Peer-review started: January 28, 2015
First decision: March 6, 2015
Revised: March 16, 2015
Accepted: April 8, 2015
Article in press: April 9, 2015
Published online: May 2, 2015
Processing time: 91 Days and 9 Hours
Mycosis fungoides is the most common form of cutaneous T-cell lymphoma (CTCL), and is characterized by a clonal expansion of malignant CD4+ T lymphocytes with skin-homing properties. Clinically and pathologically, mycosis fungoides can be categorized into patch, plaque and tumor stages. The clinical course of mycosis fungoides is usually chronic and indolent, but a proportion of patients may develop progressive disease with peripheral blood, lymph node and visceral organ involvement. Sézary syndrome is an aggressive leukemic form of CTCL characterized by a clonal population of malignant T cells in the peripheral blood. Various forms of skin-directed and systemic treatments are available for mycosis fungoides and Sézary syndrome. However, current treatments are generally not curative, and can only control the disease. Currently, the etiology and pathogenesis of mycosis fungoides and Sézary syndrome are not well defined. Proposed mechanisms include chronic antigenic stimulation by infectious agents, expression of specific adhesion molecules, altered cytokine production, mutations of oncogenes and tumor suppressor genes, and avoidance of apoptosis. In recent years, a number of chemokine receptors and their corresponding chemokine ligands have been found to contribute to the migration and survival of lymphoma cells in mycosis fungoides and Sézary syndrome, including CC chemokine receptor 4 (CCR4), CCR10, C-X-C chemokine receptor type 4 (CXCR4), CCR7, CCR3 and CXCR3. Since chemokines and chemokine receptors have been found to play important roles in the pathophysiology of mycosis fungoides and Sézary syndrome, they may be potentially useful targets for the development of new treatments for these diseases in the future.
Core tip: Mycosis fungoides and Sézary syndrome are characterized by a clonal expansion of malignant CD4+ T lymphocytes with skin-homing properties. Currently, treatment options for mycosis fungoides and Sézary syndrome are limited. The lack of effective targeted therapy results in part from the poor understanding regarding the pathophysiology of these diseases. Recently, a number of chemokines and chemokine receptors have been found to contribute to the pathogenesis of mycosis fungoides and Sézary syndrome, including the CC chemokine receptor 4 (CCR4)/chemokine (C-C motif) ligand 17 (CCL17), CCR10/CCL27, C-X-C chemokine receptor type 4/chemokine (C-X-C Motif) ligand 12 and CCR7/CCL21 axes. Therefore, these chemokines and chemokine receptors may be potentially useful targets for the treatment of these lymphomas in the future.
- Citation: Hu SCS. Mycosis fungoides and Sézary syndrome: Role of chemokines and chemokine receptors. World J Dermatol 2015; 4(2): 69-79
- URL: https://www.wjgnet.com/2218-6190/full/v4/i2/69.htm
- DOI: https://dx.doi.org/10.5314/wjd.v4.i2.69
Cutaneous T-cell lymphoma (CTCL) is a group of diseases characterized by malignant T lymphocytes infiltrating the skin, and includes mycosis fungoides, Sézary syndrome, lymphomatoid papulosis, anaplastic large cell lymphoma, subcutaneous panniculitis-like T-cell lymphoma, cutaneous natural killer/T-cell lymphoma, and primary cutaneous peripheral T-cell lymphoma[1]. This Editorial focuses on mycosis fungoides and Sézary syndrome.
Mycosis fungoides is the most common form of CTCL, and is characterized by a clonal expansion of malignant CD4+ T lymphocytes with skin-homing properties[2]. It is more common in the middle-aged and elderly, and is about twice more common in males than females[3]. However, mycosis fungoides can also occur in children and young adults[4]. Clinically and pathologically, mycosis fungoides can be categorized into patch, plaque and tumor stages. The clinical course of mycosis fungoides is usually chronic and indolent, but a subset of patients may develop progressive disease with peripheral blood, lymph node and visceral organ involvement[5]. Patients with mycosis fungoides also have a higher risk of developing a second malignancy, especially other types of lymphomas[6,7].
Sézary syndrome is an aggressive leukemic form of CTCL showing a clonal population of malignant T cells in the peripheral blood. Traditionally, mycosis fungoides and Sézary syndrome have been regarded as a spectrum of diseases with a common pathogenesis. More recently, investigations have indicated that mycosis fungoides and Sézary syndrome are two different diseases which originate from distinct T-cell subsets[8]. Mycosis fungoides is believed to be a lymphoma arising from skin resident “effector” memory T cells, in which atypical lymphocytes remain confined to the skin. On the other hand, Sézary syndrome is regarded as a lymphoma of “central” memory T cells, in which atypical lymphocytes circulate between the blood, skin and lymph nodes. This may partially account for the differences in biologic behaviors and prognosis between these two diseases.
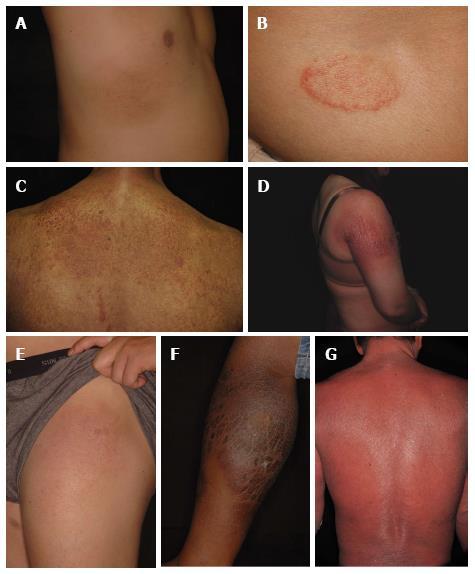
Classically, mycosis fungoides presents as erythematous patches and plaques, often associated with scaling (Figure 1). The skin lesions are usually located on non-sun exposed areas, such as the chest, abdomen, back, buttocks, groin and thigh. However, any region of the body can be affected. Pruritus is a common symptom. The skin lesions have usually been present for months to years, and they may gradually become thicker and develop into tumors. The three different types of skin lesions (patches, plaques, tumors) can sometimes be seen in a single patient concurrently. In certain patients, mycosis fungoides may progress into an erythrodermic form with generalized erythema and scaling of the skin[5].
Sézary syndrome is an aggressive leukemic form of CTCL. It is characterized by erythroderma (generalized erythema and scaling of the skin involving more than 80% of the body surface area), and a clonal population of malignant T lymphocytes in the peripheral blood[9,10]. Lymphadenopathy may or may not be present. This disease usually develops de novo without preceding mycosis fungoides.
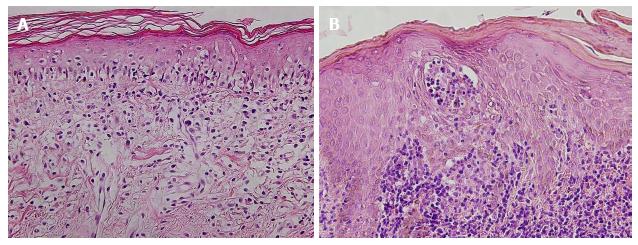
On histopathological examination, skin lesions of mycosis fungoides are characterized by atypical lymphocytes infiltrating mainly the dermis (Figure 2). The atypical lymphocytes are usually hyperchromatic, may have a haloed appearance, and show irregular, convoluted, or cerebriform nuclei[11]. They often accumulate in a band-like distribution at the dermoepidermal junction. They show a tendency for epidermotropism (migration into the epidermis without epidermal spongiosis), and may form aggregates with Langerhans cells in the epidermis (Pautrier’s microabscess) [12]. There may be an accompanying infiltrate of reactive inflammatory cells.
On immunohistochemical staining, the atypical lymphocytes in mycosis fungoides are usually CD4+, and there is an elevated CD4:CD8 ratio[11,13]. However, in a minority of cases the atypical lymphocytes are CD8+[14]. The atypical cells are also CD45RO+, which is a marker of memory T cells[15]. In addition, there is a loss of T cell antigens (including CD2, CD5, CD7 and CD26)[15]. In Sézary syndrome and certain cases of mycosis fungoides, Sézary cells can be detected in the peripheral blood by flow cytometry, and are identified as CD4+CD7- and/or CD4+CD26- cells[16,17].
The skin lesions of mycosis fungoides may show a dominant T cell clone. This is demonstrated by the presence of clonal T cell receptor gene rearrangement on polymerase chain reaction (PCR) analysis of the skin[18,19]. In Sézary syndrome patients, a large clonal population of atypical T lymphocytes may be found in the peripheral blood, determined by molecular methods (T cell receptor gene rearrangements by PCR) and flow cytometry (expression of specific T cell receptor Vβ epitopes)[10,20].
Patients should undergo a detailed physical examination to determine the total body surface area involved by lymphoma, as well as the surface area involved by patch, plaque and tumor stages[10]. This provides a measure of the skin tumor burden.
Laboratory tests that should be performed in patients with mycosis fungoides and Sézary syndrome include a complete blood count with differential counts, electrolytes and lactate dehydrogenase. A skin biopsy should be undertaken for histopathological examination, immunohistochemistry and T cell receptor gene rearrangement studies. Peripheral blood should be examined for Sézary cell count and clonality of circulating T cells. Computed tomography scans with positron emission tomography are useful for determining internal organ involvement[21]. Lymph node biopsy should also be performed in cases with lymphadenopathy.
The staging of mycosis fungoides is based on the International Society for Cutaneous Lymphomas/European Organization for Research and Treatment of Cancer system, and takes into account the extent of skin involvement (T), lymph node disease (N), visceral involvement (M), and the presence of Sézary cells in the peripheral blood (B). The TNMB classification is converted into a clinical stage[10,22].
The prognosis in patients with early stage mycosis fungoides (stage IA) is good, and these patients have a similar life expectancy as the general population[23]. Poor prognostic factors for mycosis fungoides include advanced stage, older age, elevated lactate dehydrogenase levels, presence of erythroderma, large cell transformation, presence of a clonal population of atypical lymphocytes in the peripheral blood, and high Sézary cell count[24-28]. The presence of CD8+ T cells in the skin of mycosis fungoides patients is associated with better prognosis, since this may lead to a host anti-lymphoma response[29].
The etiology and pathogenesis of mycosis fungoides and Sézary syndrome is currently not well defined. It has been proposed that mycosis fungoides is caused by chronic antigenic stimulation, which results in lymphocyte proliferation and eventually a clonal expansion of CD4+ T helper cells in the skin. Certain infections, such as Staphylococcus aureus and Chlamydia species, have been implicated in the etiology of mycosis fungoides[30-32].
Atypical lymphocytes in patients with mycosis fungoides and Sézary syndrome have been found to express the adhesion molecule cutaneous lymphocyte antigen, which may mediate the migration of lymphoma cells to the skin[33].
The cytokine profile in mycosis fungoides also changes according to the stage of the disease. In early stages of mycosis fungoides, Th1 cytokines [(interferon-γ, interleukin-12 (IL-12), IL-2] predominate[15,34]. In later stages of mycosis fungoides and Sézary syndrome, a shift from Th1 to Th2 cytokine profile is found, including IL-4, IL-5, IL-10, and IL-13[35-37].
T cell proliferation and cell cycle control may be dysregulated in patients with mycosis fungoides. Amplification and overexpression of the oncogene JUNB were found in a subset of patients with mycosis fungoides and Sézary syndrome[38]. Increased expression of the oncoproteins ras and myc has been implicated in the pathogenesis of mycosis fungoides[39].
Atypical lymphocytes in mycosis fungoides and Sézary syndrome have also been shown to be resistant to apoptosis. Fas is a death receptor which can mediate apoptosis, and studies have shown that low Fas expression and impaired Fas-mediated apoptosis may play a role in the pathogenesis of mycosis fungoides[40-42].
Chemokines and chemokine receptors were initially found to play important roles in mediating the chemotaxis (directional migration) of leukocytes[43]. Chemokines are a family of small polypeptides, and are categorized based on the location of cysteine residues near their amino termini into four families (C, CC, CXC, and CX3C). Chemokines bind to chemokine receptors, which are seven-membrane spanning, G-protein-coupled receptors. There are more than 50 different chemokines and at least 18 different chemokine receptors identified to date[44]. There are redundancies in the binding between chemokines and chemokine receptors, as some chemokines bind to multiple chemokine receptors, and vice versa. The activation of chemokine receptors by chemokines may activate various downstream signaling pathways, including the mitogen-activated protein kinase, phosphoinositide-3 kinase, and mammalian target of rapamycin (mTOR) pathways.
In recent years, various types of cancer cells have been found to express chemokine receptors, which have been shown to play important roles in cancer growth, progression and metastasis[45]. Apart from mediating cancer cell migration, chemokine receptors have also been demonstrated to mediate cancer cell proliferation, survival/apoptosis, and angiogenesis[46,47]. A number of chemokines and chemokine receptors have been found to contribute to the migration and survival of lymphoma cells in mycosis fungoides and Sézary syndrome (Table 1)[48-50].
| Chemokine receptor | Chemokine | Role in pathogenesis of mycosis fungoides and Sézary syndrome |
| CCR4 | CCL17 (TARC) CCL22 | Increased percentages of T lymphocytes expressing CCR4 in the blood and skin lesions of CTCL patients Activation of CCR4 promoted migration of mycosis fungoides cell lines CCL17 expression is upregulated in the skin lesions and serum of mycosis fungoides patients |
| CCR10 | CCL27 (CTACK) | CCR10 is expressed by malignant lymphocytes in skin lesions and peripheral blood of patients with mycosis fungoides and Sézary syndrome The level of CCL27 is increased in the serum and skin of patients with mycosis fungoides |
| CXCR4 | CXCL12 (SDF) | CXCR4 is expressed by Sézary cells, and acts as a chemotactic factor for Sézary cells Loss of the cell-surface antigen CD26 (which cleaves and deactivates the CXCL12) is a characteristic feature in Sézary syndrome |
| CCR7 | CCL19 (MIP-3b) CCL21 (SLC) | CCR7 is expressed on atypical lymphocytes of Sézary syndrome CCR7 promotes migration of Sézary cells CCR7 was expressed in mycosis fungoides skin lesions, and its expression correlated with subcutaneous extension of lymphoma cells Activation of CCR7 by its ligand CCL21 promotes MyLa (mycosis fungoides cell line) cell migration through the mTOR pathway |
| CCR3 | Eotaxin-3 Eotaxin-1 | Skin lesions of CTCL show higher expression of CCR3 and eotaxin-3 CTCL patients show higher serum levels of eotaxin-3 and eotaxin-1 |
| CXCR3 | CXCL9 CXCL10 CXCL11 | CXCR3 is expressed in low-grade mycosis fungoides |
The chemokine receptor CC chemokine receptor 4 (CCR4) has been found to be important in mediating the migration of normal lymphocytes to inflamed skin[51,52]. There are increased percentages of T lymphocytes expressing CCR4 in the blood and skin lesions of patients with CTCL (including mycosis fungoides and Sézary syndrome)[8,53-55]. CCR4 expression is also seen in mycosis fungoides tumors with large cell transformation[56]. Furthermore, CCR4 was found to be expressed by mycosis fungoides cell lines, and activation of the CCR4 receptor promoted migration of lymphoma cells[57]. The CCR4 ligand chemokine (C-C motif) ligand 17 (CCL17) (thymus and activation regulated chemokine) is produced by activated epidermal keratinocytes, dendritic cells and endothelial cells, and its expression is upregulated in the skin lesions and serum of mycosis fungoides patients[58,59]. In addition, serum CCL17 levels were found to correlate with disease activity in patients with mycosis fungoides[59]. Therefore, the interaction between CCR4 and CCL17 may play a role in the homing of mycosis fungoides cells to the skin or promote the survival of lymphoma cells.
The chemokine receptor CCR10 has been found to be expressed by normal lymphocytes which home to the skin[60,61]. CCR10 has been demonstrated to be expressed by malignant lymphocytes in skin lesions of mycosis fungoides and Sézary syndrome, and mediated migration of Sézary cell line[62,63]. In patients with mycosis fungoides and Sézary syndrome, increased numbers of lymphocytes expressing CCR10 was also found in the peripheral blood[63-65]. The CCR10 ligand CCL27 (cutaneous T-cell attracting chemokine), a skin-specific chemokine, is synthesized by epidermal keratinocytes[66,67]. The level of CCL27 was increased in the serum and skin of patients with mycosis fungoides[63,68], and may act as a therapeutic marker following interferon-α and psoralen and ultraviolet-A (PUVA) treatment[69].
The chemokine receptor C-X-C chemokine receptor type 4 (CXCR4) may also be involved in homing of mycosis fungoides and Sézary cells to skin. CXCR4 has been demonstrated to be expressed by Sézary cells, and acts as a chemotactic factor for Sézary cells[55]. CXCR4 has also been shown to be expressed in mycosis fungoides skin lesions[70]. The ligand for CXCR4 is the chemokine chemokine (C-X-C Motif) ligand 12 (CXCL12) (also known as stromal cell-derived factor 1), which is expressed by skin dermal fibroblasts and endothelial cells[71,72]. In Sézary syndrome, loss of the cell-surface antigen CD26 is a characteristic feature[16,73]. CD26 is a dipeptidyl peptidase which cleaves and deactivates CXCL12, preventing it from activating CXCR4. The downregulation of CD26 and subsequent increased levels of CXCL12 may promote CXCL12-induced chemotaxis of Sézary cells[55].
CCR7 is a chemokine receptor which has been discovered to mediate the migration of T lymphocytes and dendritic cells to lymphatic vessels and lymph nodes[74-76]. The ligands for CCR7 are CCL19 (also known as macrophage inflammatory protein-3b, MIP-3b) and CCL21 (also known as secondary lymphoid-tissue chemokine). CCR7 has been found to be involved in the lymph node metastasis of certain cancer cells[77-79]. In addition, CCR7 plays a role in cancer cell proliferation, migration and invasion.
Previous studies have indicated that CCR7 is expressed on atypical lymphocytes of Sézary syndrome[8,64,65], and may promote migration of Sézary cells[80]. CCR7 is also expressed in tumor-stage mycosis fungoides[70]. Recently, our research group found that CCR7 was expressed in 62% (13 out of 21) of mycosis fungoides skin tissue specimens, and its expression correlated with subcutaneous extension of lymphoma cells (an indication of lesion thickness). In addition, we showed that CCR7 expression was increased on the surface of MyLa cells (a human mycosis fungoides cell line) compared to peripheral blood mononuclear cells. Activation of CCR7 by its ligand CCL21 promoted MyLa cell migration but not proliferation. We also demonstrated that the CCL21-induced MyLa cell migration was mediated through the mTOR pathway[81].
It has been demonstrated that in skin lesions of CTCL (mycosis fungoides), keratinocytes, endothelial cells and dermal fibroblasts showed higher expression of eotaxin-3 compared to normal skin. In some advanced cases of CTCL, atypical lymphocytes in skin lesions were found to express CCR3, the chemokine receptor for eotaxins. These patients also show higher serum levels of eotaxin-3 and eotaxin-1. Therefore, the interaction between eotaxins and CCR3 may play a role in the pathogenesis of mycosis fungoides[82].
In addition, the chemokine receptor CXCR3 has been found to be expressed in low-grade (patch and plaque stage) mycosis fungoides, especially in the epidermotropic lymphoma cells[83-85].
There are a variety of different treatment strategies available for mycosis fungoides and Sézary syndrome (Table 2), depending on the severity of the disease and patient factors[86-89]. Since mycosis fungoides and Sézary syndrome are generally not curable, chronic management is required in order to control the disease. In early stage mycosis fungoides, (stages IA-IIA), lymphoma cells are mainly confined to the skin, and skin-directed therapies are usually used. In advanced stage mycosis fungoides (stages IIB-IVB) and Sézary syndrome, systemic therapies may be selected, including immunotherapy, targeted therapies and chemotherapy[90]. Each of the different treatment strategies are associated with various adverse effects, the discussion of which is beyond the scope of this Editorial.
| Skin-directed therapies | Systemic therapies |
| Topical corticosteroids | Oral retinoid (bexarotene) |
| Topical nitrogen mustard | IL-12 |
| Topical retinoid (bexarotene) | Interferon-α |
| Ultraviolet light phototherapy (PUVA, narrowband UVB) | Histone deacetylase inhibitors |
| Extracorporeal photopheresis | |
| Radiation therapy | Methotrexate |
| Chemotherapy | |
| Hematopoietic stem cell transplantation |
In early stage mycosis fungoides, topical corticosteroids is the most commonly used form of treatment[91]. It can also be used in combination with other therapies in advanced stages of disease. Topical nitrogen mustard (a DNA alkylating agent) or topical retinoids (bexarotene, tazarotene) may also be used in early stage mycosis fungoides[92,93].
Phototherapy with ultraviolet light may be effective for patients with early stage (stages IA-IIA) mycosis fungoides. Forms of phototherapy include PUVA with oral 8-methoxypsoralen, and narrowband ultraviolet B (311 nm)[94-96].
Ionizing radiation therapy has deeper penetration compared to ultraviolet phototherapy[97]. Total skin electron beam therapy may be used for patients with rapidly progressive or refractory disease, and plaque or tumor lesions involving large body surface area[98,99]. Localized radiotherapy may be suitable for patients with localized tumor lesions[100].
The oral retinoid bexarotene is used for the treatment of refractory mycosis fungoides and Sézary syndrome in all stages[101,102]. Bexarotene acts by modulating cell differentiation and apoptosis, and also decreases the expression of CCR4 on malignant lymphocytes, which may inhibit their ability to migrate to the skin[103,104].
In mycosis fungoides and Sézary syndrome, there is an increased expression of Th2 cytokines (including IL-4, IL-5, and IL-10). IL-12 is a Th1-promoting cytokine, and has been shown to be efficacious in some patients[105]. Interferon-α may also be effective for different stages of mycosis fungoides, and act by inducing Th1-mediated immune responses to atypical lymphocytes[106].
In extracorporeal photopheresis, the circulating lymphocytes are separated from the patients’ peripheral blood, 8-methoxypsoralen is added, and the cells are treated with ultraviolet-A light. This treatment is indicated for erythrodermic mycosis fungoides and Sézary syndrome[107,108].
Chemotherapy drugs may be used for refractory or progressive mycosis fungoides and Sézary syndrome. Methotrexate is an antifolate agent which acts by inhibiting dihydrofolate reductase and thereby inhibits proliferation of lymphocytes[109]. Hematopoietic stem cell transplantation may be used in advanced stage mycosis fungoides and Sézary syndrome, and have the potential for curing the disease[110].
Mycosis fungoides and Sézary syndrome are characterized by a clonal expansion of malignant CD4+ T lymphocytes with skin-homing properties, and have potential for lymph node, blood and visceral organ dissemination. Currently, treatment strategies for mycosis fungoides and Sézary syndrome are limited. The lack of effective targeted therapy results in part from the current poor understanding regarding the pathophysiology of these diseases. Since chemokines and chemokine receptors have been found to play important roles in the pathogenesis of mycosis fungoides and Sézary syndrome, they may be useful targets for the development of new treatments for these diseases[111]. Previously, antibodies against CCR4 which induce antibody-dependent cellular cytotoxicity have been used in the treatment of mycosis fungoides and Sézary syndrome[112]. In addition, chemokine-toxin fusion proteins (for example CCL17 ligated to the Pseudomonas exotoxin 38) have been demonstrated to selectively target and kill lymphoma cells which express CCR4[113]. Therefore, further investigations are warranted to determine whether modulation of chemokines and chemokine receptors may be potentially useful for the treatment of mycosis fungoides and Sézary syndrome in the future.
P- Reviewer: Cuevas-Covarrubias SA, Kaliyadan F, Vasconcellos C S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2714] [Cited by in RCA: 2577] [Article Influence: 128.9] [Reference Citation Analysis (2)] |
| 2. | Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350:1978-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 269] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 3. | Bradford PT, Devesa SS, Anderson WF, Toro JR. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064-5073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 484] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 4. | Laws PM, Shear NH, Pope E. Childhood mycosis fungoides: experience of 28 patients and response to phototherapy. Pediatr Dermatol. 2014;31:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Hwang ST, Janik JE, Jaffe ES, Wilson WH. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 6. | Huang KP, Weinstock MA, Clarke CA, McMillan A, Hoppe RT, Kim YH. Second lymphomas and other malignant neoplasms in patients with mycosis fungoides and Sezary syndrome: evidence from population-based and clinical cohorts. Arch Dermatol. 2007;143:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Kantor AF, Curtis RE, Vonderheid EC, van Scott EJ, Fraumeni JF. Risk of second malignancy after cutaneous T-cell lymphoma. Cancer. 1989;63:1612-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Campbell JJ, Clark RA, Watanabe R, Kupper TS. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. 2010;116:767-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 435] [Cited by in RCA: 371] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 9. | Foss F. Mycosis fungoides and the Sézary syndrome. Curr Opin Oncol. 2004;16:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Olsen EA, Whittaker S, Kim YH, Duvic M, Prince HM, Lessin SR, Wood GS, Willemze R, Demierre MF, Pimpinelli N. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 493] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 11. | McKee PH, Calonje E, Granter SR. Pathology of the Skin: With Clinical Correlations. 3rd ed. Philadelphia, Pa: Elsevier Mosby 2005; . |
| 12. | Kazakov DV, Burg G, Kempf W. Clinicopathological spectrum of mycosis fungoides. J Eur Acad Dermatol Venereol. 2004;18:397-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Berger CL, Warburton D, Raafat J, LoGerfo P, Edelson RL. Cutaneous T-cell lymphoma: neoplasm of T cells with helper activity. Blood. 1979;53:642-651. [PubMed] |
| 14. | Nikolaou VA, Papadavid E, Katsambas A, Stratigos AJ, Marinos L, Anagnostou D, Antoniou C. Clinical characteristics and course of CD8+ cytotoxic variant of mycosis fungoides: a case series of seven patients. Br J Dermatol. 2009;161:826-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Jawed SI, Myskowski PL, Horwitz S, Moskowitz A, Querfeld C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-216.e1; quiz 221-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 16. | Sokolowska-Wojdylo M, Wenzel J, Gaffal E, Steitz J, Roszkiewicz J, Bieber T, Tüting T. Absence of CD26 expression on skin-homing CLA+ CD4+ T lymphocytes in peripheral blood is a highly sensitive marker for early diagnosis and therapeutic monitoring of patients with Sézary syndrome. Clin Exp Dermatol. 2005;30:702-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Hristov AC, Vonderheid EC, Borowitz MJ. Simplified flow cytometric assessment in mycosis fungoides and Sézary syndrome. Am J Clin Pathol. 2011;136:944-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Wood GS, Tung RM, Haeffner AC, Crooks CF, Liao S, Orozco R, Veelken H, Kadin ME, Koh H, Heald P. Detection of clonal T-cell receptor gamma gene rearrangements in early mycosis fungoides/Sezary syndrome by polymerase chain reaction and denaturing gradient gel electrophoresis (PCR/DGGE). J Invest Dermatol. 1994;103:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 233] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Yang H, Xu C, Tang Y, Wan C, Liu W, Wang L. The significance of multiplex PCR/heteroduplex analysis-based TCR-γ gene rearrangement combined with laser-capture microdissection in the diagnosis of early mycosis fungoides. J Cutan Pathol. 2012;39:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Morice WG, Katzmann JA, Pittelkow MR, el-Azhary RA, Gibson LE, Hanson CA. A comparison of morphologic features, flow cytometry, TCR-Vbeta analysis, and TCR-PCR in qualitative and quantitative assessment of peripheral blood involvement by Sézary syndrome. Am J Clin Pathol. 2006;125:364-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Tsai EY, Taur A, Espinosa L, Quon A, Johnson D, Dick S, Chow S, Advani R, Warnke R, Kohler S. Staging accuracy in mycosis fungoides and sezary syndrome using integrated positron emission tomography and computed tomography. Arch Dermatol. 2006;142:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, Zackheim H, Duvic M, Estrach T, Lamberg S. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 976] [Cited by in RCA: 996] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 23. | Kim YH, Jensen RA, Watanabe GL, Varghese A, Hoppe RT. Clinical stage IA (limited patch and plaque) mycosis fungoides. A long-term outcome analysis. Arch Dermatol. 1996;132:1309-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 152] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Talpur R, Singh L, Daulat S, Liu P, Seyfer S, Trynosky T, Wei W, Duvic M. Long-term outcomes of 1,263 patients with mycosis fungoides and Sézary syndrome from 1982 to 2009. Clin Cancer Res. 2012;18:5051-5060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 25. | Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, Robson A, Calonje E, Stefanato CM, Wain EM. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 596] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 26. | Zackheim HS, Amin S, Kashani-Sabet M, McMillan A. Prognosis in cutaneous T-cell lymphoma by skin stage: long-term survival in 489 patients. J Am Acad Dermatol. 1999;40:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 183] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Fraser-Andrews EA, Russell-Jones R, Woolford AJ, Wolstencroft RA, Dean AJ, Whittaker SJ. Diagnostic and prognostic importance of T-cell receptor gene analysis in patients with Sézary syndrome. Cancer. 2001;92:1745-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Kim YH, Bishop K, Varghese A, Hoppe RT. Prognostic factors in erythrodermic mycosis fungoides and the Sézary syndrome. Arch Dermatol. 1995;131:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 82] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Hoppe RT, Medeiros LJ, Warnke RA, Wood GS. CD8-positive tumor-infiltrating lymphocytes influence the long-term survival of patients with mycosis fungoides. J Am Acad Dermatol. 1995;32:448-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 128] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Talpur R, Bassett R, Duvic M. Prevalence and treatment of Staphylococcus aureus colonization in patients with mycosis fungoides and Sézary syndrome. Br J Dermatol. 2008;159:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | Jackow CM, Cather JC, Hearne V, Asano AT, Musser JM, Duvic M. Association of erythrodermic cutaneous T-cell lymphoma, superantigen-positive Staphylococcus aureus, and oligoclonal T-cell receptor V beta gene expansion. Blood. 1997;89:32-40. [PubMed] |
| 32. | Abrams JT, Balin BJ, Vonderheid EC. Association between Sézary T cell-activating factor, Chlamydia pneumoniae, and cutaneous T cell lymphoma. Ann N Y Acad Sci. 2001;941:69-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Heald PW, Yan SL, Edelson RL, Tigelaar R, Picker LJ. Skin-selective lymphocyte homing mechanisms in the pathogenesis of leukemic cutaneous T-cell lymphoma. J Invest Dermatol. 1993;101:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Saed G, Fivenson DP, Naidu Y, Nickoloff BJ. Mycosis fungoides exhibits a Th1-type cell-mediated cytokine profile whereas Sezary syndrome expresses a Th2-type profile. J Invest Dermatol. 1994;103:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 125] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Vowels BR, Cassin M, Vonderheid EC, Rook AH. Aberrant cytokine production by Sezary syndrome patients: cytokine secretion pattern resembles murine Th2 cells. J Invest Dermatol. 1992;99:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 190] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Vowels BR, Lessin SR, Cassin M, Jaworsky C, Benoit B, Wolfe JT, Rook AH. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J Invest Dermatol. 1994;103:669-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 188] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 37. | Papadavid E, Economidou J, Psarra A, Kapsimali V, Mantzana V, Antoniou C, Limas K, Stratigos A, Stavrianeas N, Avgerinou G. The relevance of peripheral blood T-helper 1 and 2 cytokine pattern in the evaluation of patients with mycosis fungoides and Sézary syndrome. Br J Dermatol. 2003;148:709-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Mao X, Orchard G, Lillington DM, Russell-Jones R, Young BD, Whittaker SJ. Amplification and overexpression of JUNB is associated with primary cutaneous T-cell lymphomas. Blood. 2003;101:1513-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 39. | Tosca A, Linardopoulos S, Malliri A, Hatziolou E, Nicolaidou A, Spandidos DA. Implication of the ras and myc oncoproteins in the pathogenesis of mycosis fungoides. Anticancer Res. 1991;11:1433-1438. [PubMed] |
| 40. | Wu J, Nihal M, Siddiqui J, Vonderheid EC, Wood GS. Low FAS/CD95 expression by CTCL correlates with reduced sensitivity to apoptosis that can be restored by FAS upregulation. J Invest Dermatol. 2009;129:1165-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Zoi-Toli O, Vermeer MH, De Vries E, Van Beek P, Meijer CJ, Willemze R. Expression of Fas and Fas-ligand in primary cutaneous T-cell lymphoma (CTCL): association between lack of Fas expression and aggressive types of CTCL. Br J Dermatol. 2000;143:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Nagasawa T, Takakuwa T, Takayama H, Dong Z, Miyagawa S, Itami S, Yoshikawa K, Aozasa K. Fas gene mutations in mycosis fungoides: analysis of laser capture-microdissected specimens from cutaneous lesions. Oncology. 2004;67:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 507] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 44. | Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R. The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev. 2010;21:27-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 296] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 45. | Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1747] [Cited by in RCA: 1801] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 46. | Kulbe H, Levinson NR, Balkwill F, Wilson JL. The chemokine network in cancer--much more than directing cell movement. Int J Dev Biol. 2004;48:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 47. | Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA. Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer. 2006;42:768-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 317] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 48. | Lee CH, Hwang ST. Pathophysiology of chemokines and chemokine receptors in dermatological science: A focus on psoriasis and cutaneous T-cell lymphoma. Dermatologica Sinica. 2012;30:128-135. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Wu XS, Lonsdorf AS, Hwang ST. Cutaneous T-cell lymphoma: roles for chemokines and chemokine receptors. J Invest Dermatol. 2009;129:1115-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Lonsdorf AS, Hwang ST, Enk AH. Chemokine receptors in T-cell-mediated diseases of the skin. J Invest Dermatol. 2009;129:2552-2566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541-1547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 399] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 52. | Campbell JJ, O’Connell DJ, Wurbel MA. Cutting Edge: Chemokine receptor CCR4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J Immunol. 2007;178:3358-3362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 53. | Ferenczi K, Fuhlbrigge RC, Pinkus J, Pinkus GS, Kupper TS. Increased CCR4 expression in cutaneous T cell lymphoma. J Invest Dermatol. 2002;119:1405-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 206] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 54. | Fierro MT, Comessatti A, Quaglino P, Ortoncelli M, Osella Abate S, Ponti R, Novelli M, Bernengo MG. Expression pattern of chemokine receptors and chemokine release in inflammatory erythroderma and Sézary syndrome. Dermatology. 2006;213:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Narducci MG, Scala E, Bresin A, Caprini E, Picchio MC, Remotti D, Ragone G, Nasorri F, Frontani M, Arcelli D. Skin homing of Sézary cells involves SDF-1-CXCR4 signaling and down-regulation of CD26/dipeptidylpeptidase IV. Blood. 2006;107:1108-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 56. | Jones D, O’Hara C, Kraus MD, Perez-Atayde AR, Shahsafaei A, Wu L, Dorfman DM. Expression pattern of T-cell-associated chemokine receptors and their chemokines correlates with specific subtypes of T-cell non-Hodgkin lymphoma. Blood. 2000;96:685-690. [PubMed] |
| 57. | Wu CS, Wang ST, Liao CY, Wu MT. Differential CCR4 expression and function in cutaneous T-cell lymphoma cell lines. Kaohsiung J Med Sci. 2008;24:577-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Abou El-Ela M, El-Rifae Ael-A, Fawzi M, Abdel Hay R, Gohary Y, Shaker O. Thymus and activation-regulated chemokine in different stages of mycosis fungoides: tissue and serum levels. Australas J Dermatol. 2011;52:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Kakinuma T, Sugaya M, Nakamura K, Kaneko F, Wakugawa M, Matsushima K, Tamaki K. Thymus and activation-regulated chemokine (TARC/CCL17) in mycosis fungoides: serum TARC levels reflect the disease activity of mycosis fungoides. J Am Acad Dermatol. 2003;48:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 60. | Jarmin DI, Rits M, Bota D, Gerard NP, Graham GJ, Clark-Lewis I, Gerard C. Cutting edge: identification of the orphan receptor G-protein-coupled receptor 2 as CCR10, a specific receptor for the chemokine ESkine. J Immunol. 2000;164:3460-3464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Homey B, Alenius H, Müller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bünemann E. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 588] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 62. | Notohamiprodjo M, Segerer S, Huss R, Hildebrandt B, Soler D, Djafarzadeh R, Buck W, Nelson PJ, von Luettichau I. CCR10 is expressed in cutaneous T-cell lymphoma. Int J Cancer. 2005;115:641-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 63. | Fujita Y, Abe R, Sasaki M, Honda A, Furuichi M, Asano Y, Norisugi O, Shimizu T, Shimizu H. Presence of circulating CCR10+ T cells and elevated serum CTACK/CCL27 in the early stage of mycosis fungoides. Clin Cancer Res. 2006;12:2670-2675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Sokolowska-Wojdylo M, Wenzel J, Gaffal E, Lenz J, Speuser P, Erdmann S, Abuzahra F, Bowman E, Roszkiewicz J, Bieber T. Circulating clonal CLA(+) and CD4(+) T cells in Sezary syndrome express the skin-homing chemokine receptors CCR4 and CCR10 as well as the lymph node-homing chemokine receptor CCR7. Br J Dermatol. 2005;152:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 65. | Capriotti E, Vonderheid EC, Thoburn CJ, Bright EC, Hess AD. Chemokine receptor expression by leukemic T cells of cutaneous T-cell lymphoma: clinical and histopathological correlations. J Invest Dermatol. 2007;127:2882-2892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, Orozco R, Copeland NG, Jenkins NA, McEvoy LM. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci USA. 1999;96:14470-14475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 388] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 67. | Homey B, Wang W, Soto H, Buchanan ME, Wiesenborn A, Catron D, Müller A, McClanahan TK, Dieu-Nosjean MC, Orozco R. Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC). J Immunol. 2000;164:3465-3470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 240] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 68. | Kagami S, Sugaya M, Minatani Y, Ohmatsu H, Kakinuma T, Fujita H, Tamaki K. Elevated serum CTACK/CCL27 levels in CTCL. J Invest Dermatol. 2006;126:1189-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 69. | Goteri G, Rupoli S, Campanati A, Zizzi A, Picardi P, Cardelli M, Giantomassi F, Canafoglia L, Marchegiani F, Mozzicafreddo G. Serum and tissue CTACK/CCL27 chemokine levels in early mycosis fungoides may be correlated with disease-free survival following treatment with interferon alfa and psoralen plus ultraviolet A therapy. Br J Dermatol. 2012;166:948-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Kallinich T, Muche JM, Qin S, Sterry W, Audring H, Kroczek RA. Chemokine receptor expression on neoplastic and reactive T cells in the skin at different stages of mycosis fungoides. J Invest Dermatol. 2003;121:1045-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 71. | Fedyk ER, Jones D, Critchley HO, Phipps RP, Blieden TM, Springer TA. Expression of stromal-derived factor-1 is decreased by IL-1 and TNF and in dermal wound healing. J Immunol. 2001;166:5749-5754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 72. | Toksoy A, Müller V, Gillitzer R, Goebeler M. Biphasic expression of stromal cell-derived factor-1 during human wound healing. Br J Dermatol. 2007;157:1148-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 73. | Scala E, Russo G, Cadoni S, Narducci MG, Girardelli CR, De Pità O, Puddu P. Skewed expression of activation, differentiation and homing-related antigens in circulating cells from patients with cutaneous T cell lymphoma associated with CD7- T helper lymphocytes expansion. J Invest Dermatol. 1999;113:622-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 74. | Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2472-2475. [PubMed] |
| 75. | Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1812] [Cited by in RCA: 1815] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 76. | Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 746] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 77. | Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3911] [Cited by in RCA: 3966] [Article Influence: 165.3] [Reference Citation Analysis (0)] |
| 78. | Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, Inoue H, Mori M. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;62:2937-2941. [PubMed] |
| 79. | Günther K, Leier J, Henning G, Dimmler A, Weissbach R, Hohenberger W, Förster R. Prediction of lymph node metastasis in colorectal carcinoma by expressionof chemokine receptor CCR7. Int J Cancer. 2005;116:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 80. | Picchio MC, Scala E, Pomponi D, Caprini E, Frontani M, Angelucci I, Mangoni A, Lazzeri C, Perez M, Remotti D. CXCL13 is highly produced by Sézary cells and enhances their migratory ability via a synergistic mechanism involving CCL19 and CCL21 chemokines. Cancer Res. 2008;68:7137-7146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 81. | Hu SC, Lin CL, Hong CH, Yu HS, Chen GS, Lee CH. CCR7 expression correlates with subcutaneous involvement in mycosis fungoides skin lesions and promotes migration of mycosis fungoides cells (MyLa) through mTOR activation. J Dermatol Sci. 2014;74:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 82. | Miyagaki T, Sugaya M, Fujita H, Ohmatsu H, Kakinuma T, Kadono T, Tamaki K, Sato S. Eotaxins and CCR3 interaction regulates the Th2 environment of cutaneous T-cell lymphoma. J Invest Dermatol. 2010;130:2304-2311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 83. | Lu D, Duvic M, Medeiros LJ, Luthra R, Dorfman DM, Jones D. The T-cell chemokine receptor CXCR3 is expressed highly in low-grade mycosis fungoides. Am J Clin Pathol. 2001;115:413-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Yagi H, Seo N, Ohshima A, Itoh T, Itoh N, Horibe T, Yoshinari Y, Takigawa M, Hashizume H. Chemokine receptor expression in cutaneous T cell and NK/T-cell lymphomas: immunohistochemical staining and in vitro chemotactic assay. Am J Surg Pathol. 2006;30:1111-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Yamaguchi T, Ohshima K, Tsuchiya T, Suehuji H, Karube K, Nakayama J, Suzumiya J, Yoshino T, Kikuchi M. The comparison of expression of cutaneous lymphocyte-associated antigen (CLA), and Th1- and Th2-associated antigens in mycosis fungoides and cutaneous lesions of adult T-cell leukemia/lymphoma. Eur J Dermatol. 2003;13:553-559. [PubMed] |
| 86. | Keehn CA, Belongie IP, Shistik G, Fenske NA, Glass LF. The diagnosis, staging, and treatment options for mycosis fungoides. Cancer Control. 2007;14:102-111. [PubMed] |
| 87. | Whittaker SJ, Foss FM. Efficacy and tolerability of currently available therapies for the mycosis fungoides and Sezary syndrome variants of cutaneous T-cell lymphoma. Cancer Treat Rev. 2007;33:146-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 88. | Trautinger F, Knobler R, Willemze R, Peris K, Stadler R, Laroche L, D’Incan M, Ranki A, Pimpinelli N, Ortiz-Romero P. EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome. Eur J Cancer. 2006;42:1014-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 273] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 89. | Lundin J, Osterborg A. Therapy for mycosis fungoides. Curr Treat Options Oncol. 2004;5:203-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 90. | Jawed SI, Myskowski PL, Horwitz S, Moskowitz A, Querfeld C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-217.e1; quiz 240-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 221] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 91. | Freiman A, Sasseville D. Treatment of mycosis fungoides: overview. J Cutan Med Surg. 2006;10:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 92. | Kim YH. Management with topical nitrogen mustard in mycosis fungoides. Dermatol Ther. 2003;16:288-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 93. | Lain T, Talpur R, Duvic M. Long-term control of mycosis fungoides of the hands with topical bexarotene. Int J Dermatol. 2003;42:238-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 94. | Ponte P, Serrão V, Apetato M. Efficacy of narrowband UVB vs. PUVA in patients with early-stage mycosis fungoides. J Eur Acad Dermatol Venereol. 2010;24:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 95. | Ahmad K, Rogers S, McNicholas PD, Collins P. Narrowband UVB and PUVA in the treatment of mycosis fungoides: a retrospective study. Acta Derm Venereol. 2007;87:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 96. | Ohtsuka T. Narrow band UVB phototherapy for early stage mycosis fungoides. Eur J Dermatol. 2008;18:464-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 97. | de Sanctis V, Persechino S, Fanelli A, Valeriani M, Bracci S, D’Arienzo M, Monarca B, Caperchi C, Raffa S, Enrici RM. Role of radiation therapy in mycosis fungoides refractory to systemic therapy. Eur J Dermatol. 2011;21:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 98. | Jones GW, Wilson LD. Mycosis fungoides and total skin electron beam radiation. Blood. 1997;89:3062-3064. [PubMed] |
| 99. | Jones GW, Kacinski BM, Wilson LD, Willemze R, Spittle M, Hohenberg G, Handl-Zeller L, Trautinger F, Knobler R. Total skin electron radiation in the management of mycosis fungoides: Consensus of the European Organization for Research and Treatment of Cancer (EORTC) Cutaneous Lymphoma Project Group. J Am Acad Dermatol. 2002;47:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 134] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 100. | Piccinno R, Caccialanza M, Çuka E, Recalcati S. Localized conventional radiotherapy in the treatment of Mycosis Fungoides: our experience in 100 patients. J Eur Acad Dermatol Venereol. 2014;28:1040-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 101. | Otremba B. Treatment of refractory stage IV mycosis fungoides with bexarotene monotherapy. Dermatol Clin. 2008;26 Suppl 1:51-52. [PubMed] |
| 102. | Coors EA, Von den Driesch P. Treatment of mycosis fungoides with bexarotene and psoralen plus ultraviolet A. Br J Dermatol. 2005;152:1379-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 103. | Zhang C, Hazarika P, Ni X, Weidner DA, Duvic M. Induction of apoptosis by bexarotene in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. Clin Cancer Res. 2002;8:1234-1240. [PubMed] |
| 104. | Richardson SK, Newton SB, Bach TL, Budgin JB, Benoit BM, Lin JH, Yoon JS, Wysocka M, Abrams CS, Rook AH. Bexarotene blunts malignant T-cell chemotaxis in Sezary syndrome: reduction of chemokine receptor 4-positive lymphocytes and decreased chemotaxis to thymus and activation-regulated chemokine. Am J Hematol. 2007;82:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 105. | Rook AH, Wood GS, Yoo EK, Elenitsas R, Kao DM, Sherman ML, Witmer WK, Rockwell KA, Shane RB, Lessin SR. Interleukin-12 therapy of cutaneous T-cell lymphoma induces lesion regression and cytotoxic T-cell responses. Blood. 1999;94:902-908. [PubMed] |
| 106. | Olsen EA. Interferon in the treatment of cutaneous T-cell lymphoma. Dermatol Ther. 2003;16:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 107. | Knobler R, Girardi M. Extracorporeal photochemoimmunotherapy in cutaneous T cell lymphomas. Ann N Y Acad Sci. 2001;941:123-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 108. | Heald PW, Perez MI, Christensen I, Dobbs N, McKiernan G, Edelson R. Photopheresis therapy of cutaneous T-cell lymphoma: the Yale-New Haven Hospital experience. Yale J Biol Med. 1989;62:629-638. [PubMed] |
| 109. | Zackheim HS, Kashani-Sabet M, McMillan A. Low-dose methotrexate to treat mycosis fungoides: a retrospective study in 69 patients. J Am Acad Dermatol. 2003;49:873-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 110. | Duarte RF, Canals C, Onida F, Gabriel IH, Arranz R, Arcese W, Ferrant A, Kobbe G, Narni F, Deliliers GL. Allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sézary syndrome: a retrospective analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2010;28:4492-4499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 111. | Ruffini PA, Morandi P, Cabioglu N, Altundag K, Cristofanilli M. Manipulating the chemokine-chemokine receptor network to treat cancer. Cancer. 2007;109:2392-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 112. | Yano H, Ishida T, Inagaki A, Ishii T, Ding J, Kusumoto S, Komatsu H, Iida S, Inagaki H, Ueda R. Defucosylated anti CC chemokine receptor 4 monoclonal antibody combined with immunomodulatory cytokines: a novel immunotherapy for aggressive/refractory Mycosis fungoides and Sezary syndrome. Clin Cancer Res. 2007;13:6494-6500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 113. | Baatar D, Olkhanud P, Newton D, Sumitomo K, Biragyn A. CCR4-expressing T cell tumors can be specifically controlled via delivery of toxins to chemokine receptors. J Immunol. 2007;179:1996-2004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |