Peer-review started: July 6, 2015
First decision: July 31, 2015
Revised: November 2, 2015
Accepted: November 23, 2015
Article in press: November 24, 2015
Published online: February 2, 2016
Processing time: 212 Days and 24 Hours
AIM: To investigate the pharmacokinetics profile of Ivermectin 1% cream after topical treatment in patients with papulopustular rosacea (PPR).
METHODS: Ivermectin 1% cream is a new, effective, and safe treatment for PPR. The human pharmacokinetic (PK) profile of ivermectin and its circulating metabolites were assessed following topical application of ivermectin 1% cream to the face. Clinical PK assessments were conducted after 4 wk of treatment using healthy volunteers and PPR subjects. Additionally, PK sampling was conducted up to 1 year of treatment in clinical phase 3 studies. Plasma concentrations of ivermectin and ivermectin metabolites were determined using high-performance liquid chromatography with fluorescence detection after a specific derivation to increase sensitivity.
RESULTS: Systemic exposure to ivermectin was quantifiable at low levels in healthy and moderate to severe PPR subjects following the first topical application of ivermectin 1% cream (mean Cmax of 0.5 ± 0.2 ng/mL and 0.7 ± 0.5 ng/mL in healthy volunteers and PPR subjects, respectively). Ivermectin plasma levels reached a plateau after 2 wk of repeated topical application, indicating that steady-state concentrations had been reached. No further ivermectin plasma accumulation was observed during the long-term clinical studies that investigated ivermectin treatment up to 1 year. Investigation of ivermectin metabolites indicated that 2 circulating metabolites represented more than 10% of parent drug systemic exposure at steady state. Repeated topical application of ivermectin 1% cream resulted in lower systemic exposure levels when compared with orally administered ivermectin, suggesting limited transdermal absorption of ivermectin. Topically applied ivermectin is cleared from the plasma slowly (with a prolonged plasma half-life when compared to the oral route).
CONCLUSION: Applications of ivermectin 1% cream result in low systemic exposure levels. Steady–state conditions are achieved by 2 wk without further accumulation under chronic treatment.
Core tip: Papulopustular rosacea (PPR) is a chronic skin disease affecting patients face, with a dramatic impact on social and professional interactions. Ivermectin 1% cream is a new effective and safe treatment for PPR recently approved in many countries. This article presents the clinical pharmacokinetics (PK) assessments conducted during the drug development of Ivermectin 1% cream. Usually, for topical products, PK assessments are incomplete due to the low systemic exposure. For ivermectin cream, a comprehensive PK and metabolism program was conducted in healthy volunteers and PPR patients up to 1 year treatment. These provided valuable information to better assess ivermectin safety profile.
- Citation: Benkali K, Rony F, Graeber M, Jacovella J, Chappuis JP, Peirone MH, Poncet M, Delage S, Bouer R, Wagner N. Clinical pharmacokinetics profile of ivermectin 1% cream after dermal applications on the face. World J Dermatol 2016; 5(1): 57-64
- URL: https://www.wjgnet.com/2218-6190/full/v5/i1/57.htm
- DOI: https://dx.doi.org/10.5314/wjd.v5.i1.57
Ivermectin is a semi-synthetic derivative that belongs to the avermectin family of macrocytic lactones with anti-parasitic activities and is thought to have an anti-inflammatory effect by decreasing cellular and humoral immune responses[1]. The efficacy of oral ivermectin in human and animal demodicidosis and its anti-inflammatory properties suggest that ivermectin may also be effective in the treatment of papulopustular rosacea (PPR)[2,3]. Ivermectin 1% cream development has shown that this treatment is effective and safe in treating inflammatory lesions of papulopustular rosacea[4,5]. Therefore, ivermectin is now approved in the United States and in European Union member states as Soolantra® Cream 1% for treatment of papulopustular rosacea in adults[6].
Ivermectin pharmacokinetics (PK) data are well documented but mainly available for the oral marketed product for the treatment of onchocerciasis, strongyloidiasis of the intestinal tract and lymphatic filariasis[7]. In addition, ivermectin is indicated for scabies treatment in some countries[7]. After single or repeated oral dosing, peak plasma concentrations are achieved at approximately 4 to 10 h after dosing[8-10]. The plasma systemic exposures increase proportionally with doses between 6 and 120 mg[8,9]. After single 12 mg doses of oral ivermectin (tablet) in healthy volunteers, the mean peak plasma concentrations were from 23.5 to 50 ng/mL[10]. Ivermectin elimination curve might be subject to an enterohepatic recycling[11,12]. Ivermectin is widely distributed in the body with a volume of distribution about 3.1 and 3.5 L/kg, after ingesting 6 and 12 mg of ivermectin, respectively[13]. In addition, ivermectin is approximately 93% bound to plasma proteins, mainly to serum albumin[14].
Ivermectin is extensively metabolized in vitro by liver microsomal cytochrome P450 3A4 to hydroxylated and demethylated metabolites[15]. Ivermectin and its metabolites appear to be eliminated mainly in the faeces, with minimal urinary excretion (≤ 1% of the administered dose). The mean half-life of ivermectin when administered orally is ranging from about 15 to 20 h[9].
Recently, ivermectin has been approved for use in human as a topical treatment of head lice infestations with a short contact therapy (10 min application, single use)[16]. The ivermectin transdermal absorption was evaluated in a clinical study in subjects aged from 6 mo to 3 years after a single application of ivermectin 0.5% lotion on the head. The resulting systemic exposure levels after a single 10-min application were very low in comparison to the oral administration, the mean maximum exposure (Cmax) being 0.24 ± 0.23 ng/mL[17].
The present work summarizes the human PK behavior of ivermectin and its metabolites following topical applications of ivermectin 1% cream as developed recently for the treatment of PPR. A comprehensive assessment of the clinical PK profile of ivermectin following topical application was performed in healthy volunteers and PPR subjects after 4 wk of treatment. In addition, due to the anticipated chronic use of this treatment, systemic exposure levels were further investigated in long term studies of up to 1-year treatment.
A single-centre, open-label study to assess the pharmacokinetics and safety of ivermectin 1% cream has been conducted in healthy volunteers. Thirty-two male or female volunteers were enrolled in the study. A maximized dose (1 g of ivermectin 1% cream) was applied under nurses’ supervision on the whole face as a single application (Group 1: 8 subjects) or as repeated applications once (Group 2: 12 subjects) or twice (Group 3: 12 subjects) daily for 28 d. The treatment was followed by a 28 d or 56 d follow-up treatment-free period for the single and repeated dose respectively.
For the single application group (Group 1), blood samples for the determination of ivermectin plasma levels were collected over a 24 h period post dose and during a 28 d follow-up period.
For the repeated application groups [Group 2 (QD) and Group 3 (BID)], blood samples for the determination of ivermectin plasma levels were collected over a 24 hperiod on day 0 (first drug application), 14 and 28. In addition, pre-dose blood samples (residual levels) were collected on day 7 and 21. Blood samples were also collected during the 56 d follow-up period.
This study was a multi-centre, open label study, involving approximately 15 adult male or female subjects with severe PPR, i.e., with at least 25 inflammatory lesions and an Investigator Global Assessment (IGA) of rosacea of severe (score 4 on a 5-point rating scale from 0 to 4). Subjects were treated by nurses once daily on the whole face with 1 g of ivermectin 1% cream during a 4 wk period. The treatment was followed by a 28 d treatment-free follow-up period.
Blood samples were collected over 24 h in day 0 (first drug application), 14, and 28 to investigate the pharmacokinetics of ivermectin (and its related metabolites) in the plasma. In addition, pre-dose samples were collected at day 7 and 21. Blood draws were also sampled during the 4 wk following the last treatment application.
Two phase 3 studies of same design (Study #1 and Study #2) enrolled a total of 1371 adult subjects with moderate to severe PPR. The design of these studies has been previously described by Stein et al[4]. Overall, 1 group of subjects was treated with ivermectin 1% cream once daily for 52 wk, the remaining subjects were treated with the vehicle (during the first 12 wk of treatment) followed by an active treatment, azelaic acid 15% gel twice daily (from wk 13 to 52 of the study). Blood samples to assess ivermectin systemic levels were collected in a subset of subjects at approximately 12 h after the drug application at week 12, 32, 52 and at week 56 (4 wk after treatment stop).
In all clinical trials, ivermectin plasma concentrations were measured, after a solid-phase extraction, using the same validated high-performance liquid chromatography method (using fluorescence detection after a specific derivation to increase sensitivity). The limit of quantification of the method was 0.05 ng/mL. In addition, an estimation of concentration levels of the ivermectin metabolites was performed from the human plasma samples collected in the maximal use PK study.
Pharmacokinetic parameters (Cmin, Cmax, Tmax, AUC and t1/2) were calculated for each subject using a non-compartmental method (KineticaTM software, version 4.3, InnaPhase Corporation, Philadelphia, United States). Descriptive statistics were performed on PK parameters. In addition, selected PK parameters (from healthy volunteers and PPR PK studies) were transformed into natural logarithms (Ln) and submitted to an analysis of variance including subject and time as factors in the model to assess the steady state conditions. The statistical review of the study was performed by a biomedical statistician.
Thirty-two healthy volunteer subjects were enrolled. There was an equal repartition of males (50%) and females (50%) in each group. The mean age (± SD) was 30 ± 8 years (Table 1).
| Healthy volunteers PK study (n = 32) | PPR subjects maximal use PK study(n = 17) | |
| Age (yr) | ||
| Mean ± SD | 30 ± 8 | 54 ± 12 |
| (Min-max) | (18-45) | (35–74) |
| Gender (male/female) | 16/16 | 6/11 |
| IGA score: 4 (n %) | NA | 17 (100%) |
| Inflammatory lesion count | ||
| Mean ± SD | NA | 40.5 ± 14.3 |
| (Min-max) | (27.0-88.0) |
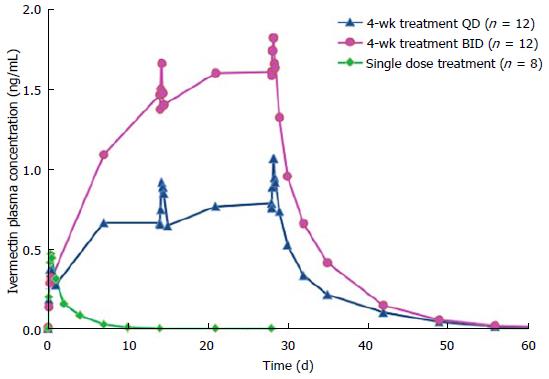
After a single topical application of ivermectin 1% cream, ivermectin plasma levels were quantifiable in all subjects (Figure 1). The mean values of AUC0-12h and AUC0-24 h were 3.8 ± 1.4, 8.3 ± 2.5 ng × h/mL, respectively (Table 2). The mean maximum plasma concentration of ivermectin peaked at 9 h after dosing (mean Cmax: 0.49 ± 0.15 ng/mL) and slowly decreased thereafter (Figure 1). The mean plasma terminal half-life was 45 h (range 32 to 130 h).
| Group | Time Point | Cmax(ng/mL) | Tmax(h) | AUC0-12 h(ng × h/mL) | AUC0-24 h(ng × h/mL) |
| 1: Single dose | NA | 0.49 ± 0.15 | 9 ± 3 | 3.8 ± 1.4 | 8.3 ± 2.5 |
| 2: QD 4 wk treatment | Day 0 | 0.41 ± 0.17 | 10 ± 5 | 3.1 ± 1.5 | 6.9 ± 2.9 |
| Day 14 | 0.93 ± 0.35 | NA | 9.8 ± 3.4 | 19 ± 7 | |
| Day 28 | 1.08 ± 0.43 | NA | 11 ± 5 | 21 ± 8 | |
| 3: BID 4 wk treatment | Day 0 | 0.34 ± 0.16 | 11 ± 2 | 2.6 ± 1.5 | NA |
| Day 14 | 1.70 ± 0.66 | NA | 18 ± 6 | NA | |
| Day 28 | 1.90 ± 0.76 | NA | 20 ± 9 | 38 ± 17 |
Following repeated topical applications of ivermectin 1% cream, systemic exposure was higher than that found after a single application (Table 2 and Figure 1). However, ivermectin systemic levels reached a plateau at day 14 of treatment for both QD and BID dosage regimen groups (Table 2 and Figure 1). In addition, the comparison of PK parameters (AUC and Cmax) calculated at d 14 and 28 have shown that there were no statistical differences in both dosage regimen groups, evidencing that the steady-state was already reached at day 14 (Table 2 and Figure 1).
After twice daily repeated topical applications of ivermectin 1% cream, the systemic exposure parameters (Cmax and AUC0-24 h) were 1.8-fold higher than parameters calculated for the once daily dosage regimen, suggesting a dose proportionality trend with the applied dose (Table 2). In addition, no gender effect on PK parameters was observed in this study (data not shown). After the last topical application, ivermectin was slowly eliminated with a mean half-life of 87 h (range 28 to 180 h) and 97 h (range 55 to 163 h) for the QD and the BID groups respectively.
From the 17 subjects enrolled, 2 discontinued the study prematurely, and 15 subjects completed the study. The mean age of all 17 subjects was 54 ± 12 years, and the majority of subjects were females (64.7%). All subjects presented a severe PPR with an IGA score of 4 and mean facial lesion count of 40.5 ± 14.3 (Table 1).
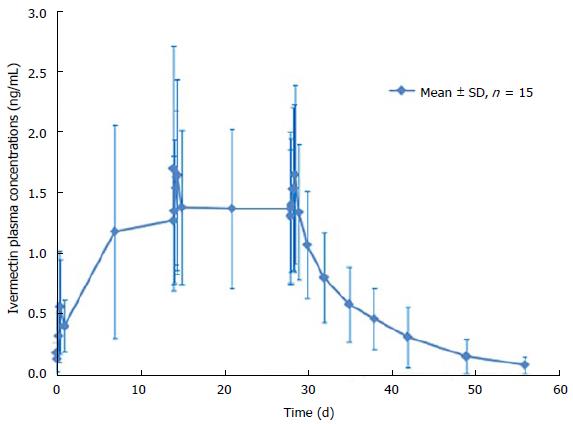
After 1 single topical application of ivermectin 1% cream, quantifiable ivermectin levels (> 0.05 ng/mL) were detected in the plasma of all subjects (Figure 2). Maximum plasma concentrations of ivermectin were observed within 9 h post dose with a mean Cmax of 0.69 ± 0.49 ng/mL and then slowly decreased thereafter to 0.37 ± 0.21 ng/mL, 24 h post dose (Cmin) (Table 3). After repeated topical application, ivermectin maximum concentration reached a plateau with a Cmax of 2.10 ± 1.04 ng/mL and 1.74 ± 0.77 ng/mL at day 14 and 28 (Figure 2). In addition, residual concentrations (Cmin) were also stable from day 7 to day 28 ranging from 1.17 ± 0.88 ng/mL to 1.36 ± 0.63 ng/mL.
Overall, all assessed systemic exposure PK parameters (Cmin, Cmax and AUC0-24 h) were stable through the treatment duration (Table 3). Indeed, after repeated topical applications of ivermectin 1% cream in subjects with severe PPR, exposure to ivermectin was similar at day 14 (AUC0-24 h of 36 ± 16 ngh/mL) and at day 28 (AUC0-24 h of 35 ± 14 ngh/mL), indicating that steady-state conditions were reached by 2 wk of treatment. Furthermore, the statistical analysis demonstrated that steady state conditions were achieved after 2 wk of treatment, as evidenced by the geometric mean ratio of AUC0-24 h of day 28-14 (0.99, 90%CI: 0.82-1.18).
At the end of the 28 d application period, ivermectin was slowly cleared from the plasma (Figure 2). The mean value for the apparent terminal half-life was 145 h (range 92 to 238 h).
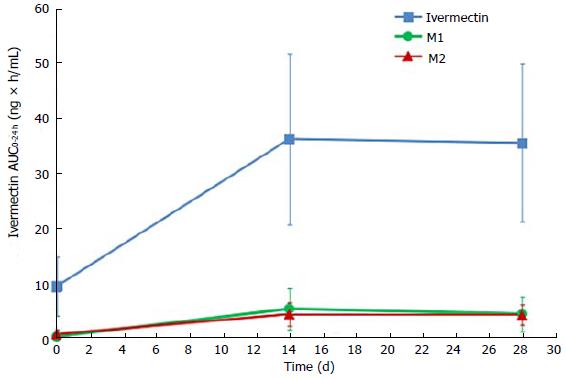
Ivermectin metabolites investigation has shown that 2 circulating metabolites represented more than 10% of parent drug systemic exposure at steady state. According to FDA guidance on safety testing of drug metabolites, these 2 metabolites are considered as major[18]. These 2 metabolites were identified as a 3’’O-demethyl ivermectin (M1) and 4a hydroxy ivermectin (M2). The systemic exposures of M1 at day 14 (AUC0-24 h of 5.2 ± 3.8 ng × h/mL) and at day 28 (AUC0-24 h of 4.3 ± 3.2 ng × h/mL) were similar, indicating that steady state was already reached by day 14. The same tendency was observed with M2, which had similar systemic exposures at day 14 (AUC0-24 h of 4.2 ± 2.1 ng × h/mL) and day 28 (AUC0-24 h of 4.1 ± 1.8 ng × h/mL) (Figure 3). At the end of the 28 d application period, the metabolites were slowly cleared from the plasma, with the last quantifiable concentration being observed 4 to 8 d after the last application.
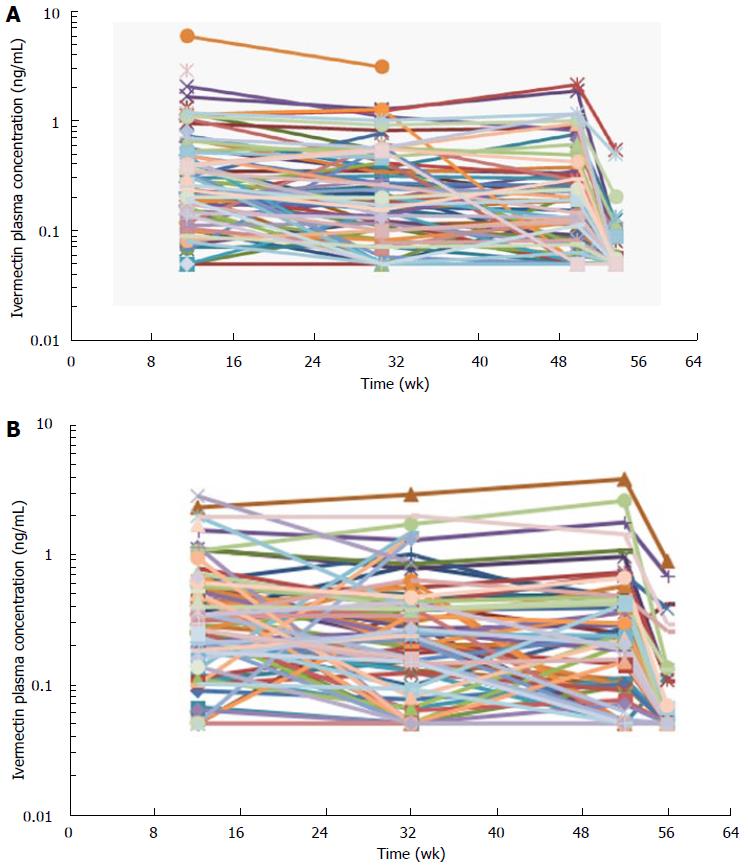
Blood samples for the assessment of ivermectin levels were collected in 197 subjects in the 2 phase 3 studies (Study #1 and Study #2). Ivermectin concentrations were stable through the 1-year treatment duration with concentrations means ranging from 0.3-0.5 ng/mL (Table 4 and Figure 4). Four weeks after the last treatment application (at week 56), ivermectin plasma concentration had decreased to mean concentrations of 0.07 and 0.1 ng/mL in Study #1 and #2, respectively (Figure 4). In addition, only 26% of subjects still had quantifiable low levels of ivermectin 4 wk after the last application, ranging from 0.05-0.89 ng/mL.
Overall, ivermectin 1% cream was safe and well tolerated after repeated topical treatment in both healthy volunteers and PPR subjects after 4 wk or 1 year treatment periods. With regards to ivermectin exposure, systemic levels were low and stable through the 1-year treatment duration without any further accumulation.
The pharmacokinetics investigation of ivermectin 1% cream was conducted on both healthy volunteers and subjects with moderate to severe rosacea (PPR). In addition, to assess ivermectin systemic levels under chronic use conditions, blood samples were collected during a treatment period up to 1 year. The PK studies conducted in healthy volunteers and PPR subjects showed that after the first topical administration ivermectin was not completely eliminated at the time of the second application (24 h after the first dose when considering a once daily dosage regimen). Subsequently, ivermectin plasma concentrations were higher during the second dosing interval. However, after repeated topical application, plasma concentrations of ivermectin increased progressively until reaching a plateau after 2 wk (i.e., steady state conditions) (Figures 1 and 2). After repeated topical applications of ivermectin 1% cream in healthy subjects, the PK behavior of ivermectin could be accurately predicted from single dose data, confirming that the PK profile of ivermectin was not affected by the repeated topical applications (time stationarity). Moreover, systemic exposure in healthy volunteers increased proportionally to the daily dose of ivermectin (dose proportionality) (Figure 1 and Table 2).
The PK study in PPR subjects was conducted under maximal use conditions to ensure the assessment of the maximal exposure. Then, the maximum body surface area involved in the pathology (whole face) and the maximum therapeutic dose (1 g) were used. In addition, subjects with PPR presented the upper level of severity (at least 25 lesions and IGA score of 4 in all subjects). Overall, ivermectin systemic exposure levels obtained in PPR subjects under maximized conditions were much lower than those observed after oral administration. The mean Cmax in PPR subjects treated under maximal use conditions was 1.74 ± 0.77 ng/mL after 4 wk treatment, while the means Cmax after 12 mg oral dose were from 23.5 to 50 ng/mL[10]. Overall, these data evidenced the limited ivermectin transdermal absorption.
The repeated topical applications of ivermectin 1% cream in this study resulted in similar exposure after 2 or 4 wk of treatment (AUC0-24 h of 36 ± 16 ng × h/mL at week 2 and AUC0-24 h of 35 ± 14 ng × h/mL at week 4), confirming that steady state was reached by 2 wk as was observed in healthy volunteers. In addition, at steady state levels, 2 metabolites, 3’’O-demethyl ivermectin and 4a hydroxy ivermectin, were considered as “major” because their systemic exposures were greater than 10% of ivermectin systemic exposure (parent compound)[18]. These 2 metabolites were previously characterized consecutive to oral administration of ivermectin[15]. In addition, these 2 metabolites were present in the same ratios (metabolite/parent) after oral ivermectin administration (data not shown).
With regard to impact of disease severity on ivermectin systemic exposure, no trend of correlation was observed between the number of inflammatory lesions and systemic ivermectin levels. From the maximal use PK study, the patient presenting the highest level of severity (subject with 88 inflammatory lesions) had a lower systemic level of ivermectin (Cmax of 1 ng/mL and an AUC0-24 h of 23 ng × h/mL) than the most exposed subject who had 35 inflammatory lesions at baseline (Cmax of 4 ng/mL and an AUC0-24 h of 75 ng × h/mL). In addition, the time to reach the peak of exposure (Tmax) and the time to reach the steady state conditions were similar between healthy volunteers and subjects with PPR. However, ivermectin systemic exposure levels in PPR subjects were slightly higher than those observed in healthy volunteers (1.6-fold higher). Nevertheless, considering the high variability and the limited number of subjects, no firm conclusions could be drawn on the impact of rosacea skin on ivermectin transdermal penetration.
At the end of the 4 wk treatment period, ivermectin was slowly cleared from the plasma in both healthy subjects and subjects with severe PPR. Under maximal use conditions, the half-life (t½) of ivermectin was approximately 6 d (range: 92-238 h), and the last quantifiable concentration was observed approximately 24 d after ivermectin application. This prolonged apparent half-life indicates that ivermectin was slowly cleared from plasma after the last treatment application. This terminal half-life is more prolonged than the one published for an oral administration of ivermectin oral tablets (15 to 20 h)[9]. This increase in terminal half-life observed by topical route suggests that absorption is the limiting step for ivermectin elimination. The term flip flop is used to describe this phenomenon[19]. Therefore, ivermectin elimination is limited by its slow absorption process through the skin (absorption dependent elimination): After the last application, ivermectin is slowly cleared from plasma, the low absorption phase becoming the limiting factor for its elimination. However, to confirm that no accumulation of ivermectin occurred in deeper body compartments and to confirm that steady state conditions are achieved, plasma samples were collected over longer treatment duration (up to 52 wk) in subjects with moderate to severe PPR. Overall, the ivermectin mean plasma concentrations measured at weeks 12, 32, and 52 were similar (Table 4 and Figure 4), supporting the assumption that steady state was achieved after 2 wk of treatment with no further accumulation.
Repeated topical application of ivermectin 1% cream resulted in lower systemic exposure levels in comparison to those observed after ivermectin oral administration, evidencing the limited ivermectin transdermal absorption. In addition, the steady state conditions were achieved by 2 wk of treatment and no accumulation occurred under chronic treatment as evidenced in long term use clinical studies for up to 1-year treatment. The pharmacokinetic behavior of ivermectin applied topically (prolonged plasma half-life) is consistent with a slow release of ivermectin from the skin rather than an accumulation in a deeper body compartment.
Pharmacokinetics investigations of topical drugs are of a high interest during drug development. The characterization of the transcutaneous penetration helps to assess the pathology effect of drug systemic exposure; and therefore, define accurately safety margins and the potential for drug-drug interactions.
For a long time due to the limited sensitivity of analytical methods, the pharmacokinetics behaviors of dermatological drugs were not investigated thoroughly. Therefore, limited information on drug safety was available. However, recent technological innovations in the bioanalytical field now allow the accurate quantification of very low levels of circulating compounds. Then, pharmacokinetics of topical drugs and their metabolites became feasible.
This article describes the comprehensive assessment of the ivermectin’s pharmacokinetics, a topical drug, recently approved for the treatment of papulopustular rosacea. This assessment includes metabolites investigation and the determination of the drug exposure in chronic use up to 1 year.
Pharmacokinetics results presented in this article will provide prescribers with valuable information about the systemic safety of this new treatment.
Pharmacokinetics is the study of the drug absorption, distribution, metabolism and elimination. These information are useful to establish treatment conditions and bring important knowledge on the drug safety.
This is an interesting and well written article regarding the pharmacokinetics of 1% Ivermectin cream.
P- Reviewer: Aksoy B, Feroze K S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Stankiewicz M, Cabaj W, Jonas WE, Moore LG, Millar K, Ng Chie W. Influence of ivermectin on cellular and humoral immune responses of lambs. Vet Immunol Immunopathol. 1995;44:347-358. [PubMed] |
| 2. | Forstinger C, Kittler H, Binder M. Treatment of rosacea-like demodicidosis with oral ivermectin and topical permethrin cream. J Am Acad Dermatol. 1999;41:775-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Rebora A. The management of rosacea. Am J Clin Dermatol. 2002;3:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Stein L, Kircik L, Fowler J, Tan J, Draelos Z, Fleischer A, Appell M, Steinhoff M, Lynde C, Liu H. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13:316-323. [PubMed] |
| 5. | Taieb A, Ortonne JP, Ruzicka T, Roszkiewicz J, Berth-Jones J, Peirone MH, Jacovella J. Superiority of ivermectin 1% cream over metronidazole 0·75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172:1103-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 6. | Deeks ED. Ivermectin: A Review in Rosacea. Am J Clin Dermatol. 2015;16:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 7. | Omura S, Crump A. Ivermectin: panacea for resource-poor communities? Trends Parasitol. 2014;30:445-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Porras AG, Chiou R, Kukovetz W, Hall-Gregg M, Stubbs RJ, Meisinger M, Beubler A, Jaeger A. Dose proportionality of the anthelmintic ivermectin in man. Pharmaceutical Res. 1987;4:S95. |
| 9. | Guzzo CA, Furtek CI, Porras AG, Chen C, Tipping R, Clineschmidt CM, Sciberras DG, Hsieh JY, Lasseter KC. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol. 2002;42:1122-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 222] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | González Canga A, Sahagún Prieto AM, Diez Liébana MJ, Fernández Martínez N, Sierra Vega M, García Vieitez JJ. The pharmacokinetics and interactions of ivermectin in humans--a mini-review. AAPS J. 2008;10:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 236] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 11. | Fink DW, Porras AG. Pharmacokinetics of ivermectin in animals and humans. Ivermectin and Abamectin. New-York: Springer-Verlag 1989; 113-130. [DOI] [Full Text] |
| 12. | Baraka OZ, Mahmoud BM, Marschke CK, Geary TG, Homeida MM, Williams JF. Ivermectin distribution in the plasma and tissues of patients infected with Onchocerca volvulus. Eur J Clin Pharmacol. 1996;50:407-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Krishna DR, Klotz U. Determination of ivermectin in human plasma by high-performance liquid chromatography. Arzneimittelforschung. 1993;43:609-611. [PubMed] |
| 14. | Klotz U, Ogbuokiri JE, Okonkwo PO. Ivermectin binds avidly to plasma proteins. Eur J Clin Pharmacol. 1990;39:607-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Zeng Z, Andrew NW, Arison BH, Luffer-Atlas D, Wang RW. Identification of cytochrome P4503A4 as the major enzyme responsible for the metabolism of ivermectin by human liver microsomes. Xenobiotica. 1998;28:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 16. | Pariser DM, Meinking TL, Bell M, Ryan WG. Topical 0.5% ivermectin lotion for treatment of head lice. N Engl J Med. 2012;367:1687-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Hazan L, Berg JE, Bowman JP, Murray JV, Ryan WG. Pharmacokinetics and safety of 0.5% ivermectin lotion for head louse infestations. Pediatr Dermatol. 2013;30:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | United States Food and Drug Administration. FDA guidance on Safety Testing of Drug Metabolites, February 2008; ICH Topic M3(R2) Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals. [accessed. New-York: Springer-Verlag 2015; Aug 12] Available from: http//www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm079266.pdf. |
| 19. | Toutain PL, Bousquet-Mélou A. Plasma terminal half-life. J Vet Pharmacol Ther. 2004;27:427-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |