INTRODUCTION
The role of the gut microbiome as an important determinant of human health and disease has emerged as an exciting niche of research in many areas of medicine. An imbalance in the gut microbiome has been linked to obesity, type 2 diabetes, atopy and inflammatory bowel disease (IBD)[1]. Furthermore, the relationship between normally residing intestinal bacteria (the gut microbiota) and their potential role in the pathogenesis of skin diseases is an area of research for which we are only now starting to gain an understanding. The small and large intestines provide residence for a vast community of bacteria and their metabolites and by-products, which we call the gut microbiome. Similarly, thousands of microbial organisms and their by-products inhabit the skin, referred to as the skin microbiome. In both the gut and the skin, a harmonial balance in these microflora is important in maintaining homeostasis[2]. The skin and the gut have more similarities than one would suppose, and in fact, there is budding interest in learning how the skin and gut communicate and influence the health of one another[3]. Both contain rich vascular supply, diverse microbial communities, and act as vital interfaces between the internal human body and the external environment. Additionally, the skin and gut both operate as neuro-immuno-endocrine organs, and participate in essential communication with the nervous system, immune system, and endocrine system. The “brain-gut axis” has been documented extensively in the literature, and was first described in 1930 when Stokes and Pillsbury attributed depression to altering the gut microbiome, leading to inflammatory skin diseases[4]. However, the “skin-gut axis” is a newly emerging and important avenue of investigation, still sparse in pathobiological explanations. This review will introduce and describe the intestinal microbiome as it relates to skin health in a complex communication network between the immune system, endocrine system, metabolic system, and nervous system.
HUMAN INTESTINAL MICROBIOME
The “gut microbiome” refers to the diverse community of microbial organisms that normally inhabit the bowel and their metabolites/byproducts[5]. There are more than 100 trillion bacteria present in the human gastrointestinal tract, consisting of over one thousand different species colonizing the intestines[6,7]. A large proportion of the organisms found in the gut microbiome belong to two phyla: Firmicutes and Bacteroidetes[5].The density of the bacterial populations within the bowel differs by anatomical location. For instance, the density is approximately 102-3 colony forming units (CFU) per gram in the proximal ileum and jejunum, compared to the ascending colon which has approximately 1011-12 CFU per gram[8]. There is significant variation in the gut microbiome communities among healthy individuals[9]. The gut microbiome is relatively stable, however, studies have demonstrated that antibiotic therapy, international travel, and illness can all alter the normal gut microbiome. Aging can also lead to a shift in the predominant species within the gut microbiome. Research currently suggests that our long-term dietary patterns could have a large impact on the composition of our gut microbiome[6].
The role of the gut microbiome is thought to include proper development and functioning of the immune system, protection against infections, digestion of polysaccharides, and synthesis of vitamins[7]. The symbiotic relationship between resident gut bacterial flora and the host is vital to the normal immune system development and homeostasis of the host and regulation of epithelial growth and differentiation[10].
PROBIOTICS/PREBIOTICS
Probiotic supplementation has become increasingly popular, with many commercially available products in capsule, powder, beverage, and food forms. According to the Food and Agricultural Organization of the United Nations and the World Health Organization, probiotics are considered to be “live microorganisms which when administered in adequate amounts confer a health benefit on the host”[11]. The most frequently used bacteria are from the Lactobacillus and Bifidobacterium genera[12]. There has been evidence to suggest that they are useful in the treatment of irritable bowel syndrome (IBS), diarrhea, and lactose intolerance[13]. Probiotics may alleviate abnormal alterations of the gut microbiome, referred to as “dysbiosis”. Dysbiosis of the gut microbiome has been linked to metabolic disorders, gastrointestinal infections, IBD, and irritable bowel syndrome (IBS)[14]. Probiotics are thought to provide therapeutic benefits via multiple mechanisms. Firstly they are believed to prevent pathogenic bacteria from colonizing the gastrointestinal tract, which would otherwise subsequently lead to disease. Secondly, they are thought to improve the barrier function of the colonic mucosa. Thirdly, probiotics may help modulate the immune system, which may help shift away from pro-inflammatory immune reactivity[12]. Fourth, they may synthesize and secrete metabolites that may have nutritional benefits and anti-inflammatory effects[15]. Lastly, probiotics may even play a role in modulating central nervous system and enteric nervous system functions. In fact, in a randomized controlled trial patients with Alzheimer’s disease who received probiotic supplementation for 12 wk had significant improvement in mental status score and had a significant decrease in serum c-reactive protein (Akbari, 2016 #991). Additionally, probiotic supplementation has demonstrated improvement in multiple sclerosis symptoms and exacerbations (Dolan, 2016 #992).
Probiotics have not yet been widely studied in the treatment of dermatological diseases. Two meta-analyses failed to demonstrate any clinically significant changes in the severity of atopic dermatitis (AD) in children treated with probiotic supplementation[16,17]. However, Lee et al[16] found a significant risk reduction (up to 61%) of pediatric AD in those who were treated with prenatal and/or postnatal probiotics. There are even fewer studies available regarding the treatment of adults with AD using probiotics. These small studies have demonstrated that there may be a clinical benefit in adults[18-20]; however, larger trials are needed before any conclusions can be drawn. Probiotics are postulated to help in atopic dermatitis by improving the diversity of the intestinal flora, increase the barrier function of the skin and mucosa and by producing a mainly Th1 response[13].
Prebiotics are non-digestible carbohydrates that help stimulate the growth of certain bacteria in the gut, which can lead to an improvement in the health of the host[21]. A review by Osborn et al[22] of four clinical trials found that there was a statistically significant reduction in the incidence of infant eczema with prebiotic supplementation of galactoligosaccharides and fructooligosaccharides (RR 0.68). It has been demonstrated that milk glycoproteins are able to select for and stimulate the growth of Bifidobacteria longum infantis (B. infantis) in the gut microbiome[23]. This is of clinical importance as B. infantis supplementation can reduce the risk of necrotizing enterocolitis in preterm infants. B. infantis colonization of the gastrointestinal tract is associated with improved immune response to vaccination and weight gain[24].
However, further studies need to be conducted into the use of prebiotics and probiotics before recommendations regarding their use in the treatment or prevention of dermatological diseases can be made.
LINK BETWEEN SKIN DISEASE AND THE GUT
Gastrointestinal disorders can present with dermatological skin findings. IBD is linked to skin manifestations such as pyoderma gangrenosum, erythema nodosum, Sweets Syndrome and oral lesions[23]. Celiac disease is associated skin manifestations such as dermatitis herpetiformis, alopecia, vitiligo and oral mucosal lesions. Furthermore, psoriasis is more commonly found in patients with Crohn’s disease than healthy people[24].
There is emerging evidence linking certain dermatological disorders to gut dysbiosis. However, this is not a novel topic and in fact, in 1911 a gastroenterologist named Milton H. Mack wrote, “Acne and eczema are both traceable to this fountainhead of diseases… if in a case of urticarial we look to the intestinal track, why not in eczema and acne?” [25]. Simultaneous gut and skin microbiome dysbiosis has been observed in several inflammatory skin diseases, such as rosacea, psoriasis, and atopic dermatitis[26].
Psoriasis
Interestingly, patients with psoriatic arthritis are at increased risk of developing IBD and have subclinical evidence of gut inflammation[27]. A recent clinical study including 16 patients with psoriatic arthritis, 15 with psoriasis and 17 healthy controls analysed the gut microbiome across these three groups. The gut microbiome was less diverse in the psoriasis and psoriatic arthritis groups; with a decrease in the Coprococcus spp. Those with psoriatic arthritis experienced a reduction in important bacterial enterotypes such as Akkermansia, Ruminococcus, and Pseudobutyrivibrio. It is thought that these taxanomic changes cause to a reduction in the ability of the gut to regulate immune responses, which may lead to systemic or localized inflammation[28].
In addition, a clinical trial has shown that treating psoriasis patients with probiotic Bifidobacterium infantis 35624 for eight weeks improved C-reactive protein (CRP), TNF-alpha and IL-6 levels. However, during this study no clinical assessments were performed after baseline. These results suggest that probiotic supplementation could modulate inflammation in this disorder[29].
Rosacea
Rosacea has been linked to Helicobacter pylori (H. pylori) infection, however the efficacy of H. pylori eradication in rosacea therapy is unclear[30]. Moreover, a study of 113 rosacea patients demonstrated that those with rosacea have a higher incidence of small intestinal bacterial overgrowth (SIBO) when compared to controls. Those with SIBO were treated with either rifaximin therapy for 10 d or placebo. Those who were treated with antibiotic therapy experienced an improvement in their symptoms for at least nine months[31].
Atopic dermatitis
There is a well-documented association between gut microbiome dysbioses and low diversity within the gut microbiota with the development of allergic diseases (Melli, 2016 #993). Conversely, increased microbial diversity within the gut has been associated with reduced flares in inflammatory skin diseases, such as atopic dermatitis (Marrs, 2016 #994).
PROPOSED MECHANISMS REGARDING THE SKIN-GUT AXIS
At present, there is clinical evidence suggesting a close relationship between intestinal dysbiosis and dermatologic conditions. However, the mechanistic basis behind these observations has yet to be confirmed. The association between the gut and skin likely involves a complex and multifactorial interplay between the nervous, immune, and endocrine systems as well as environmental factors such as diet and medications (Figure 1).
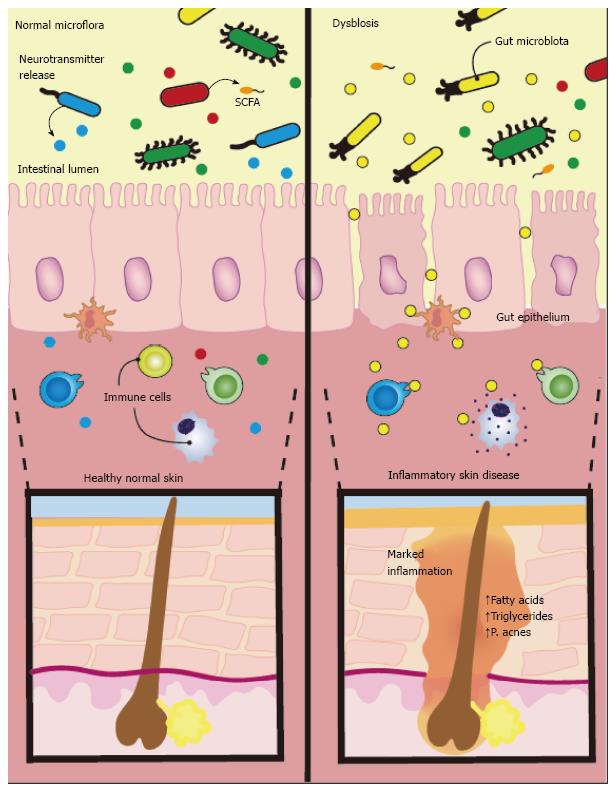
Figure 1 There is emerging evidence linking dermatological disorders to alterations in gut bacteria.
Studies hypothesize intestinal flora produce neurotransmitters in response to stress that can modulate skin function. These neurotransmitters cross the intestinal epithelium enter the bloodstream and induce systemic effects. Along with neurotransmitters, the gut microflora also release short chain fatty acids (SCFAs), which can also enter systemic circulation and affect the skin. Additionally, diet may influence inflammation in the skin though nutrient signalling and release of long chain fatty acids, leading to excessive stimulation of sterol regulatory element-binding protein 1 and increased synthesis of fatty acids and triglycerides promoting Propionibacterium acnes overgrowth.
Skin-gut axis and the neuroendocrine system
The “brain-gut-skin axis” has been eloquently documented by Arck et al[32] and Bowe and Logan[4]. It is known that psychosocial stress is implicated in both exacerbation and the initiation of various skin conditions[33]. It is plausible that the intestinal microflora produce neurotransmitters in response to stress and other external stimuli that could modulate skin function via neural pathways. For instance, commensal organisms in the gut can produce norepinephrine, serotonin, and acetylcholine or may evoke the release of neuropeptides from nearby enteroendocrine cells[34]. These neurotransmitters might cross the intestinal epithelium into the bloodstream and induce systemic effects[35]. Along with neurotransmitters, the gut microflora also release short chain fatty acids (SCFAs), including propionic acid, butyric acid, acetic acid, and lactic acid derived from polysaccharide fermentation from food we eat[36]. The majority of these SCFAs are produced in the large intestine, where the colon is highly efficient in the reabsorption of fatty acids, only allowing approximately 10% to remain in expelled feces[37]. The true systemic levels of SCFA derived from the colon depend on individual dietary habits, rate of SCFA production by gut microbes, and the degree of absorption through the large intestine. It is not known whether these metabolites, along with many others produced by gut microbes, are able to reach clinically significant levels in the bloodstream in order to impact the skin[38].
Immune system modulation
Health, including skin health and overall well being, require tightly integrated immune and hormone feedback systems that allow beneficial microbial to dominate in the gut and on the skin[39]. The normal gut microbial residents continuously interact with the immune system to support host homeostasis. In general, immune system homeostasis requires a proper balance of pro-inflammatory and anti-inflammatory signals and molecules in response to internal and external environmental changes. If the microbiome composition changes for any given reason, the immune system reactivity could subsequently shift and eventually lead to inflammatory skin diseases[40]. This idea was exemplified in a mouse study by Zanvit et al which demonstrated that mice treated with antibiotics neonatally had exacerbated imiquimod-induced psoriasis as an adult, while mice treated with the same antibiotics in adulthood had improved psoriasis (Zanvit, 2015 #990). This study demonstrates the importance of how neonatal gut dysbioses can affect skin inflammation, potentially triggering or exacerbating inflammatory skin diseases such as psoriasis later in adulthood. Interleukin-10 (IL-10) is generally considered to decrease pro-inflammatory molecules, such as IL-17[41]. Animal models have shown that probiotic supplementation up regulates IL-10 and provides beneficial skin effects[42]. In a recent article, Zákostelská et al[43] hypothesize that certain beneficial families of intestinal bacteria, such as lactobacilli, are able to supress the IL-23/Th17 axis, which is believed to play an important role in inflammation involved in psoriasis[43]. This suppression may occur through certain gut commensal organisms’ ability to down regulate IL-23 and transforming growth factor-beta (TGF-β) expression, and preventing Th17 cell-mediated release of proinflammatory IL-17[44]. As a result of immune system dysfunction and deficiency in T regulatory cells, some autoimmune diseases can result in rampant inflammation and severe dermatitis, such as in IPEX syndrome (Halabi-Tawil, 2009 #996). The intestinal microbiome is responsible for regulating the expansion of T regulatory cells, Th1 and Th2 type cells to provide immune system homeostasis, and there has been recent research investigating how treating the gut microbiome could improve these types of skin conditions (He, 2017 #995). These are examples demonstrating the complex interplay between the immune system and gut commensal organisms. The true connection between skin health and gut bacteria induced immune system reactivity is poorly understood and still requires more extensive investigation.
Diet
Recent research continues to reveal the influence of the “western diet” in the obesity epidemic, and researchers have hypothesized that alterations in the gut microbiome due to high dietary fat intake could be partly to blame (Murphy, 2015 #997). In the literature, it is generally accepted that high fat diets lead to gut dysbioses, reflected by a decrease in Bacteroidetes species and an increase in Firmicutes species (Zhang, 2012 #998). Although the exact mechanisms are still under investigation, “western diet” induced gut dysbioses may be associated with cancer (Schulz, 2014 #999), atherosclerosis and heart disease (Gregory, 2015 #1000), insulin resistance (Carvalho, 2012 #1001), and even disorders of the central nervous system (Scheperjans, 2015 #1002). Until recently, conflicting opinions and inconclusive evidence have predominated regarding the link between diet and skin conditions. Although more mechanistic studies are warranted, there is growing evidence that diet plays an important role in the pathogenesis of skin diseases, with acne vulgaris being an example. For example, the western diet consisting of large amounts of saturated fats and high glycemic load has been strongly associated with acne[45,46]. Researchers hypothesize this occurs from problems in nutrient signalling, ultimately leading to excessive stimulation of sterol regulatory element-binding protein 1 (SREBP-1) and increased synthesis of fatty acids (ex - free oleic acid) and triglycerides in sebum that promotes flourishing Propionibacterium acnes growth[47]. The strong association between atopic dermatitis and food sensitivities similarly exemplifies the importance of food on the gut-skin relationship[48]. The ability of diet to both positively and negatively influence skin function demonstrates the undeniable link between the skin and gut, however, the mechanisms surrounding this connection is likely multifactorial and at present based primarily on theory. Indeed, it is difficult to detangle the direct effects of food on the skin versus food’s modulation of the intestinal microflora.
CONCLUSION
The intimate relationship between the gut and skin is undeniable. Possibly, both the intestinal bacteria themselves and their metabolic by-products influence skin physiology. The mechanisms are still under study but there are a few theories: (1) bacterial products and diet could alter the physiology of the gut epithelium, resulting in different secretory products that might circulate systemically and reach the skin; (2) neurotransmitters, hormones, and other bioactive chemicals such as SCFAs derived from the gut could all act on receptors within the skin and directly alter the skin or alter the skin’s commensal bacteria; and (3) ingested compounds and chemicals may absorb and have a direct effect on the skin’s appearance or function[49].
Although not a new avenue of research, the relationship between the gut microbiome and skin health is emerging as an important and intriguing topic in dermatology and gastroenterology alike. It is especially important to understand how diet, medications, and psychosocial stress can influence or contribute to altered microbial communities in the gut, which may directly or indirectly affect skin health.
Manuscript source: Invited manuscript
Specialty type: Dermatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Antonakopoulos N, Rhoads JM S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ