnosis is essential for determining correct antifungal treatment.
RCM caused by R. oryzae is a relatively rare disease. Through more case reports, clinicians will better understand this intractable disease. The early detection and etiological diagnosis with early use of amphotericin B - supplemented with lesion removal and supportive treatment for any primary diseases – will significantly reduce the mortality of this fungal infection.
Manuscript source: Unsolicited manuscript
Specialty type: Dermatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moschovi MA S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Kwon-Chung KJ. Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clin Infect Dis. 2012;54 Suppl 1:S8-S15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 2. | Hammond SP, Marty FM, Bryar JM, DeAngelo DJ, Baden LR. Invasive fungal disease in patients treated for newly diagnosed acute leukemia. Am J Hematol. 2010;85:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Auberger J, Lass-Flörl C, Ulmer H, Nogler-Semenitz E, Clausen J, Gunsilius E, Einsele H, Gastl G, Nachbaur D. Significant alterations in the epidemiology and treatment outcome of invasive fungal infections in patients with hematological malignancies. Int J Hematol. 2008;88:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Turner JH, Soudry E, Nayak JV, Hwang PH. Survival outcomes in acute invasive fungal sinusitis: a systematic review and quantitative synthesis of published evidence. Laryngoscope. 2013;123:1112-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 5. | Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1793] [Cited by in RCA: 1966] [Article Influence: 98.3] [Reference Citation Analysis (1)] |
| 6. | Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 996] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 7. | Pagano L, Offidani M, Fianchi L, Nosari A, Candoni A, Picardi M, Corvatta L, D'Antonio D, Girmenia C, Martino P, Del Favero A; GIMEMA (Gruppo Italiano Malattie EMatologiche dell'Adulto) Infection Program. Mucormycosis in hematologic patients. Haematologica. 2004;89:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Vidovic A, Arsic-Arsenijevic V, Tomin D, Djunic I, Jakovic R, Loncar Z, Barac A. Proven invasive pulmonary mucormycosis successfully treated with amphotericin B and surgery in patient with acute myeloblastic leukemia: a case report. J Med Case Rep. 2013;7:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Mohammadi R, Nazeri M, Sayedayn SM, Ehteram H. A successful treatment of rhinocerebral mucormycosis due to Rhizopus oryzae. J Res Med Sci. 2014;19:72-74. [PubMed] |
| 10. | Najafi N, Kermani F, Gholinejad Ghadi N, Aghili SR, Seifi Z, Roilides E, Shokohi T. Fatal rhinocerebral mucormycosis in a patient with ulcerative colitis receiving azathioprine and corticosteroid. Curr Med Mycol. 2019;5:37-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Muggeo P, Calore E, Decembrino N, Frenos S, De Leonardis F, Colombini A, Petruzziello F, Perruccio K, Berger M, Burnelli R, Zanazzo GA, Santoro N, Cesaro S. Invasive mucormycosis in children with cancer: A retrospective study from the Infection Working Group of Italian Pediatric Hematology Oncology Association. Mycoses. 2019;62:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | El-Mahallawy HA, Khedr R, Taha H, Shalaby L, Mostafa A. Investigation and Management of a Rhizomucor Outbreak in a Pediatric Cancer Hospital in Egypt. Pediatr Blood Cancer. 2016;63:171-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Mulki R, Masab M, Eiger G, Perloff S. Lethargy and vision loss: successful management of rhinocerebral mucormycosis. BMJ Case Rep. 2016;2016:bcr2016215855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Langford S, Trubiano JA, Saxon S, Spelman D, Morrissey CO. Mucormycete infection or colonisation: experience of an Australian tertiary referral centre. Mycoses. 2016;59:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | El Zein S, El-Cheikh J, El Zakhem A, Ibrahim D, Bazarbachi A, Kanj SS. Mucormycosis in hospitalized patients at a tertiary care center in Lebanon: a case series. Infection. 2018;46:811-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Erami M, Shams-Ghahfarokhi M, Jahanshiri Z, Sharif A, Razzaghi-Abyaneh M. Rhinocerebral mucormycosis due to Rhizopus oryzae in a diabetic patient: a case report. J Mycol Med. 2013;23:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Hilal AA, Taj-Aldeen SJ, Mirghani AH. Rhinoorbital mucormycosis secondary to Rhizopus oryzae: a case report and literature review. Ear Nose Throat J. 2004;83:556, 558-560, 562. [PubMed] |
| 18. | Artal R, Agreda B, Serrano E, Alfonso JI, Vallés H. [Rhinocerebral mucormycosis: report on eight cases]. Acta Otorrinolaringol Esp. 2010;61:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Rajagopalan S. Serious infections in elderly patients with diabetes mellitus. Clin Infect Dis. 2005;40:990-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Gebremariam T, Lin L, Liu M, Kontoyiannis DP, French S, Edwards JE, Filler SG, Ibrahim AS. Bicarbonate correction of ketoacidosis alters host-pathogen interactions and alleviates mucormycosis. J Clin Invest. 2016;126:2280-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Liu M, Spellberg B, Phan QT, Fu Y, Fu Y, Lee AS, Edwards JE, Filler SG, Ibrahim AS. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest. 2010;120:1914-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 22. | Parikh SL, Venkatraman G, DelGaudio JM. Invasive fungal sinusitis: a 15-year review from a single institution. Am J Rhinol. 2004;18:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 23. | Lerchenmüller C, Göner M, Büchner T, Berdel WE. Rhinocerebral zygomycosis in a patient with acute lymphoblastic leukemia. Ann Oncol. 2001;12:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Li HT, Lin BC, Huang ZF, Yang CZ, Huang WM. [Clinical value of droplet digital PCR in rapid diagnosis of invasive fungal infection in neonates]. Zhongguo Dang Dai Er Ke Za Zhi. 2019;21:45-51. [PubMed] |
| 25. | Ramadorai A, Ravi P, Narayanan V. Rhinocerebral Mucormycosis: A Prospective Analysis of an Effective Treatment Protocol. Ann Maxillofac Surg. 2019;9:192-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Chen CY, Wen H. Antifungal mechanism of amphotericin B and its liposomes. Zhongguo Zhenjunxue Zazhi. 2006;5:312-314. |
| 27. | Chamdine O, Gaur AH, Broniscer A. Effective treatment of cerebral mucormycosis associated with brain surgery. Pediatr Infect Dis J. 2015;34:542-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Macedo D, Leonardelli F, Dudiuk C, Theill L, Cabeza MS, Gamarra S, Garcia-Effron G. Molecular Confirmation of the Linkage between the Rhizopus oryzae CYP51A Gene Coding Region and Its Intrinsic Voriconazole and Fluconazole Resistance. Antimicrob Agents Chemother. 2018;62:e00224-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |