Peer-review started: September 27, 2016
First decision: October 15, 2016
Revised: December 16, 2016
Accepted: January 11, 2017
Article in press: January 12, 2017
Published online: February 2, 2017
Processing time: 147 Days and 16.3 Hours
Vacuum-assisted closure, sometimes referred to as microdeformational wound therapy or most commonly negative pressure wound therapy (NPWT), has significantly improved wound care over the past two decades. NPWT is known to affect wound healing through four primary mechanisms (macrodeformation, microdeformation, fluid removal, and alteration of the wound environment) and various secondary mechanisms (including neurogenesis, angiogenesis, modulation of inflammation, and alterations in bioburden) which are described in this review. In addition, the technique has many established uses, for example in wound healing of diabetic and pressure ulcers, as well as burn and blast wounds. This therapy also has many uses whose efficacy has yet to be confirmed, for example the use in digestive surgery. Modifications of the traditional NPWT have also been established and are described in detail. This therapy has various considerations and contraindications which are summarized in this review. Finally, future perspectives, such as the optimal cycling of the treatment and the most appropriate interface material, are touched upon in the final segment. Overall, despite the fact that questions remain to be answered about NPWT, this technology is a major breakthrough in wound healing with significant potential use both in the hospital but also in the community.
Core tip: Negative pressure wound therapy has been very beneficial in the wound care of many different kinds of wounds, from pressure ulcers to open fractures mostly due to its mechanism of action which we explain in detail. We explain the original purpose of this technology and going into detail about the many different ways it is currently being used in a clinical setting. Our review also explains its advantages and disadvantages and how they could be overcome. The last part of this review discusses the future of this technology and how it will keep impacting the field of wound care.
- Citation: Panayi AC, Leavitt T, Orgill DP. Evidence based review of negative pressure wound therapy. World J Dermatol 2017; 6(1): 1-16
- URL: https://www.wjgnet.com/2218-6190/full/v6/i1/1.htm
- DOI: https://dx.doi.org/10.5314/wjd.v6.i1.1
Since its introduction 19 years ago by Argenta and Morykwas, negative pressure wound therapy (NPWT) has emerged as a common treatment for acute and chronic wounds, including diabetic wounds, pressure ulcers, and burns[1]. In simple terms, NPWT refers to any device that tightly seals the wound creating a near airtight environment to which a vacuum can be applied resulting in a series of biological reactions that enhance wound healing.
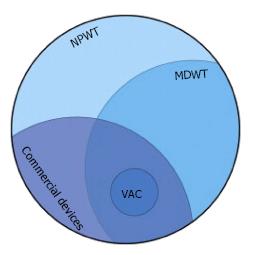
The terms Vacuum Assisted Closure (VAC, KCI, San Antonio, TX) and microdeformational wound therapy (MDWT) are sometimes used interchangeably with NPWT. MDWT refers to devices (generally foam) that substantially deform the wound surface[2]. VAC now commonly refers to a family of devices using a highly porous foam based on the first commercially available NPWT device. Much of the clinical and basic science literature is based on these early devices (Figure 1). “Negative pressure” is somewhat of a misnomer as technically all pressure values should be positive.
Research on the application of NPWT in treating chronic non-healing wounds has largely taken the form of case studies, single-center studies, non-randomized controlled trials, with few randomized controlled trials (RCTs). This paper will analyze the available literature in order to summarize the current understanding of NPWT in terms of its mechanism of action, its applications, complications, contraindications and its future.
In NPWT the wound is first filled with a porous material such as foam or gauze, that facilitates pressure transmission within the wound. A drainage port is then attached above the porous material and the wound is sealed with an adhesive film dressing. The drainage port is connected to a controlled vacuum pump which maintains negative pressure, usually ranging from -50 to -150 mmHg[2,3]. The pressure can be applied in a continuous, intermittent, or variable mode, with the continuous type being the most frequently used. In the variable mode, the suction level changes but is never turned off, whereas in the intermittent mode the pressure is switched on and off throughout the course of treatment.
The specific interface material that contacts the wound surface affects the biological response of the system. The most commonly used material is a reticulated open-pore polyurethane (PU) foam that forms a structure resembling a three-dimensional net. This lattice formation allows the vacuum to be evenly distributed throughout the foam and improves fluid drainage.
Three foam types are used in the VAC systems. Black polyurethane ether (VAC GranuFoam, KCI) is the most commonly used foam, and black polyurethane ester (VAC VeraFlow, KCI) is used in instillation systems. The white polyvinyl alcohol (VAC WhiteFoam, KCI) foam has very small pore sizes and is used to protect critical structures without inducing microdeformations, which will be discussed in the following section.
Usage of gauze in NPWT is based on the Chariker-Jeter method of application, which uses a moistened antimicrobial gauze (AMD; Covidien, Hampshire, United Kingdom) as a wound interface, along with 80 mmHg of negative pressure and a silicone drain[4]. In one retrospective study with a mixed group of patients with challenging wounds, gauze used as a wound filler material was found to achieve reductions in wound size and volume comparable with published data from polyurethane foam-based systems[5].
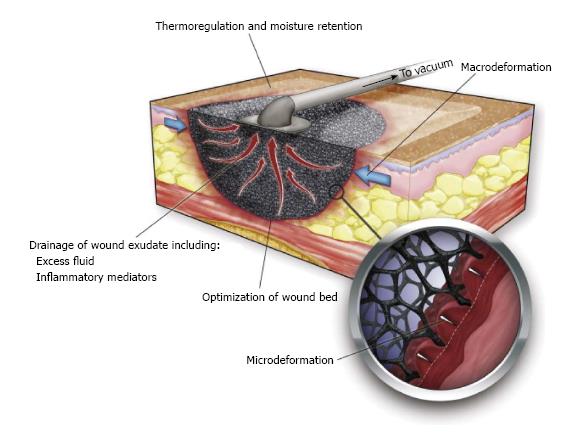
NPWT is thought to promote wound healing via four primary mechanisms: (1) macrodeformation; (2) microdeformation; (3) fluid removal; and (4) alteration of the wound environment (Figure 2).
Macrodeformation: Macrodeformation, or simply induced wound shrinkage, occurs when suction is applied to the foam causing pore collapse. This results in deformational forces being exerted on the wound edges, which draws them together. Macrodeformation can also induce compressive forces such as when these devices are used circumferentially on extremities[6,7].
Studies in a porcine model showed that suction of 125 mmHg can decrease the volume of a PU foam by approximately 80% resulting in a substantial shrinkage of the wound[8,9]. The extent of contraction is largely dependent on the deformability of the wound[2].
The inherent tension in the dermis, which can cause wound margins to pull apart, and the attachment of the dermis to underlying structures vary in different parts of the body. Consequently different wounds contract to different degrees. For example, scalp skin is constrained by attachments to the underlying skull resulting in minimal deformation of the surrounding tissue when a foam based NPWT device is applied. In contrast, when a large open abdominal wound in an obese patient is treated with a similar device, the wound edges can be brought together in close approximation.
Microdeformation: Microdeformation describes the mechanical changes that occur on the microscopic scale when suction is applied to the porous material resulting in an undulated wound surface. For PU foam interfaces, treating wounds for several days results in a cobble-stone appearance of the wound surface. Models that mimic the strain applied to a wound by the opposing forces of the suction and the sponge have been designed to investigate these mechanical changes. Using finite element analysis (FEA), these models have shown that at 110 mmHg, MDWT results in a 5%-20% strain across the wound surface. This strain directly corresponds to the percentage change in length of the material exposed to the external forces[10].
Mechanical forces, which include compression and tension from the foam, shear and hydrostatic forces from the extracellular fluid, and the effect of gravity, are transmitted throughout the tissue via the extracellular cell matrix (ECM). These forces vary greatly across the wound surface. For example, the tissue just underneath the foam struts is exposed to focal high compression, whereas the wound surface centrally in the pore is focally exposed to high tension[10]. Microdeformation is the morphology that occurs due to the interplay between these forces.
Shear forces affect the cytoskeleton and activate a signaling cascade that upregulates granulation tissue formation and, hence, enhances wound healing[11-13]. Furthermore, microdeformation is believed to stimulate vessel sprouting towards the wound[2]. This is described in further detail in the secondary effects of NPWT. Microdeformation causes localized hypoxia that causes an increase in local vascularity. Factors known to affect the efficiency of microdeformation include the level of suction, the pore size and the consistency of the foam, the tissue being treated and the deformability of the surrounding tissues.
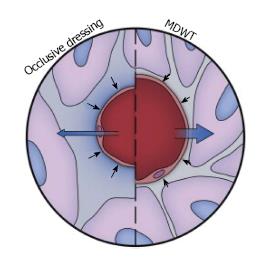
Fluid removal: Fluid accumulation in the extracellular space or edema, which often occurs in chronic wounds, inhibits healing by compressing local cells and tissues. For example, during wound healing in peripheral diabetic ulcers, cell proliferation occurs due to the intrinsic tension generated in the cells by the interaction of their cytoskeleton and the ECM[14]. Fluid accumulation in the extracellular space elevates pressure in the interstitium and inhibits proliferation by decreasing the buildup of intrinsic tension. Since the fluids in the extracellular space communicate with the surface of the wound, vacuum application can remove fluid from the wound. Depending on the type of wound, significant amounts of fluid can be removed, as is the case with open abdominal and fasciotomy wounds. By removing fluid, the compression forces acting on the microvasculature allow increased blood flow and perfusion of the tissue (Figure 3)[1]. The adhesive film dressing covering the wound is semipermeable and hence allows some air to enter the system preventing a fluid lock and enabling continuous fluid removal. Other devices have been designed to let a small amount of air into the system through a remote port.
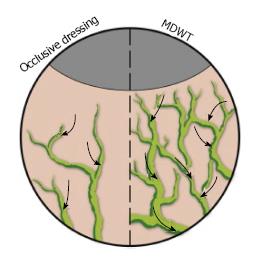
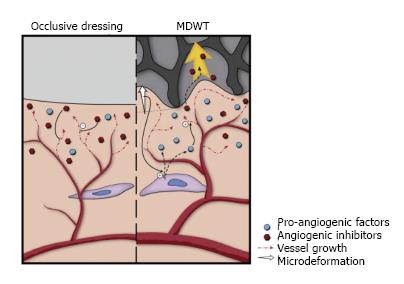
NPWT is believed to affect the lymphatic system via two mechanisms. First, since edema is cleared via the lymphatic system, by removing fluid, NPWT concurrently reduces the burden on the lymphatic system. Second, NPWT promotes lymphatic drainage by inducing a gradual increase in the density of the lymphatics at the wound edges (Figure 4)[15].
Alteration of the wound environment: When fluid is evacuated, electrolytes and proteins are removed that may stabilize osmotic and oncotic gradients at the wound surface[14]. The foam and drape act as insulators maintaining a warm wound environment[2]. The drape is semipermeable and helps maintain a sterile, moist environment by reducing wound contamination with microorganisms and minimizes water evaporation from the wound. In addition, NPWT can be more comfortable to patients by reducing the number of dressing changes. Special types of NPWT have been designed that serve to address specific issues in healing. For example, foam can be bound with antimicrobial silver or bioactive factors.
The four primary mechanisms of NPWT affect various wound healing processes including neurogenesis, hemostasis, angiogenesis, modulation of inflammation, cellular proliferation, differentiation, and migration, granulation formation, and alterations in bioburden.
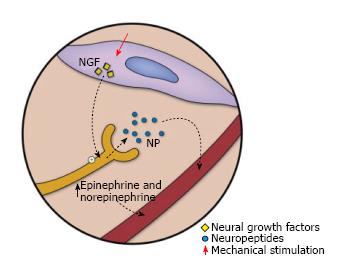
Neurogenesis: MDWT has been linked to enhanced neural growth and neuropeptide expression through upregulation of neurotrophin nerve growth factor, substance P, and calcitonin gene-related peptide[16]. Epinephrine and nonepinephrine show a transient elevation, which is followed by a slower but more long-lasting elevation of substance P and neuropeptide Y (Figure 5). Neuropeptides are believed to be key homeostatic factors in the skin which play a role in the secondary effects of NPWT. The extent of neurogenesis has been directly linked to the level of microdeformation. In addition, intermittent suction results in greater neurogenesis than continuous MDWT[16].
Hemostasis: NPWT is postulated to promote haemostasis via two mechanisms. First, the negative pressure is believed to constrict and occlude small blood vessels mechanically reducing hemorrhage. It should be noted that this constrictive effect persists even after negative pressure is discontinued. Second, compression due to negative pressure strongly apposes the dressings to the wound surface favoring clot formation by the gauze[17]. In terms of the appropriate usage of the device, suction is applied when hemostasis is nearly complete, taking special care in patients with coagulopathies. In addition to the filler material, dressing, connecting tube and the vacuum pump, most systems also have a fluid collection canister which sounds an alarm when full, alerting clinicians of excessive blood loss[18].
Angiogenesis and blood flow: MDWT treatment of chronic wounds results in increased microvessel density[19]. Microdeformation causes temporary hypoperfusion to the wound edge resulting in localized hypoxia of the tissues, subsequent upregulation of hypoxia-inducible factor-1α and in turn increased VEGF expression[20]. Ultimately this leads to increased angiogenesis (Figure 6). Similar results to angiogenic response stimulation have been replicated in in vitro studies using intermittent MDWT[21]. Furthermore, in vivo studies in patients have shown a difference between the initial and final stages of wound healing. Initially MDWT results in upregulation of angiogenin-2 (Ang-2) expression and downregulation of angiogenin-1 (Ang-1) expression, hence leading to decreased ratios of Ang-1/Ang-2. This favors destabilization and regression of microvessels leading to increased angiogenesis. In contrast, in the latter stages, Ang-1 is increased and the ratio of Ang-1/Ang-2 also increases. Phosphorylation of tyrosine kinase receptor-2 is activated, enhancing microvessel stabilization and promoting microvessel maturation[22].
Using a deep tissue wound in a porcine model it was shown that a maximum fourfold increase in blood perfusion occurs when suction of 125 mmHg is applied to a PU foam. It was also shown that higher suction levels of 400 mmHg and above inhibit blood flow as the capillaries distort. In healthy human skin suction levels of up to 300 mmHg applied to a PU foam cause a fivefold increase of blood flow while suction on a Polyvinyl Alcohol (PVA) foam results in a threefold increase[23].
Topical negative pressure has been shown to stimulate vessel proliferation and neo-angiogenesis. Topical negative pressure applied to chronic wounds of 16 patients (-125 mmHg) in preparation for reconstruction with free or pedicled flaps has been shown to considerably increase blood vessel density, reaching a maximum of approximately 200% in contrast to the vessel density prior to treatment[24].
Modulation of inflammation: MDWT promotes active wound healing by simultaneously inducing inflammation while removing harmful components of inflammation such as infiltrating leucocytes, cytokines, and matrix metalloproteinases. Wounds treated with MDWT display increased cellularity of wound exudate with elevated erythrocytes and leukocytes, along with increased gene expression of leukocyte chemoattractants, such as CXCL5 and IL-8[25].
Topical negative pressure with reticulated open cell foam (ROCF) has been shown to increase expression of various pro-healing anabolism-related genes. This includes increased expression of extracellular matrix genes resulting in increased production of the proteoglycans epiphycan and fibronectin. Other genes found to be upregulated include CD163 and macrophage scavenger receptor 1 which are involved in macrophage signalling[26].
Cellular responses-proliferation, differentiation, and migration: Cells have long been known to undergo proliferation and division when exposed to mechanical stresses[27]. Consequently, cells can be induced to undergo these cellular functions by exposure to dynamic physical inputs. MDWT is largely based on this principle; the tissue microdeformation stimulates cellular proliferation enhancing wound healing. This effect has been shown to upregulate angiogenesis and epithelialization in chronic wounds in a rabbit model[28].
Studies using a diabetic mouse model found that application of short (6 h) intermittent MDWT results in increased expression of Ki-67, a marker for proliferation. The level of strain induced in the tissue by MDWT is the same level of strain required to induce cell proliferation in vitro[13].
It is believed that isometric tension is heavily involved in promoting cellular proliferation[29]. In the absence of isometric tension, growth factors and cell attachment to ECM proteins are essential but insufficient for cellular proliferation. Chronic wounds tend to lack the structural scaffolding needed for cell adherence and hence the development of isometric tension. Consequently cells undergo spherization and apoptosis. Suction applied during MDWT is believed to generate the necessary forces within the tissues that enable isometric tension and hence cellular proliferation[13].
In addition, gene ontology enrichment analysis of tissue treated with MDWT has shown increased epithelial cell migration in the tissue[25]. Analysis of histologic penetration depth showed increased endothelial migration in tissues, whereas migration assays indicated enhanced dermal fibroblast migration[30,31]. MDWT also enhances migration of resident skin mesenchymal cells and circulating progenitor cells into granulation tissue[32].
Interestingly, although migration and proliferation of epithelial cells is increased by MDWT, differentiation is decreased. By downregulating keratin genes, such as KRT1 and KRT2, and major cornified envelope genes, such as annexin A9 and loricrin, MDWT inhibits keratinocyte differentiation[25]. This decrease is believed to be due to the changes induced in the wound tissue matrix as mesenchymal stem cell lineages are highly specific to the mechanical characteristics of the ECM. For example, mesenchymal stem cell neurogenesis, myogenesis, and osteogenesis are guided along soft, stiffer and rigid matrices, respectively[33]. Overall, MDWT is believed to promote wound healing by modulating cellular proliferation and migration, whilst inhibiting epidermal development and maturation[25].
Granulation tissue formation: MDWT affects the proliferation stage of repair by inducing robust tissue granulation, cell proliferation, and blood vessel sprouting. Research on mast cell-deficient mice has shown that all three processes require early and continuous activation by mast cells[34]. Collagen maturation is strictly dependent on mast cells in order to proliferate and remodel. Interestingly, MDWT application in mast cell-deficient mice, has no effect on collagen maturation, as expected, but does induce an increase in collagen production. In the control mice, both production and maturation were increased. Consequently, collagen production is not believed to be dependent on mast cells[34].
MDWT relies heavily on mechanotransduction, whereby mechanical forces are transduced by cells into biological triggers for various processes, including gene expression[35,36]. Mechanotransduction signalling in MDWT is a relatively new area of study. The current theory supports that molecules in the hypoxia pathway, such as nitric oxide, are involved[18].
In vitro studies using tissue engineering bioreactors have held a dominant role in simulating the in vivo micromechanical environment and the foam-wound interface. For example, tissue analogues subjected to topical MDWT for 48 h in a 3-dimensional bioreactor model of the wound bed environment displayed that fibroblast cell bodies undergo morphological change, from elongated bipolar to thickened morphology. Another observation following this treatment was the presence of dense actin cortical structures[37].
Research on a 3-dimensional fibrin matrix model found that MDWT increases cytochrome c oxidase levels, energy charge, and the adenosine triphosphate (ATP)-adenosine diphosphate (ADP) ratio in fibroblasts. The increased energy was found to be utilized by healing biomechanisms. Two important factors required for collagen production during granulation formation, growth factor TGF-β and platelet-derived growth factors (PDGF) α and β, were also shown to increase with simultaneous application of subatmospheric pressure and a reticulated open-cell foam. In addition to upregulating collagen formation, PDGF α and β upregulate glycosaminoglycans and fibronectin synthesis in fibroblasts[31]. Upregulation of fibroblast growth factor, TGF-β1, Type I collagen α1, and smooth muscle actin α2 messenger RNA expression has also been observed in cells 48 h after been exposed to a suction, foam or perfusion bioreactor[32].
The rate of granulation formation with NPWT therapy with a PU foam was measured in a porcine model by determining the decrease in wound volume over time. Increased rates of granulation formation were seen with continuous (63%) and intermittent (103%) application of suction. Continuous treatment is believed to be less effective than intermittent treatment because the cells in the wound become accommodated, and hence less responsive, to continuous physical forces[38].
Intermittent suction application inactivates capillary autoregulation, hence increasing tissue perfusion, and enables the production of new cellular components by allowing time between cycles of cell division for the proliferating cells to rest. Continuous stimulation on the other hand is believed to switch off mitosis. Despite this, many clinicians prefer to use continuous treatment for the first 48 h, before switching to the intermittent mode, because it is better tolerated by patients[39].
Alterations in bioburden: Changes in bioburden occur as a result of NPWT. However, studies related to this have produced mixed results. One study showed that NPWT results in decreased presence of non-fermentative gram-negative bacilli, but increased load of Staphylococcus aureus[40]. Other studies found no significant difference in bacterial levels when foam dressings were used with and without suction. These experiments, however, used nonviable tissue and focused primarily on the effect of suction on bacterial load[41]. It is believed that the decreased bacterial load occurs due to an interplay of multiple factors, not just due to the effect of suction[42,43]. Experiments on foam material found high bacterial loads in sonicated foams, and very high polymicrobial bacterial loads in all foams studied[44]. Porous PU foams on high suction (125 mmHg) were found to have a lower level of bacteria than PVA foams on lower suction. In addition, increased angiogenesis and blood perfusion may increase infection resistance by increasing inflow of oxygen in the wound tissue.
NPWT has been used to treat wounds in numerous different anatomical locations, with different levels of complexity and varying pathologies. The following section will review the evidence available on the application of NPWT in different wounds.
Basic applications of NPWT: At its most basic application, NPWT has been used in the management of open wounds, where the foam is directly applied to the wound bed. Common targets are poorly healing ulcers such as those caused by diabetes, venous or arterial pathologies and pressure necrosis.
More specifically, NPWT has been found to promote wound area reduction, wound bed granulation and clearance of microbial infection in diabetic foot ulcers[45]. NPWT has been associated with a higher rate of limb salvage[46]. Furthermore, treatment of diabetic, arterial and venous ulcers in high-risk patients using NPWT results in a higher rate of successful closure, with the greatest difference seen in venous ulcers[47]. When NPWT is applied earlier on in the treatment these wounds display faster healing times[47]. In nonoperative treatment of scleroderma ulcers, NPWT has been found to prevent digit amputation[48]. In the treatment of pressure ulcers, NPWT has been shown to reduce the surface area, volume and depth of wounds, to enhance granulation and to decrease the likelihood of hospitalisation[39,49,50]. Vowden K NPWT may also be effective in treating nonhealing deep-pressure ulcers covered by soft-necrotic tissue which require rapid formation of granulation tissue[51]. It is important to be noted that NPWT needs to be used concurrently with disease specific treatment, for example medical treatment for vasculitis and pyoderma gangrenosum[52].
In the treatment of surgical wounds, NPWT often acts as the pre-treatment before a skin flap or graft, or before secondary closure with NPWT. More specifically, in the excision of melanoma, NPWT enhances both functional (improves vascularity) and cosmetic (scar reduction) outcomes[53], whereas in the postoperative treatment of lymphangioma in children it is believed to decrease the risk of recurrence and infection[54].
Skin graft and dermal scaffold recipient site preparation: NPWT is often used to prepare a recipient site for skin grafts and dermal scaffolds. Large wounds, where granulation tissue spans the entire wound, can be rapidly closed with autologous skin grafting. One prospective RCT investigated the efficacy of NPWT prior to skin grafting in patients with acute traumatic wounds. NPWT improved total successful graft uptake, decreased regrafting, and required shorter lengths of hospital stay[55].
Dermal scaffolds are often used in wounds where tendon or bone is exposed to induce vascularization of the wound bed in preparation for skin grafting[56,57]. Concurrent treatment with NPWT and dermal scaffolds (Matriderm, Dr Suwelack Skin and Health Care AG, Billerbeck, Germany) enhances the contact between the wound surface and the scaffold and is believed to result in scars with higher elasticity and more natural skin pigmentation, and a decreased occurrence of postoperative wound contamination one year post-operatively[58].
Combination therapy: Various bioactive factors have been incorporated in NPWT to enhance efficacy.
Silver has been used in various wound dressings and has proven useful in burn care[59]. In NPWT therapy, silver was added to the coating of the PU foam in order to decrease the bacterial load in the wound. In a goat model of complex infected orthopedic wounds, silver dressings placed beneath the negative pressure dressings resulted in a decrease in the bacterial load, most notably in the numbers of S. aureus[60]. The MDWT foam can be modified to contain silver in order to act as an antimicrobial agent, as has been used in wound bed preparation for substantial split-thickness skin grafts (STSGs) to treat recalcitrant venous stasis ulcers[61]. However, silver-infused dressings are not always indicated[62].
A combined treatment of NPWT, platelet-rich plasma (PRP), STSGs and bilayered acellular matrix grafting was found to completely heal a large necrotizing fasciitis wound in a patient with diabetes[63]. Furthermore, one study used a combination therapy of PRP and NPWT on patients with sternal osteomyelitis and sinus tract after thoracotomy. The treatment regimen was PRP gel on the day of surgery followed by continuous NPWT for 20 d. This combination therapy was found to shorten the sinus tract sealing time, wound healing time, and length of hospital stay. Secondary repair surgery was also avoided[64].
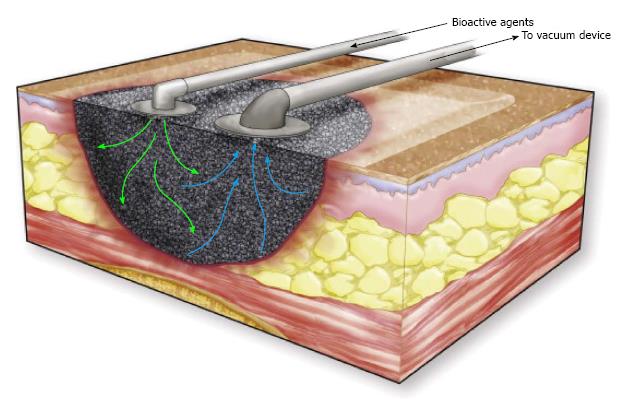
Instillation therapy is the injection of fluid, such as normal saline, into the wound through a port on the NPWT connecting tube to enhance wound healing (Figure 7). This technique has been successfully applied in massive venous stasis wounds to reduce bacterial concentrations in the wound prior to STSG. A single-delivery-instillation system, whereby a series of intermittent cycles of MDWT was followed by a single injection of dilute sodium hypochlorate solution, appeared to create good wound bed preparation[65].
In continuous-instillation MDWT a second port is connected to the continuous-drip system, which can allow continuous instillation of a fluid, for example insulin, to decrease the time required for wound healing[66]. In full-thickness excisional wounds in a porcine model, NPWT with simultaneous irrigation with polyhexanide biguanide (PHMB) or saline showed improved wound healing with either irrigation solution when compared with NPWT alone[67]. Other factors which can be instilled are dilute Betadine, doxycycline, phenytoin and lactoferrin. Further research is needed to investigate the efficacy of this concept[68]. Instillation MDWT has been recommended in patients with multiple comorbidities with difficult wounds, although without high level of evidence to support these recommendations[69].
Further examples of adjuvants include platelet gel, activated protein C, arginine-rich dietary supplements, and Manuka and Leptospermum honeys. Platelet gel, added to the wound bed following initial NPWT, has been used in the treatment of a nonhealing ileocutaneous fistula leading to complete wound healing[70]. In orthopedic wounds, activated protein C, an anticoagulant, was injected into the wound bed resulting in a decrease in the area and depth of the wound and an increase in granulation tissue formation[71]. Studies on the use of arginine-rich dietary supplements, which are believed to enhance local circulation at the wound bed, have shown that treatment with the supplements results in complete healing of infection-induced wound dehiscence with only one month of treatment and no recurrence at the 6-mo follow-up[72]. Leptospermum and Manuka honeys have been used in the treatment of a nonhealing postsurgical wound and an abdominal phlegmonous lesion, respectively[73,74].
NPWT in burns: NPWT has been found to preserve perfusion in acute partial-thickness hand burns[75]. It has also been used as a dressing over a dermal substitute in burn wounds, where it was believed to have no effect on graft adherence, but did improve long-term scar elasticity[76]. When NPWT was used with porcine acellular dermal matrix (ADM) dressing as combination therapy in deep burn wounds it was found to decrease wound exudate and bacterial load and promote wound healing[77]. NPWT has also been used in a patient with major third and fourth-degree high voltage electrical burns to enhance granulation tissue formation in preparation for skin grafting. Through the use of NPWT, major chronic soft tissue defects in the right leg were covered and amputation was avoided[78].
Treatment of deep infected wounds: The efficacy of NPWT in deep wounds has been studied using soft tissue blast injuries in porcine models. In these models, NPWT was found to decrease bacterial load, inhibit infection-induced tissue necrosis, and induce early initiation of granulation tissue formation[79]. Research on humans has shown that NPWT is effective in controlling infection, specifically in thoracic and abdominal wounds.
NPWT in open fractures: NPWT has also been used in the treatment of open wounds with exposed bone or joints, where it is believed to keep the wound moist, warm and sterile by preventing external contamination. The rate of wound healing in open fractures has also been shown to be expedited with NPWT[80]. Furthermore, the rate of deep infection in open tibial fractures is believed to be lower in NPWT treated wounds than in conventional treatment[81]. Treatment with NPWT in an open left knee-joint wound, induced formation of granulated wound bed which fully covered the exposed bones and joint[82]. NPWT is especially important in cases where free-flap transfer is contraindicated[82,83]. NPWT in Gustilo grade IIIB open tibial fracture treatment helps reduce the size of the flap required but can also eliminate the need for a flap transfer all together. However, it should be noted that treatment with duration longer than 7 d was associated with higher likelihood of infection and amputation[84]. NPWT in patients with grade III open fractures significantly reduced bacterial load at the wound site, as well as decreasing the risk of recurrent infection[85].
Deep sternal wound infection: Use of NPWT as the first line of therapy in deep sternal wound infections (DSWI) has been shown to decrease rates of early reinfections, as well as reducing numbers of late chronic sternal infections and mortality[86]. The length of hospital stay is also shortened by 1 wk[87]. In methicillin-resistant DSWIs following cardiovascular surgery, MDWT has been shown to decrease length of hospital stay, healing time, and infection recurrence[88].
NPWT is believed to have the ability to stabilize the thoracic cage, improving hemodynamics and pulmonary status. NPWT in conjunction with a tissue flap can provide adequate control of infection preventing sepsis and hemodynamic instability[89]. In the presence of poststernotomy osteomyelitis, adequate debridement and antibiotic therapy is still necessary with the use of NPWT[90,91].
NPWT as an augmented surgical drain: NPWT enables improved drainage of the fluid which builds up in anatomical cavities or abscesses in deep wound infections. For example, application of NPWT in the treatment of a deep neck abscess reduced the need for open thoracotomy by preventing accumulation of purulent material[92]. Concurrent open window thoracotomy and NPWT was found to eradicate local infection and hence control sepsis in postoperative or recurrent pleural empyema. In complex chest wall wounds NPWT inhibited empyema recurrence and enhanced lung expansion[93]. A modified NPWT therapy has been used to treat deep cavitary defects, such as those caused by blast injuries or high-velocity projectiles. By connecting a superficial foam dressing to the surgical drain, deep cavitary defects can be converted into superficial ones. This modified therapy can apply suction deep in the wound cavity and result in a decrease in the dead space, reduced edema and lower risk of infection. In comparison to traditional VAC therapy, draining is enhanced, and the risk of deeper cavities closing off is minimized[94]. VAC therapy has been successfully used in cases of Hidradenitis suppurativa where immediate primary closure was not possible secondary to the large size of the defect. An internal VAC was found to accelerate delayed closure and reduce the rate of recurrence in hidradenitis excisions[95].
NPWT as biologic sampling device: NPWT is increasingly being used as a biologic sampling device, where mediastinal fluid is collected from the wound and cultured for microorganisms. In one study, NPWT was found to increase the rate of detection of microorganisms and was recommended as a replacement to traditional biologic sampling devices[96]. On the other hand, microbiology of NPWT specimens in patients with prosthetic vascular graft infections was found to have limited diagnostic value, with anaerobe species being the most poorly identified in NPWT foam samples[97]. Additional studies are, however, required before general conclusions on the efficacy of NPWT as a sampling device can be drawn.
Intra-abdominal NPWT: A further potential target for NPWT are deep intra-abdominal wound infections.
Specifically, in a case of acute necrotizing pancreatitis, placement of NPWT foam dressing in the opening created during lesser sac marsupialization in classical laparotomy was found to accelerate wound closure, hence improving patient outcome[98]. In addition, examination of wound secretions improved, abdominal compartment syndrome was prevented and care was overall simplified. NPWT has also been successfully used endoscopically in the treatment of rectal wall anastomotic disruptions[99].
NPWT and gynecological laparotomy: NPWT has also been used as a prophylactic measure in laparotomy wounds in patients with gynecologic malignancies. The rate of wound complications was similar in patients who received traditional treatment and those receiving prophylactic NPWT dressing, despite those receiving NPWT having significantly higher BMIs[100].
NPWT and soft-tissue sarcomas: NPWT has been shown to be safe and effective as an adjunct to wound closure in cases of wide tumor resection for soft-tissue sarcomas. Continuous suction, with pressures from -200 to -300 mmHg, was applied on the soft-tissue defects as preparation for wound closure. This treatment was found to decrease wound complications, such as post-operative infection and recurrence, while also reducing edema, draining exudate, and promoting granulation tissue formation[101].
NPWT and congenital deformities: NPWT holds potential for the treatment of congenital deformities such as giant omphalocele[102] or complex gastroschisis[103]. However the efficacy for this has yet to be established and the treatment is not approved by the Food and Drug Administration (FDA). In pediatric patients, the granulation tissue response is often much more robust than in adults, often leading to more frequent dressing changes to avoid ingrowth into the interface material.
NPWT and digestive surgery: Most recently, there have been suggestions of using NPWT in digestive surgery, however this use is yet to be established. One previous pilot study investigated NPWT use following ileocecal resection in Crohn’s disease and found that NPWT shortened length of hospital stay by 70%-80%[104]. It is important to note that one major concern of NPWT use in digestive surgery is the development of enteric fistulas due to negative pressure[105]. Prophylactic NPWT at the ostomy closure wound in patients with ulcerative colitis was found to be safe with no enterocutaneous fistula formation or postoperative bleeding. However, in this particular study, no effect on the duration of wound healing was observed and the prophylactic efficacy of NPWT could not be proven[106]. Further studies are needed to prove the efficacy of NPWT in digestive surgery.
The literature supporting the use of NPWT over clean incisions has mixed results. Clean, closed surgical incisions displayed decreased rates of seroma and hematoma formation in porcine models[107]. Decreased rates of seroma, as well as haematoma formation have also been reported in post-bariatric patients receiving incisional NPWT[108]. Topical NPWT applied to closed incisions also decreases the risk of infection[109-112]. In total hip and knee arthroplasty, NPWT has been shown to be beneficial by decreasing excessive hospital stay and achieving a more predictable length of hospital stay. Wound complications, such as superficial wound infections and prolonged wound exudate, were also reduced[113]. Furthermore, in total hip arthroplasty incisional NPWT was found to decrease the rate of postoperative seromas[114], whereas in abdominal wall reconstruction it was found to reduce the incidence of incisional wound dehiscence[114,115]. Incisional NPWT in the reconstructive surgery of poststernotomy mediastinitis was found to decrease the duration of required therapy, length of hospital stay, and failure of treatment[116]. Peri-incisional lateral stress is reduced by approximately 50% following NPWT application and the directions of these stress vectors mimicked the distribution found in intact tissue[117]. Evidence from this research has supported the development of systems such as Prevena™ Incision Management System (KCI, an Acelity company, San Antonio, TX) which is specifically designed to be used in incisional wounds. Prevena™ has successfully been used in closed sternal incisions in cardiac patients where it has been shown to result in favorable outcomes within 30 d post-surgery[118]. A recent literature review has recommended the use of incisional NPWT in all patients with high risk of developing surgical site occurrences and those undergoing a high-risk procedure or a procedure that would have morbid consequences if complications occurred[119].
In contrast, VAC therapy in high risk patients with lower extremity and abdominal wound incisions had no significant effect on infection and dehiscence rates[120]. It should be noted that one prospective analysis of 21 patients who received NPWT post primary knee arthroplasty found no benefit in wound healing with NPWT, with the only notable benefit being less wound leakage and better protection of the incisional site. This study was, however, limited by the small sample size and the results need to be validated by a larger prospective RCT[121].
NPWT is used in STSGs in the place of a bolster, which is traditionally used to immobilize the graft by applying gentle pressure[122,123]. NPWT stabilizes the graft, drains excess fluids, and promotes better contact for graft integration enhancing vascularisation[122-125]. NPWT has been shown to decrease the risk of reoperation in cases of congested lower extremity pedicle and free flaps by decreasing venous insufficiency and tissue edema, promoting granulation and, hence, preventing further flap necrosis[126]. Furthermore, NPWT decreases venous congestion in random local flaps used in complex ankle wounds, hence decreasing the likelihood for ischemia and distal necrosis and enhancing their viability[127]. NPWT is also used in the treatment of large back donor sites for head and neck free flaps where it is believed to decrease complications. NPWT has successfully been used in degloving injuries to immobilize skin grafts[128,129], or as adjuvant treatment with a dermal regeneration template[130].
NPWT has been used in various wound types and its use in poorly healing wounds is FDA approved. The KCI VAC therapy system, which is widely used, lists acute, subacute, chronic, traumatic, dehisced wounds, ulcers, partial-thickness burns, grafts and flaps as indications for use in its manufacturer guidelines[131].
Use of NPWT is contraindicated in untreated osteomyelitis, when necrotic tissue or malignancy is present in the wound, in nonenteric and unexplored fistulas, and when there is exposed vasculature, nerves, anastomotic sites, or organs[107]. It should be noted that NPWT has been used successfully in contraindicated cases such as in cases of osteomyelitis[91], exposed organs[98], and exposed anastomotic sites[132].
Numerous potential patient risk factors that require consideration have also been identified. NPWT is contraindicated in patients with high risk of bleeding or hemorrhage or those who are on chronic anticoagulation or antiplatelet treatment[133]. In addition, the patient’s body habitus needs to be considered.
Direct contact of exposed tendons, nerves, vasculature and organs with PU foam under vacuum forces can also result in complications. Hence, in cases where NPWT needs to be applied in close proximity to exposed structures, these structures can be covered with a nonadherent barrier layer such as petroleum gauze and nonadherent dressings[134]. In addition, an isolation sterile bag has been described for intra-abdominal dressings in which the VAC foam is placed inside a sterile bag (3M SteriDrape Isolation Bag) whose surface has been perforated, allowing fluid drainage whilst simultaneously protecting the surrounding tissue[97].
Complications that have been mentioned in the literature include infection and sepsis, foam retention in the wound, tissue adherence, bleeding, and pain (Figure 8). Although in the most serious cases bleeding and infection have led to death, these complications occur very rarely[107]. Death associated with NPWT application at home or in long-term care facilities, is most commonly due to massive bleeding. Consequently, care needs to be exercised when selecting patients to apply NPWT at home, particularly in wounds of high risk for bleeding. In open abdominal wounds the tension generated on the proximal bowel of the stoma during NPWT has been seen to cause stomal mucocutaneous dehiscence[135].
The frequency of dressing changes can also affect treatment outcome. For example, in a case of NPWT treatment of a stage IV ischial pressure ulcer, spreading infections were masked, resulting in necrotizing fasciitis, as the dressing was changed at 5 d intervals[136].
Special consideration should be taken when treating blast wounds with NPWT, as application has been linked to increased rates of sepsis[137]. If applied before complete debridement is performed, it should be changed more frequently until the debridement is complete. Blast wounds have deep cavitary defects and the tail of the foam placed within the cavity has a higher chance of retention[94]. Currently, most interface materials do not have any indicator that can be visualized on X-ray. Great care must therefore be taken when changing the interface material to ensure complete removal.
Various interfaces have been developed through the years. At the moment, reticulated open-pore foams have been the most carefully studied and are considered to be able to transmit suction over long distances and to induce tissue microdeformation. Furthermore, the pore size in the foam material is believed to be directly related to the level of granulation tissue formation, where larger pores induce higher production of granulation tissue[138]. Further research related to optimal interface materials for different clinical situations is necessary.
To date, research supports that short, intermittent NPWT induces a more robust tissue response in biological systems than continuous mechanical forces[16]. However, in one study, continuous NPWT application and variable application (every 2 d for 4 h) were found to induce a similar granulation tissue response. Furthermore, very fast cycle times seem to decrease granulation tissue formation by causing damage to nascent granulation tissue[8,139,140]. Intermittent therapy is also often not adhered to by patients because of patient discomfort. Additionally, optimal cycling varies with the type of wound. A chronic ulcer, for example, may be best treated with continuous suction throughout treatment, whereas an acute wound, may respond better to continuous suction for 48 h, followed by cycles of intermittent therapy[135].
There has yet to be a definitive study that states the optimal NPWT suction level. A lower pressure is believed to be best for circumferential wounds and in cases where NPWT is used in conjunction with a free flap.
A limitation of the currently available NPWT devices is the inability to obtain a good seal at the edges of the device which compromises the maintenance of suction. Further development of materials that enable better adhesion on curved and moist surfaces is necessary to allow use of the devices in difficult wounds.
NPWT may have proven successful as an adjunctive therapy in a wide variety of wounds. However, the currently available systems are still novel, and the number of high-level clinical studies investigating NPWT is lacking. More RCTs are needed to elucidate the details of NPWT efficacy, particularly in terms of its different indications and modalities. Overall, NPWT continues to hold great promise and with further research on the optimal parameters of its application this management option stands to continue to improve wound healing and patient care.
We would like to acknowledge Mr. Christian Jones an MD candidate at the School of Clinical Medicine, University of Cambridge, Cambridge CB2 0SP United Kingdom for recording the Core Tip.
Manuscript source: Invited manuscript
Specialty type: Dermatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Horch RE, Ichioka S, Lu SL S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38:563-576; discussion 577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1531] [Cited by in RCA: 1383] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 2. | Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. 2014;51:301-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 310] [Article Influence: 28.2] [Reference Citation Analysis (2)] |
| 3. | Malmsjö M, Borgquist O. NPWT settings and dressing choices made easy. Wounds International. 2010;1:1-6. |
| 4. | Chariker ME, Jeter KF, Tintle TE, Ottsford JE. Effective management of incisional and cutaneous fistulae with closed suction wound drainage. Contemp Surg. 1989;34:59 63. |
| 5. | Campbell PE, Smith GS, Smith JM. Retrospective clinical evaluation of gauze-based negative pressure wound therapy. Int Wound J. 2008;5:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Kairinos N, Solomons M, Hudson DA. Negative-pressure wound therapy I: the paradox of negative-pressure wound therapy. Plast Reconstr Surg. 2009;123:589-598; discussion 599-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Kairinos N, Solomons M, Hudson DA. The paradox of negative pressure wound therapy--in vitro studies. J Plast Reconstr Aesthet Surg. 2010;63:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Scherer SS, Pietramaggiori G, Mathews JC, Prsa MJ, Huang S, Orgill DP. The mechanism of action of the vacuum-assisted closure device. Plast Reconstr Surg. 2008;122:786-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 211] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 9. | Borgquist O, Ingemansson R, Malmsjö M. The influence of low and high pressure levels during negative-pressure wound therapy on wound contraction and fluid evacuation. Plast Reconstr Surg. 2011;127:551-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg. 2004;114:1086-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Quinn TP, Schlueter M, Soifer SJ, Gutierrez JA. Cyclic mechanical stretch induces VEGF and FGF-2 expression in pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L897-L903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Rivilis I, Milkiewicz M, Boyd P, Goldstein J, Brown MD, Egginton S, Hansen FM, Hudlicka O, Haas TL. Differential involvement of MMP-2 and VEGF during muscle stretch- versus shear stress-induced angiogenesis. Am J Physiol Heart Circ Physiol. 2002;283:H1430-H1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Urschel JD, Scott PG, Williams HT. The effect of mechanical stress on soft and hard tissue repair; a review. Br J Plast Surg. 1988;41:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Orgill DP, Manders EK, Sumpio BE, Lee RC, Attinger CE, Gurtner GC, Ehrlich HP. The mechanisms of action of vacuum assisted closure: more to learn. Surgery. 2009;146:40-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Lancerotto L, Bayer LR, Orgill DP. Mechanisms of action of microdeformational wound therapy. Semin Cell Dev Biol. 2012;23:987-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Younan G, Ogawa R, Ramirez M, Helm D, Dastouri P, Orgill DP. Analysis of nerve and neuropeptide patterns in vacuum-assisted closure-treated diabetic murine wounds. Plast Reconstr Surg. 2010;126:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 17. | Kheirabadi BS, Terrazas IB, Williams JF, Hanson MA, Dubick MA, Blackbourne LH. Negative-pressure wound therapy: a hemostatic adjunct for control of coagulopathic hemorrhage in large soft tissue wounds. J Trauma Acute Care Surg. 2012;73:1188-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Saxena V, Orgill D, Kohane I. A set of genes previously implicated in the hypoxia response might be an important modulator in the rat ear tissue response to mechanical stretch. BMC Genomics. 2007;8:430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Greene AK, Puder M, Roy R, Arsenault D, Kwei S, Moses MA, Orgill DP. Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg. 2006;56:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Erba P, Ogawa R, Ackermann M, Adini A, Miele LF, Dastouri P, Helm D, Mentzer SJ, D’Amato RJ, Murphy GF. Angiogenesis in wounds treated by microdeformational wound therapy. Ann Surg. 2011;253:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 21. | Potter MJ, Banwell P, Baldwin C, Clayton E, Irvine L, Linge C, Grobbelaar AO, Sanders R, Dye JF. In vitro optimisation of topical negative pressure regimens for angiogenesis into synthetic dermal replacements. Burns. 2008;34:164-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Ma Z, Shou K, Li Z, Jian C, Qi B, Yu A. Negative pressure wound therapy promotes vessel destabilization and maturation at various stages of wound healing and thus influences wound prognosis. Exp Ther Med. 2016;11:1307-1317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Timmers MS, Le Cessie S, Banwell P, Jukema GN. The effects of varying degrees of pressure delivered by negative-pressure wound therapy on skin perfusion. Ann Plast Surg. 2005;55:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Malsiner CC, Schmitz M, Horch RE, Keller AK, Leffler M. Vessel transformation in chronic wounds under topical negative pressure therapy: an immunohistochemical analysis. Int Wound J. 2015;12:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Nuutila K, Siltanen A, Peura M, Harjula A, Nieminen T, Vuola J, Kankuri E, Aarnio P. Gene expression profiling of negative-pressure-treated skin graft donor site wounds. Burns. 2013;39:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 26. | Leffler M, Derrick KL, McNulty A, Malsiner C, Dragu A, Horch RE. Changes of anabolic processes at the cellular and molecular level in chronic wounds under topical negative pressure can be revealed by transcriptome analysis. J Cell Mol Med. 2011;15:1564-1571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Ingber DE. The mechanochemical basis of cell and tissue regulation. Mech Chem Biosyst. 2004;1:53-68. [PubMed] |
| 28. | Fabian TS, Kaufman HJ, Lett ED, Thomas JB, Rawl DK, Lewis PL, Summitt JB, Merryman JI, Schaeffer TD, Sargent LA. The evaluation of subatmospheric pressure and hyperbaric oxygen in ischemic full-thickness wound healing. Am Surg. 2000;66:1136-1143. [PubMed] |
| 29. | Huang S, Ingber DE. The structural and mechanical complexity of cell-growth control. Nat Cell Biol. 1999;1:E131-E138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 536] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 30. | Baldwin C, Potter M, Clayton E, Irvine L, Dye J. Topical negative pressure stimulates endothelial migration and proliferation: a suggested mechanism for improved integration of Integra. Ann Plast Surg. 2009;62:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 31. | McNulty AK, Schmidt M, Feeley T, Kieswetter K. Effects of negative pressure wound therapy on fibroblast viability, chemotactic signaling, and proliferation in a provisional wound (fibrin) matrix. Wound Repair Regen. 2007;15:838-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Lu F, Ogawa R, Nguyen DT, Chen B, Guo D, Helm DL, Zhan Q, Murphy GF, Orgill DP. Microdeformation of three-dimensional cultured fibroblasts induces gene expression and morphological changes. Ann Plast Surg. 2011;66:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9969] [Cited by in RCA: 9658] [Article Influence: 508.3] [Reference Citation Analysis (0)] |
| 34. | Younan GJ, Heit YI, Dastouri P, Kekhia H, Xing W, Gurish MF, Orgill DP. Mast cells are required in the proliferation and remodeling phases of microdeformational wound therapy. Plast Reconstr Surg. 2011;128:649e-658e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Ingber DE. Tensegrity-based mechanosensing from macro to micro. Prog Biophys Mol Biol. 2008;97:163-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 36. | Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35:564-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 535] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 37. | Wilkes RP, McNulty AK, Feeley TD, Schmidt MA, Kieswetter K. Bioreactor for application of subatmospheric pressure to three-dimensional cell culture. Tissue Eng. 2007;13:3003-3010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Morykwas MJ, Faler BJ, Pearce DJ, Argenta LC. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg. 2001;47:547-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 234] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 39. | Vowden K. Conservative management of pressure ulcers. Vacuum Assisted Closure TM Therapy: Science and Practice. London: MEP Ltd 2006; . |
| 40. | Mouës CM, Vos MC, van den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. 2004;12:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 357] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 41. | Assadian O, Assadian A, Stadler M, Diab-Elschahawi M, Kramer A. Bacterial growth kinetic without the influence of the immune system using vacuum-assisted closure dressing with and without negative pressure in an in vitro wound model. Int Wound J. 2010;7:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1526] [Cited by in RCA: 1442] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 43. | Morykwas MJ, Simpson J, Punger K, Argenta A, Kremers L, Argenta J. Vacuum-assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg. 2006;117:121S-126S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 261] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 44. | Yusuf E, Jordan X, Clauss M, Borens O, Mäder M, Trampuz A. High bacterial load in negative pressure wound therapy (NPWT) foams used in the treatment of chronic wounds. Wound Repair Regen. 2013;21:677-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Nather A, Hong NY, Lin WK, Sakharam JA. Effectiveness of bridge V.A.C. dressings in the treatment of diabetic foot ulcers. Diabet Foot Ankle. 2011;2:5893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Ulusal AE, Sahin MS, Ulusal B, Cakmak G, Tuncay C. Negative pressure wound therapy in patients with diabetic foot. Acta Orthop Traumatol Turc. 2011;45:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Yao M, Fabbi M, Hayashi H, Park N, Attala K, Gu G, French MA, Driver VR. A retrospective cohort study evaluating efficacy in high-risk patients with chronic lower extremity ulcers treated with negative pressure wound therapy. Int Wound J. 2014;11:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Patel RM, Nagle DJ. Nonoperative management of scleroderma of the hand with tadalafil and subatmospheric pressure wound therapy: case report. J Hand Surg Am. 2012;37:803-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Joseph E, Hamori CA, Bergman S, Roaf E, Swann NF, Anastasi GW. A prospective, randomized trial of vacuum- assisted closure versus standard therapy of chronic non healing wounds. Wounds. 2000;12:60-67. |
| 50. | Schwien T, Gilbert J, Lang C. Pressure ulcer prevalence and the role of negative pressure wound therapy in home health quality outcomes. Ostomy Wound Manage. 2005;51:47-60. [PubMed] |
| 51. | Nakayama M. Applying negative pressure therapy to deep pressure ulcers covered by soft necrotic tissue. Int Wound J. 2010;7:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Zutt M, Haas E, Kruger U, Distler M, Neumann C. Successful use of vacuum-assisted closure therapy for leg ulcers caused by occluding vasculopathy and inflammatory vascular diseases--a case series. Dermatology. 2007;214:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Oh BH, Lee SH, Nam KA, Lee HB, Chung KY. Comparison of negative pressure wound therapy and secondary intention healing after excision of acral lentiginous melanoma on the foot. Br J Dermatol. 2013;168:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Katz MS, Finck CM, Schwartz MZ, Moront ML, Prasad R, Timmapuri SJ, Arthur LG. Vacuum-assisted closure in the treatment of extensive lymphangiomas in children. J Pediatr Surg. 2012;47:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Saaiq M, Hameed-Ud-Din MI, Chaudhery SM. Vacuum-assisted closure therapy as a pretreatment for split thickness skin grafts. J Coll Physicians Surg Pak. 2010;20:675-679. [PubMed] |
| 56. | Heit YI, Lancerotto L, Cortes R, Mesteri I, Ackermann M, Hollander R, Li Q, Douaiher J, Konerding MA, Orgill DP. Early kinetics of integration of collagen-glycosaminoglycan regenerative scaffolds in a diabetic mouse model. Plast Reconstr Surg. 2013;132:767e-776e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Kang GC, Por YC, Tan BK. In vivo tissue engineering over wounds with exposed bone and tendon: Autologous dermal grafting and vacuum-assisted closure. Ann Plast Surg. 2010;65:70-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Bloemen MC, van der Wal MB, Verhaegen PD, Nieuwenhuis MK, van Baar ME, van Zuijlen PP, Middelkoop E. Clinical effectiveness of dermal substitution in burns by topical negative pressure: a multicenter randomized controlled trial. Wound Repair Regen. 2012;20:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Khundkar R, Malic C, Burge T. Use of Acticoat dressings in burns: what is the evidence? Burns. 2010;36:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 60. | Stinner DJ, Waterman SM, Masini BD, Wenke JC. Silver dressings augment the ability of negative pressure wound therapy to reduce bacteria in a contaminated open fracture model. J Trauma. 2011;71:S147-S150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Gerry R, Kwei S, Bayer L, Breuing KH. Silver-impregnated vacuum-assisted closure in the treatment of recalcitrant venous stasis ulcers. Ann Plast Surg. 2007;59:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Leaper D. Appropriate use of silver dressings in wounds: international consensus document. Int Wound J. 2012;9:461-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Deng W, Boey J, Chen B, Byun S, Lew E, Liang Z, Armstrong DG. Platelet-rich plasma, bilayered acellular matrix grafting and negative pressure wound therapy in diabetic foot infection. J Wound Care. 2016;25:393-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Hao D, Feng G, Li T, Chu W, Chen Z, Li S, Zhang X, Zhao J, Zhao F. [Curative effects of platelet-rich plasma combined with negative-pressure wound therapy on sternal osteomyelitis and sinus tract after thoracotomy]. Zhonghua Shaoshang Zazhi. 2016;32:331-335. [PubMed] |
| 65. | Raad W, Lantis JC, Tyrie L, Gendics C, Todd G. Vacuum-assisted closure instill as a method of sterilizing massive venous stasis wounds prior to split thickness skin graft placement. Int Wound J. 2010;7:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Scimeca CL, Bharara M, Fisher TK, Kimbriel H, Mills JL, Armstrong DG. Novel use of insulin in continuous-instillation negative pressure wound therapy as “wound chemotherapy”. J Diabetes Sci Technol. 2010;4:820-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Davis K, Bills J, Barker J, Kim P, Lavery L. Simultaneous irrigation and negative pressure wound therapy enhances wound healing and reduces wound bioburden in a porcine model. Wound Repair Regen. 2013;21:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 68. | Kim PJ, Attinger CE, Steinberg JS, Evans KK, Lehner B, Willy C, Lavery L, Wolvos T, Orgill D, Ennis W. Negative-pressure wound therapy with instillation: international consensus guidelines. Plast Reconstr Surg. 2013;132:1569-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 69. | Kim PJ, Attinger CE, Olawoye O, Crist BD, Gabriel A, Galiano RD, Gupta S, Lantis Ii JC, Lavery L, Lipsky BA. Negative Pressure Wound Therapy With Instillation: Review of Evidence and Recommendations. Wounds. 2015;27:S2-S19. [PubMed] |
| 70. | Scala M, Spagnolo F, Trapasso M, Strada P, Moresco L, Santi P. Association of vacuum-assisted closure and platelet gel for the definitive surgical repair of an enterocutaneous fistula: a case report. In Vivo. 2012;26:147-150. [PubMed] |
| 71. | Wijewardena A, Vandervord E, Lajevardi SS, Vandervord J, Jackson CJ. Combination of activated protein C and topical negative pressure rapidly regenerates granulation tissue over exposed bone to heal recalcitrant orthopedic wounds. Int J Low Extrem Wounds. 2011;10:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Masumoto K, Nagata K, Oka Y, Kai H, Yamaguchi S, Wada M, Kusuda T, Hara T, Hirose S, Iwasaki A. Successful treatment of an infected wound in infants by a combination of negative pressure wound therapy and arginine supplementation. Nutrition. 2011;27:1141-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Rudzka-Nowak A, Łuczywek P, Gajos MJ, Piechota M. Application of manuka honey and GENADYNE A4 negative pressure wound therapy system in a 55-year-old woman with extensive phlegmonous and necrotic lesions in the abdominal integuments and lumbar region after traumatic rupture of the colon. Med Sci Monit. 2010;16:CS138-CS142. [PubMed] |
| 74. | 74Ganacias-Acuna EF. Active Leptospermum honey and negative pressure wound therapy for nonhealing postsurgical wounds. Ostomy Wound Manage. 2010;56:10-12. [PubMed] |
| 75. | Kamolz LP, Andel H, Haslik W, Winter W, Meissl G, Frey M. Use of subatmospheric pressure therapy to prevent burn wound progression in human: first experiences. Burns. 2004;30:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 76. | Honari S, Gibran NS, Engrav LH. Three years’ experience with 52 Integra (artificial skin) patients since FDA approval. J Burn Care Rehabil. 2000;21:190. |
| 77. | Liu W, Li F, Chen X, Pan Q. [Clinical efficacy of negative-pressure wound therapy combined with porcine acellular dermal matrix for repairing deep burn wounds in limbs]. Zhonghua Shaoshang Zazhi. 2016;32:356-362. [PubMed] |
| 78. | Tevanov I, Enescu DM, Bălănescu R, Sterian G, Ulici A. Negative Pressure Wound Therapy (NPWT) to Treat Complex Defect of the Leg after Electrical Burn. Chirurgia (Bucur). 2016;111:175-179. [PubMed] |
| 79. | Li J, Topaz M, Tan H, Li Y, Li W, Xun W, Yuan Y, Chen S, Li X. Treatment of infected soft tissue blast injury in swine by regulated negative pressure wound therapy. Ann Surg. 2013;257:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Arti H, Khorami M, Ebrahimi-Nejad V. Comparison of negative pressure wound therapy (NPWT) & amp; conventional wound dressings in the open fracture wounds. Pak J Med Sci. 2016;32:65-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Blum ML, Esser M, Richardson M, Paul E, Rosenfeldt FL. Negative pressure wound therapy reduces deep infection rate in open tibial fractures. J Orthop Trauma. 2012;26:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 82. | Lee SY, Niikura T, Miwa M, Sakai Y, Oe K, Fukazawa T, Kawakami Y, Kurosaka M. Negative pressure wound therapy for the treatment of infected wounds with exposed knee joint after patellar fracture. Orthopedics. 2011;34:211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 83. | Barnett TM, Shilt JS. Use of vacuum-assisted closure and a dermal regeneration template as an alternative to flap reconstruction in pediatric grade IIIB open lower-extremity injuries. Am J Orthop (Belle Mead NJ). 2009;38:301-305. [PubMed] |
| 84. | Hou Z, Irgit K, Strohecker KA, Matzko ME, Wingert NC, DeSantis JG, Smith WR. Delayed flap reconstruction with vacuum-assisted closure management of the open IIIB tibial fracture. J Trauma. 2011;71:1705-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Krtička M, Ira D, Nekuda V, Švancara J, Mašek M. [Effect of Negative Pressure Wound Therapy on Infectious Complications in Grade III Open Fractures]. Acta Chir Orthop Traumatol Cech. 2016;83:117-122. [PubMed] |
| 86. | Steingrimsson S, Gottfredsson M, Gudmundsdottir I, Sjögren J, Gudbjartsson T. Negative-pressure wound therapy for deep sternal wound infections reduces the rate of surgical interventions for early re-infections. Interact Cardiovasc Thorac Surg. 2012;15:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 87. | Damiani G, Pinnarelli L, Sommella L, Tocco MP, Marvulli M, Magrini P, Ricciardi W. Vacuum-assisted closure therapy for patients with infected sternal wounds: a meta-analysis of current evidence. J Plast Reconstr Aesthet Surg. 2011;64:1119-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 88. | De Feo M, Vicchio M, Nappi G, Cotrufo M. Role of vacuum in methicillin-resistant deep sternal wound infection. Asian Cardiovasc Thorac Ann. 2010;18:360-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 89. | Morisaki A, Hosono M, Murakami T, Sakaguchi M, Suehiro Y, Nishimura S, Sakon Y, Yasumizu D, Kawase T, Shibata T. Effect of negative pressure wound therapy followed by tissue flaps for deep sternal wound infection after cardiovascular surgery: propensity score matching analysis. Interact Cardiovasc Thorac Surg. 2016;23:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 90. | Tocco MP, Ballardini M, Masala M, Perozzi A. Post-sternotomy chronic osteomyelitis: is sternal resection always necessary? Eur J Cardiothorac Surg. 2013;43:715-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | Tocco MP, Costantino A, Ballardini M, D’Andrea C, Masala M, Merico E, Mosillo L, Sordini P. Improved results of the vacuum assisted closure and Nitinol clips sternal closure after postoperative deep sternal wound infection. Eur J Cardiothorac Surg. 2009;35:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 92. | Gallo O, Deganello A, Meccariello G, Spina R, Peris A. Vacuum-assisted closure for managing neck abscesses involving the mediastinum. Laryngoscope. 2012;122:785-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 93. | Sziklavari Z, Grosser C, Neu R, Schemm R, Kortner A, Szöke T, Hofmann HS. Complex pleural empyema can be safely treated with vacuum-assisted closure. J Cardiothorac Surg. 2011;6:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 94. | Rispoli DM, Horne BR, Kryzak TJ, Richardson MW. Description of a technique for vacuum-assisted deep drains in the management of cavitary defects and deep infections in devastating military and civilian trauma. J Trauma. 2010;68:1247-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 95. | Chen YE, Gerstle T, Verma K, Treiser MD, Kimball AB, Orgill DP. Management of hidradenitis suppurativa wounds with an internal vacuum-assisted closure device. Plast Reconstr Surg. 2014;133:370e-377e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 96. | Cagnoni G, Rimoldi SG, Pagani C, Savi C, Stefani F, Terzi R, Olivieri P, Tosi G, Parravicini C, Di Gregorio A. Can Drainage Using a Negative-Pressure Wound Therapy Device Replace Traditional Sample Collection Methods? Surg Infect (Larchmt). 2016;17:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 97. | Scherrer AU, Bloemberg G, Zbinden R, Zinkernagel AS, Fuchs C, Frauenfelder S, Rancic Z, Mayer D, Hasse B. Prosthetic Vascular Graft Infections: Bacterial Cultures from Negative-Pressure-Wound-Therapy Foams Do Not Improve Diagnostics. J Clin Microbiol. 2016;54:2190-2193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | Sermoneta D, Di Mugno M, Spada PL, Lodoli C, Carvelli ME, Magalini SC, Cavicchioni C, Bocci MG, Martorelli F, Brizi MG. Intra-abdominal vacuum-assisted closure (VAC) after necrosectomy for acute necrotising pancreatitis: preliminary experience. Int Wound J. 2010;7:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 99. | Bemelman WA. Vacuum assisted closure in coloproctology. Tech Coloproctol. 2009;13:261-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 100. | Lynam S, Mark KS, Temkin SM. Primary Placement of Incisional Negative Pressure Wound Therapy at Time of Laparotomy for Gynecologic Malignancies. Int J Gynecol Cancer. 2016;26:1525-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 101. | Chen YU, Xu SF, Xu M, Yu XC. Use of negative pressure wound therapy as an adjunct to the treatment of extremity soft-tissue sarcoma with ulceration or impending ulceration. Oncol Lett. 2016;12:757-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 102. | Kilbride KE, Cooney DR, Custer MD. Vacuum-assisted closure: a new method for treating patients with giant omphalocele. J Pediatr Surg. 2006;41:212-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 103. | Choi WW, McBride CA, Kimble RM. Negative pressure wound therapy in the management of neonates with complex gastroschisis. Pediatr Surg Int. 2011;27:907-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Pellino G, Sciaudone G, Candilio G, Campitiello F, Selvaggi F, Canonico S. Effects of a new pocket device for negative pressure wound therapy on surgical wounds of patients affected with Crohn’s disease: a pilot trial. Surg Innov. 2014;21:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 105. | Atema JJ, Gans SL, Boermeester MA. Systematic review and meta-analysis of the open abdomen and temporary abdominal closure techniques in non-trauma patients. World J Surg. 2015;39:912-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 106. | Uchino M, Hirose K, Bando T, Chohno T, Takesue Y, Ikeuchi H. Randomized Controlled Trial of Prophylactic Negative-Pressure Wound Therapy at Ostomy Closure for the Prevention of Delayed Wound Healing and Surgical Site Infection in Patients with Ulcerative Colitis. Dig Surg. 2016;33:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 107. | Kilpadi DV, Cunningham MR. Evaluation of closed incision management with negative pressure wound therapy (CIM): hematoma/seroma and involvement of the lymphatic system. Wound Repair Regen. 2011;19:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 108. | Horch RE. Incisional negative pressure wound therapy for high-risk wounds. J Wound Care. 2015;24:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 109. | Stannard JP, Volgas DA, McGwin G, Stewart RL, Obremskey W, Moore T, Anglen JO. Incisional negative pressure wound therapy after high-risk lower extremity fractures. J Orthop Trauma. 2012;26:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 267] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 110. | Zaidi A, El-Masry S. Closed incision negative pressure therapy in high-risk general surgery patients following laparotomy: a retrospective study. Colorectal Dis. 2016; Jul 15; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 111. | Grauhan O, Navasardyan A, Hofmann M, Müller P, Stein J, Hetzer R. Prevention of poststernotomy wound infections in obese patients by negative pressure wound therapy. J Thorac Cardiovasc Surg. 2013;145:1387-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 112. | Semsarzadeh NN, Tadisina KK, Maddox J, Chopra K, Singh DP. Closed Incision Negative-Pressure Therapy Is Associated with Decreased Surgical-Site Infections: A Meta-Analysis. Plast Reconstr Surg. 2015;136:592-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 113. | Karlakki SL, Hamad AK, Whittall C, Graham NM, Banerjee RD, Kuiper JH. Incisional negative pressure wound therapy dressings (iNPWTd) in routine primary hip and knee arthroplasties: A randomised controlled trial. Bone Joint Res. 2016;5:328-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 114. | Pachowsky M, Gusinde J, Klein A, Lehrl S, Schulz-Drost S, Schlechtweg P, Pauser J, Gelse K, Brem MH. Negative pressure wound therapy to prevent seromas and treat surgical incisions after total hip arthroplasty. Int Orthop. 2012;36:719-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 115. | Condé-Green A, Chung TL, Holton LH, Hui-Chou HG, Zhu Y, Wang H, Zahiri H, Singh DP. Incisional negative-pressure wound therapy versus conventional dressings following abdominal wall reconstruction: a comparative study. Ann Plast Surg. 2013;71:394-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 116. | Karlakki S, Brem M, Giannini S, Khanduja V, Stannard J, Martin R. Incisional negative pressure wound therapy in reconstructive surgery of poststernotomy mediastinitis. Bone Joint Res. 2013;2:276, 284. |
| 117. | Wilkes RP, Kilpad DV, Zhao Y, Kazala R, McNulty A. Closed incision management with negative pressure wound therapy (CIM): biomechanics. Surg Innov. 2012;19:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 118. | Reddy VS. Use of Closed Incision Management with Negative Pressure Therapy for Complex Cardiac Patients. Cureus. 2016;8:e506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 119. | Willy C, Agarwal A, Andersen CA, Santis G, Gabriel A, Grauhan O, Guerra OM, Lipsky BA, Malas MB, Mathiesen LL. Closed incision negative pressure therapy: international multidisciplinary consensus recommendations. Int Wound J. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 120. | Masden D, Goldstein J, Endara M, Xu K, Steinberg J, Attinger C. Negative pressure wound therapy for at-risk surgical closures in patients with multiple comorbidities: a prospective randomized controlled study. Ann Surg. 2012;255:1043-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 121. | Manoharan V, Grant AL, Harris AC, Hazratwala K, Wilkinson MP, McEwen PJ. Closed Incision Negative Pressure Wound Therapy vs Conventional Dry Dressings After Primary Knee Arthroplasty: A Randomized Controlled Study. J Arthroplasty. 2016;31:2487-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 122. | Petkar KS, Dhanraj P, Kingsly PM, Sreekar H, Lakshmanarao A, Lamba S, Shetty R, Zachariah JR. A prospective randomized controlled trial comparing negative pressure dressing and conventional dressing methods on split-thickness skin grafts in burned patients. Burns. 2011;37:925-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 123. | Azzopardi EA, Boyce DE, Dickson WA, Azzopardi E, Laing JH, Whitaker IS, Shokrollahi K. Application of topical negative pressure (vacuum-assisted closure) to split-thickness skin grafts: a structured evidence-based review. Ann Plast Surg. 2013;70:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 124. | Llanos S, Danilla S, Barraza C, Armijo E, Piñeros JL, Quintas M, Searle S, Calderon W. Effectiveness of negative pressure closure in the integration of split thickness skin grafts: a randomized, double-masked, controlled trial. Ann Surg. 2006;244:700-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 125. | Moisidis E, Heath T, Boorer C, Ho K, Deva AK. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg. 2004;114:917-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 126. | Vaienti L, Gazzola R, Benanti E, Leone F, Marchesi A, Parodi PC, Riccio M. Failure by congestion of pedicled and free flaps for reconstruction of lower limbs after trauma: the role of negative-pressure wound therapy. J Orthop Traumatol. 2013;14:213-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 127. | Goldstein JA, Iorio ML, Brown B, Attinger CE. The use of negative pressure wound therapy for random local flaps at the ankle region. J Foot Ankle Surg. 2010;49:513-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 128. | Meara JG, Guo L, Smith JD, Pribaz JJ, Breuing KH, Orgill DP. Vacuum-assisted closure in the treatment of degloving injuries. Ann Plast Surg. 1999;42:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 129. | Morris M, Schreiber MA, Ham B. Novel management of closed degloving injuries. J Trauma. 2009;67:E121-E123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 130. | Dini M, Quercioli F, Mori A, Romano GF, Lee AQ, Agostini T. Vacuum-assisted closure, dermal regeneration template and degloved cryopreserved skin as useful tools in subtotal degloving of the lower limb. Injury. 2012;43:957-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 131. | V.A.C.s Therapy Forms and Brochures. [accessed 2016 Dec 8]. Available from: http: //www.kci-medical.co.uk/UK-ENG/vacformsandbrochures. |
| 132. | Weidenhagen R, Gruetzner KU, Wiecken T, Spelsberg F, Jauch KW. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc. 2008;22:1818-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 133. | V.A.C. Therapy Indications and Contraindications. [accessed 2016 Dec 8]. Available from: http: //www.kci-medical.sg/SG-ENG/indications. |
| 134. | Mendez-Eastman S. Guidelines for using negative pressure wound therapy. Adv Skin Wound Care. 2001;14:314-322; quiz 324-325. [PubMed] |
| 135. | Steenvoorde P, den Outer A, Neijenhuis P. Stomal mucocutaneous dehiscence as a complication of topical negative pressure used to treat an open abdomen: a case series. Ostomy Wound Manage. 2009;55:44-48. [PubMed] |
| 136. | Citak M, Backhaus M, Meindl R, Muhr G, Fehmer T. Rare complication after VAC-therapy in the treatment of deep sore ulcers in a paraplegic patient. Arch Orthop Trauma Surg. 2010;130:1511-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 137. | Marsh DJ, Abu-Sitta G, Patel H. The role of vacuum-assisted wound closure in blast injury. Plast Reconstr Surg. 2007;119:1978-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 138. | Heit YI, Dastouri P, Helm DL, Pietramaggiori G, Younan G, Erba P, Münster S, Orgill DP, Scherer SS. Foam pore size is a critical interface parameter of suction-based wound healing devices. Plast Reconstr Surg. 2012;129:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 139. | Dastouri P, Helm DL, Scherer SS, Pietramaggiori G, Younan G, Orgill DP. Waveform modulation of negative-pressure wound therapy in the murine model. Plast Reconstr Surg. 2011;127:1460-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |