Peer-review started: September 22, 2014
First decision: November 1, 2014
Revised: November 29, 2014
Accepted: December 16, 2014
Article in press: December 17, 2014
Published online: February 2, 2015
Processing time: 120 Days and 14 Hours
Atopic dermatitis (AD) is a chronic inflammatory skin disorder which can precede asthma and allergic rhinitis in a disease trajectory known as the atopic march. The pathophysiology of AD includes cutaneous inflammation, disrupted epidermal barrier function, xerosis and propensity to secondary infections. AD had previously been thought to arise from the systemic atopic immune response and therapies are therefore directed towards ameliorating Th2-mediated inflammation. However in recent years the focus has shifted towards primary defects in the skin barrier as an initiating event in AD. Links between loss-of-function variants in the gene encoding filaggrin and disrupted activity of epidermal serine proteases and AD have been reported. Based on these observations, a mechanism has been described by which epidermal barrier dysfunction may lead to inflammation and allergic sensitization. Exogenous and endogenous stressors can further exacerbate inherited barrier abnormalities to promote disease activity. Pathways underlying progression of the atopic march remain unclear, but recent findings implicate thymic stromal lymphopoietin as a factor linking AD to subsequent airway inflammation in asthma. This new appreciation of the epidermis in the development of AD should lead to deployment of more specific strategies to restore barrier function in atopic patients and potentially halt the atopic march.
Core tip: Atopic diseases [including atopic dermatitis (AD), allergic rhinitis and asthma] are characterised by Th2-type inflammation. Research over the past decade has highlighted a crucial role for primary skin barrier impairment in the pathogenesis of AD and associated atopic phenotypes. Notably, the epidermal protein, filaggrin, epidermal serine proteases, and the pro-Th2 cytokine thymic stromal lymphopoietin, have been implicated in disease development. We review the evidence upholding a role for epidermal defects in the initiation of skin inflammation in AD, allergic sensitization and pathogenesis of the “atopic march”, and discuss the clinical implications of these findings.
- Citation: Gillespie RM, Brown SJ. From the outside-in: Epidermal targeting as a paradigm for atopic disease therapy. World J Dermatol 2015; 4(1): 16-32
- URL: https://www.wjgnet.com/2218-6190/full/v4/i1/16.htm
- DOI: https://dx.doi.org/10.5314/wjd.v4.i1.16
Atopic diseases are reaching epidemic proportions[1-4], affecting up to 20%-30% of children in developed nations[5-7]. Prominent among these disorders are atopic dermatitis (AD, synonymous with atopic eczema), atopic asthma and allergic rhinitis. Atopic diseases constitute a major source of physical and psychosocial distress[8-10] and account for a large portion of general paediatric practice[11]. Atopic diseases demonstrate complex inheritance patterns and extensive phenotypic variation, and as such their aetiology is poorly understood[12]. However the disorders appear to be underpinned by some common features: they are often associated with elevated levels of total serum IgE and with “atopy” - a personal and/or familial tendency to become sensitized and produce specific IgE against environmental allergens[13] - but this association is hotly debated[14,15]. Atopic diseases are thus presumed to arise as a result of interplay between inherited disposition and environmental factors[16,17].
Longitudinal studies have revealed that approximately half of patients with AD develop asthma later in life and two-thirds go on to exhibit allergic rhinitis[18]. This phenomenon, dubbed the “atopic march” describes the tendency for AD (usually apparent within the first two years of life) to precede the development of food allergies, asthma and allergic rhinitis in a typical temporal sequence[17,19,20]. As AD is now a recognised “gateway” to the atopic march, its diagnosis in infants often prompts parental enquiries about disease prognosis as regards the development of subsequent disorders[17]. AD therefore represents an important focus for interventions which may modify the natural course of atopic disease in high-risk patients.
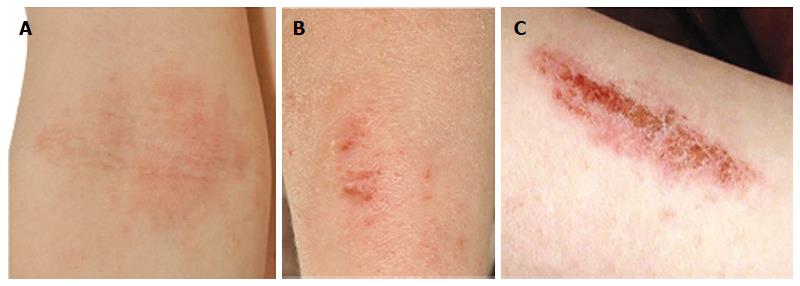
AD is a chronic, inflammatory skin disease affecting an estimated 10%-20% of children and 1%-3% of adults[6]. The skin of AD patients shows widespread xerosis (dryness) and a disturbance of epidermal barrier function. AD lesions (Figure 1A and B) are additionally characterised by pruritus and a propensity to secondary infections (Figure 1C). Th2-deviated inflammation is widely accepted in the pathogenesis of atopic disease however inflammation in AD may be biphasic, with an initial Th2 response leading to a Th0/Th1 dominated phase in chronic lesions[21]. Traditionally it has been presumed that epidermal barrier dysfunction in AD is a downstream consequence of primary immunologic abnormality (the “inside-outside” hypothesis)[22]. In recent years this view has been challenged, with new evidence shifting the focus towards an “outside-inside” model in which epidermal abnormality is not the result but rather the stimulus of inflammation[23]. In light of this concept, this review will evaluate the evidence upholding a pivotal role for the epidermis in atopic disease pathogenesis, and consider the practical implications for therapy.
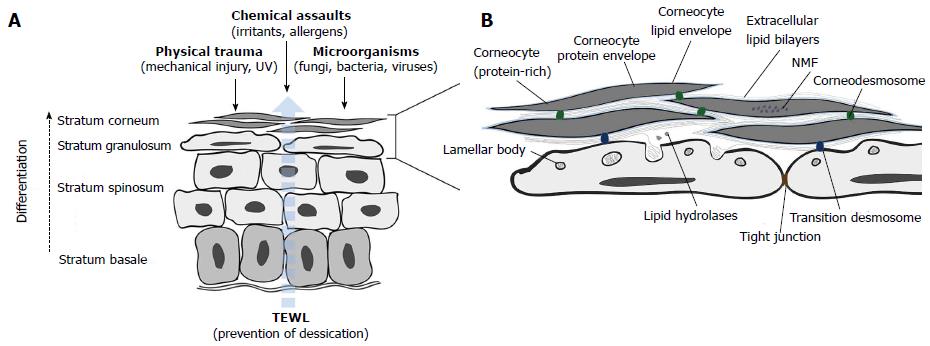
The skin forms an essential barrier between the interior of the body and the external environment. Multiple protective roles are fulfilled by the epidermis and many are mediated by its outermost layer (and end product of keratinocyte differentiation), the stratum corneum (SC; Figure 2)[24,25].
The SC comprises layers of protein-rich anucleate corneocytes interconnected by corneodesmosomes and enclosed within a coat of cross-linked proteins and lipids which together form the cornified envelope (CE)[26]. The CE replaces the plasma membrane during terminal differentiation of keratinocytes into corneocytes and is in turn surrounded by a matrix of intercellular lamellar sheets enriched by 50% ceramides, 25% cholesterol and 15% free fatty acids (FFAs)[27]. This amalgam of highly hydrophobic lipids, together with the CE, provides selective permeability to the epidermis.
Intercellular lipids are secreted as precursors from a unique epidermal organelle, the lamellar body (LB), along with the hydrolytic enzymes required for precursor transformation[24]. At the granular cell-to-corneocyte transition, LBs fuse with the plasma membrane and discharge their contents into the intercellular space by exocytosis. LB extrusion also delivers antimicrobial peptides (AMPs) and the proteases and inhibitors which together orchestrate corneodesmosome cleavage during desquamation. Beneath the SC, the stratum granulosum (SG) provides a second barrier to environmental stressors; keratinocytes in the outer SG layers are intimately connected by tight junctions (TJs) - multi-protein complexes which control paracellular transport.
AD is characterised by widespread skin barrier dysfunction in both lesional and non-lesional skin[28], as indicated by increases in transepidermal water loss (TEWL)[29-31] and percutaneous penetration[32]. This enhanced permeability has been attributed to abnormalities in the composition and architecture of extracellular lipid bilayers, reductions in total lipid and ceramide content[33] and average ceramide chain length[34], as well as an altered ceramide profile[33,35]. The resultant barrier defect renders the skin of AD patients more permissive to the ingress of irritants, allergens and pathogens.
Atopic skin is more susceptible to bacterial and viral infections[10], reflecting defects in the antimicrobial barrier. 80%-100% of AD patients show colonisation by Staphylococcus aureus (S. aureus) compared with 5%-30% of non-atopic individuals[36-38]. Flare-ups of AD are often associated with S. aureus infection, but whether infection represents a cause or consequence of inflammation remains unclear.
In healthy individuals, the desiccating surface, acidic pH and resident microflora of the skin cooperate with AMPs and lipids to provide protection against invading pathogens[39]. In AD, a number of factors - including the defective epidermal barrier, attenuated innate immune response and increased bacterial adhesion - may promote skin colonisation by S. aureus[40]. Among the human AMPs, levels of the cathelicidin, LL-37, and human beta-defensin-2 (hBD-2) are reduced in AD lesions[41], and deficiency of sphingosine - a natural ceramide metabolite and potent anti-S. aureus agent - is also evident in the SC[42]. Furthermore, in vitro studies have suggested that the Th2 inflammatory response in AD may feed back to the epidermis to promote bacterial colonisation[41,43]. For instance, IL-4, which is over-expressed in AD skin, has been reported to increase skin expression of fibronectin and fibrinogen - receptors which may facilitate attachment of S. aureus to the SC[43].
Finally, it should be noted that several other protective functions of the skin barrier are impaired in AD. The skin of AD patients shows disrupted SC integrity (reflected by excess scale[31]) and widespread xerosis, indicated by reduced SC water content[30]. Additionally, pruritus - a prominent characteristic of AD[44] - indirectly aggravates skin barrier impairment via the resultant scratching. The itch sensation is believed to result from cross-talk between the SC, keratinocytes, immune cells and nerve fibres[45]. Excoriations directly disrupt the mechanical barrier of skin, creating additional portals of entry for pathogens. Moreover, it has been reported that a subset of AD patients develop serum IgE which is auto-reactive against a variety of keratinocyte proteins[46]. Thus damage to the epidermis may itself intensify pruritus, driving the vicious “itch-scratch” cycle of AD[45].
The outside-inside phenomenon of AD drew sharp attention from the research community when a significant link between loss-of-function variants in the gene encoding filaggrin (FLG) and AD was demonstrated in three European case collections[47]. FLG mutations were originally identified as the cause of ichthyosis vulgaris (IV)[48] and have since been demonstrated to be the strongest known risk factor for AD in European and Asian populations[49-56]. Cases of AD associated with FLG mutations are more likely to be severe, persistent[57-60], and complicated by secondary infections[61,62] than non-FLG-related cases. Importantly, FLG mutations are now established as an independent risk factor at every step of the atopic march including allergic sensitization[60,63-68], allergic rhinitis[60,64,66-68], food allergies[69,70] and the sub-phenotype of asthma associated with AD[7,60,63,64,66-68].
Profilaggrin is a large precursor molecule (> 400 kDa) containing 10, 11 or 12 tandem repeats of the 37 kDa filaggrin peptide[71]. Insoluble, heavily phosphorylated profilaggrin is the main constituent of keratohyalin granules in the SG. During terminal differentiation, profilaggrin is dephosphorylated and cleaved in a multistep process to release filaggrin monomers, which bind and aggregate keratin filaments, facilitating the collapse of the cytoskeleton and contributing to the flattening of keratinocytes to produce corneocytes[72]. Filaggrin, along with several other cytosolic proteins, is cross-linked into the CE by transglutaminases. As corneocytes move outwards through the SC, filaggrin detaches from the CE and undergoes further degradation within the cytosol, ultimately generating a hygroscopic pool of amino acids and derivatives thereof [including pyrrolidone carboxylic acid (PCA) and trans-urocanic acid (UCA)], contributing to natural moisturising factor (NMF)[73]. NMF appears to play a role in multiple aspects of epidermal homeostasis including SC hydration[73,74], UV photo-protection[75], immunosuppression[76,77] and by acting as a natural acidifier, modulation of enzymatic activity[78-80] and antimicrobial defence[81].
Each of the reported null mutations in FLG has an equivalent biological effect, producing a truncated profilaggrin molecule[82]. This precursor cannot be fully processed into filaggrin monomers thus individuals with two FLG null alleles (homozygous or compound heterozygous, FLG-/-) exhibit an almost complete absence of functional filaggrin[82]. Inherited filaggrin deficiency results in both intracellular and extracellular changes in keratinocyte architecture and altered epidermal physiology. Histological examination of skin from FLG-deficient AD and IV patients reveals increased SC thickness[83] and a granular cell layer that is either strongly reduced or absent[84]. At the ultrastructural level, reduction in filaggrin correlates with perinuclear retraction of granular cell keratin filaments, impaired corneocyte integrity and reduced corneodesmosome density, concomitant with reduced SC cohesion[84]. The molecular mechanisms by which deficiency in filaggrin - an intracellular protein - impairs the paracellular skin barrier in AD remains unclear. However it is plausible that the cytoskeletal abnormalities associated with filaggrin deficiency impede the granular cell-to-corneocyte transition and thus formation of the SC extracellular environment. Consistent with this hypothesis, impaired cargo loading into LBs, partially compromised LB secretion and disorganised lamellar bilayers are observed in the skin of AD and IV patients[84,85]. Reduced expression of SG TJ proteins[84] is also likely to further contribute to barrier impairment.
Alternatively or in addition, it may be the biochemical consequences of filaggrin deficiency that are important in AD pathogenesis. FLG null mutations effect a dose-dependent reduction in SC NMF levels[86-89], which in turn correlate inversely with skin surface pH[87,89] and TEWL[89]. Additionally, FLG exhibits intragenic copy number variation, and a lower number of repeats correlates significantly with AD risk[90], SC UCA levels[90] and the presence of self-perceived “dry skin”[91]. Thus enhanced TEWL in FLG-associated AD can be explained in part by reduced SC hydration; deficiency of hygroscopic NMF components would be expected to result in lower SC water content hence a steeper water gradient across the epidermis. In addition, altered skin pH is likely to perturb the natural balance of enzymatic activities in the SC. The elevated pH of AD patient skin would be predicted to favour the net activity of SC-resident serine proteases (SPs)[78,80] whilst reducing that of key SC lipid biosynthesis enzymes. SP hyperactivity drives premature degradation of corneodesmosomes and lipid-processing enzymes[80], likely contributing to defective lamellar bilayer formation. In line with the proposed mechanisms, it has been demonstrated that levels of filaggrin breakdown products correlate with aberrant SC lipid organisation and decreased barrier function in AD patients[34,86,92]. A recent study using a reconstructed human epidermis model has suggested that filaggrin deficiency may also promote enhanced epidermal sensitivity to UVB[93], but this connection remains to be demonstrated in patients.
Finally, filaggrin deficiency in AD has implications for the antimicrobial skin barrier. As described, the natural acidity of the SC in healthy individuals provides innate antimicrobial protection - a function which is likely to be diminished in NMF-deficient AD patients. Furthermore, recent data suggest that filaggrin may play a unique role in protection against S. aureus infection, by mediating keratinocyte secretion of sphingomyelinase - an enzyme which reduces the number of S. aureusα-toxin binding sites on the keratinocyte surface[94]. These findings indicate a mechanism by which filaggrin-deficient skin may be preferentially targeted by S. aureus-induced cytotoxicity. Clinically, the consequences of FLG null mutations in AD manifest as a 7-fold increase in the risk of recurrent bacterial infections relative to wild-type FLG patients[62].
Thus our understanding of the mechanisms by which FLG genotype translates to disease phenotype remains incomplete. Furthermore, recent findings indicate additional levels of complexity to the FLG-AD relationship which are likely to influence the pathogenic mechanisms discussed above. For instance, preliminary data indicate that epigenetic, as well as genetic variation can influence disease outcome. Indeed, a recent study has shown that methylation of a specific CpG site adjacent to FLG can modify the influence of FLG null mutations on AD risk[95]. Whether inherent variation in enzymes involved in profilaggrin biosynthesis and maturation can also affect atopic disease pathogenesis has yet to be ascertained. Future lines of investigation should clarify how individual variations in filaggrin biology at the DNA, RNA and protein levels interact to determine distinct atopic phenotypes.
Whilst inherited variation in FLG undoubtedly contributes to skin barrier dysfunction in AD, FLG null variants are carried by less than one-third of European patients with AD[66] and broad defects in epidermal differentiation are characteristic of the disease regardless of FLG genetic status[96]. Taken together, these observations suggest that other factors must modify epidermal homeostasis. Genetic association studies have identified links between AD and gene variants distinct from FLG but also located on chromosome 1q21 within the Epidermal Differentiation Complex - a region comprising over sixty genes essential for epidermal structure and function[97]. Of note, a single nucleotide polymorphism (SNP) 7 kb downstream of the gene encoding hornerin (HRNR) and an 8-amino acid insertion in the gene encoding small proline-rich protein 3 (SPRR3) have been identified as risk factors for AD[98,99]. Additionally, a recent study by Margolis et al[100] using whole-exome sequencing and targeted analysis in an African American cohort has identified mutations in FLG2 (encoding filaggrin-2) that show a significant association with persistent AD - the first established link between a skin barrier gene and AD in subjects of African descent. However, it should be noted that these risk variants lie within a block of linkage disequilibrium and it remains possible that they are tagging unidentified variants within FLG. Hornerin and filaggrin-2 are S100-fused type proteins, and thus share a structural organisation similar to filaggrin. Both proteins mirror the subcellular localisation of filaggrin in the differentiating epidermis are believed ultimately (along with SPRR3) to become incorporated into the CE[97]. The precise contributions of these proteins to skin barrier function remain unknown, but available data indicate that filaggrin, hornerin and filaggrin-2 have overlapping or complementary functions in the epidermis[97] and the expression of each may be down-regulated in AD skin[96].
TJs comprise both cytoplasmic and transmembrane proteins, key among which are the claudins, representing the main determinants of barrier selectivity against macromolecules[101]. Claudin-1 expression is down-regulated in non-lesional AD skin and inversely correlated with Th2 cytokines. Variants within the claudin-1 gene, CLDN1, have shown association with AD in two ethnically distinct North American populations[102]. Interestingly, this study also demonstrated links between CLDN1 variants and AD severity, total serum IgE and asthma in subjects of African, but not European ancestry. Given that at present, FLG null mutations appear to be considerably less prevalent in African populations relative to European or Asian cohorts[89], these findings (together with those of Margolis et al[100] regarding FLG2) indicate population specificity in the genetic mechanisms which dominate skin barrier dysfunction in AD.
Finally, protein regulators of lamellar bilayer formation have been implicated in the pathogenesis of AD. Mutations in the gene encoding fatty acid transporter 4 (FATP4)[103] and MATT (encoding mattrin, a component of the LB secretory system in flaky tail (maft) mice[104]) are associated with increased risk of AD[105]. Maft mice harbour mutations in both Flg and Matt genes, and have been used for many years as an experimental model of AD. However, it has recently been shown that ma/ma mice, which carry mutations in Matt but not in Flg, exhibit enhanced TEWL and decreased SC hydration, and develop the spontaneous dermatitis and atopy exhibited by maft mice to a greater extent than the filaggrin-null (Flg-/-) mice[104-106]. Matt may therefore play a greater role than Flg in driving the dermatitis phenotype in maft mice, supporting a key role for SC lipid secretion in the development of AD.
Thus skin barrier genes may act alone or in combination with FLG to modify AD pathogenesis. Whether or not the same genes are also associated with subsequent steps of the atopic march remains to be elucidated.
Maintenance of epidermal physiology is dependent on the coordinated activities of skin-resident proteases and anti-proteases. Perturbation of this balance in favour of protease hyperactivity can result in pathogenic barrier disruption, as exemplified in Netherton syndrome (NS). NS is an autosomal recessive disorder featuring ichthyosis and atopic manifestations, which is caused by loss-of-function mutations in SPINK5[107] - the gene encoding lympho-epithelial Kazal-type-related inhibitor (LEKTI)[108]. In healthy skin, proteolytic LEKTI fragments specifically co-localise with and regulate the activity of multiple SPs, including members of the kallikrein (KLK) family. KLKs are central to desquamation[109] and also indirectly promote profilaggrin proteolysis[110]. In the skin of NS patients, residual LEKTI expression correlates inversely with enhanced KLK activity[111] resulting in dramatic SC thinning and attenuation of the permeability barrier through unrestricted degradation of corneodesmosomes[109] and lipid-processing enzymes [111] respectively. Permeability barrier function may additionally be compromised through activation of protease-activated receptor 2 (PAR-2), which is expressed in nucleated epidermal layers and can be induced by specific SPs to down-regulate LB secretion[112-114].
A number of association studies have identified SNPs in SPINK5 which are associated with AD risk in different ethnicities[115-119]. In particular, one such variant, LEKTI E420K, has also been linked to elevated serum IgE[118], food allergies[116], AD severity[116] and AD-associated asthma[120]. In vitro analysis has shown protease hyperactivity resulting from the E420K mutation to result in increased corneodesmosomal destabilisation and premature profilaggrin proteolysis[121], suggesting a functional pathway by which E420K may contribute to filaggrin deficiency and the development of AD. In addition to SPINK5 polymorphisms, a mutant allele of the CSTA gene (which encodes the skin-resident cysteine protease inhibitor, cystatin A) has been reported to associate with AD in a small UK cohort[122]. Fewer data exist for associations between AD and epidermal protease genes; an association between AD and a putative gain-of-function insertion in the 3’ UTR of KLK7 has been described in a British case-control study[122] but failed to be confirmed in subsequent investigations[123,124].
The above pathways together may account for the skin barrier phenotype in AD, but the mechanisms linking epidermal disruption and concomitant allergic inflammation remain unclear. The maft and more recently generated Flg-/- mouse models[106,125] serve as useful systems in which to study the aetiology of the immune response in the context of inherited barrier impairment. Although showing differences in disease phenotype, both mice exhibit increased percutaneous allergen penetration and a reduced inflammatory threshold to skin irritants and allergens[106,126]. Based on these observations, it is widely postulated that inflammation in AD is a secondary reaction to increased entry of allergens and irritants through the compromised skin barrier. Whilst this is yet to be confirmed in human subjects, it is worth noting that AD patients with FLG null mutations have a significantly increased risk of allergic sensitization[60] and irritant contact dermatitis[127], and display elevated numbers of allergen-specific CD4+ T cells compared with wild-type FLG patients[128]. Furthermore, it seems likely that in addition to the inflammatory response to penetrating antigens, barrier impairment itself may intrinsically promote downstream inflammation. For instance, elevated SP activity (induced in AD along the pathways described above) not only promotes epidermal barrier breakdown but leads to increased release of active IL-1α and IL-1β[129,130] from the corneocyte cytoplasm, thereby initiating inflammation. Furthermore, SP hyperactivity may stimulate inflammation indirectly by accelerating the degradation of transition desmosomes, leading to secretion of IL-1β, IL-8 and TNF-α from mechanically stressed keratinocytes[131]. Finally, accumulated data indicate that following activation by SPs, PAR-2[131], together with pro-inflammatory cytokines, induces NF-κB-mediated over-expression of the pro-Th2 cytokine, thymic stromal lymphopoietin (TSLP)[132], and IL-6[133]. Evidence in support of the latter pathway has been observed in studies of maft mice and FLG knock-down keratinocytes in vitro[134], suggesting that this immunologic cascade may operate downstream of a primary filaggrin deficiency.
Whilst these data are compelling, the same pathogenic mechanisms have yet to be demonstrated in human AD. Nonetheless, the strength and number of independent associations between FLG and atopic disorders greatly surpass those of any other gene expressed in the skin to date[123]. As such, filaggrin deficiency is widely regarded as a primary abnormality leading to skin inflammation in AD[135-138]. Leading on from this, a putative pathogenic pathway has been described in which the reduction in filaggrin acts as a central stimulus for increased SP activity, which in turn triggers the inflammatory response[139]. AD patients with FLG null mutations exhibit increased skin levels of IL-1 cytokines, in a manner inversely correlating with NMF levels[87]. This observation has been attributed to pH-induced stimulation of SPs, which can promote inflammation by the mechanisms described above[139]. However, no correlation between SC pH and levels of either IL-1α or IL-1β could be demonstrated in the same study, and recent findings have indicated that FLG status is not an essential determinant of SC pH[79,93,106]. Thus whilst these data do not rule out a role for skin pH changes in initiation of inflammation in FLG-related AD, it is likely that protease activity and consequently IL-1 levels are modulated by additional factors. For instance, Kezic et al[87] have proposed that increased SC calcium concentration (resulting from reduced SC hydration) may favour the activation of calcium-dependent SPs, but based on our current knowledge, this pathophysiological pathway remains entirely speculative.
Despite the undisputed involvement of FLG null variants in the atopic march, it is important to note that filaggrin deficiency is observed in AD patients even in the absence of known FLG mutations[96]. Whilst this may be explained in part by other forms of genetic regulation, it is apparent that a number of additional factors can reduce expression of functional filaggrin, resulting in barrier disruption. In particular, accumulating evidence points to components of the acquired immune response as key players in endogenous barrier impairment, prompting the proposition of a self-sustaining “outside-inside-outside” pathogenic loop in AD[139].
Studies in maft mice have shown that following initial sensitization, further defects in barrier function occur, suggesting exacerbation of the primary barrier impairment by the induced inflammatory response[126]. Consistent with this, the Th2 cytokines, IL-4, IL-13, IL-22 and IL-25, which are over-expressed in AD lesions, have been shown to inhibit expression of filaggrin[136-138] and profilaggrin-processing enzymes[140,141] in vitro. Inflammation in AD may also compromise skin barrier function by a number of filaggrin-independent mechanisms. The epidermal AMPs, LL-37, hBD-2 and hBD-3, are down-regulated in a Th2-dependent manner[41,142-144] and roles for Th2 cytokines in disruption of SC lipid synthesis[145-147], SC protease activity[148,149] and multiple processes in epidermal differentiation[147,149-151] have been reported. Finally, whilst not endogenously perturbing the skin barrier per se, the cytokines TSLP and IL-31 induce itch[152,153], thereby aggravating the itch-scratch cycle.
The avoidance of exogenous irritants is an important part of atopic disease management. Diverse environmental factors are known to exacerbate atopic disorders, but several are now recognised as having detrimental effects on the skin barrier. For instance, prolonged exposure to reduced ambient humidity (as may occur in centrally heated homes) has been shown to accelerate TEWL and promote profilaggrin proteolysis[73], potentially driving further depletion of cutaneous filaggrin[139]. External modifiers of skin surface pH may also aggravate disease activity via enzyme-mediated pathways; use of neutral-to-alkaline soaps is known to induce SC thinning and precipitate flares of AD[154]. Protease activity in the epidermis of AD patients may be further intensified by airborne proteins; proteolytic allergens produced by house dust mites and cockroaches have been shown to penetrate the skin and can exacerbate barrier dysfunction[155,156] both directly, by degrading barrier components[157,158] and indirectly, through activation of PAR-2[159-161]. Staphylococcal infection also has a number of implications for skin barrier function. Essential S. aureus surface proteins confer resistance to the bactericidal action of human epidermal FFAs and AMPs[162] and once established on the skin, coagulase-negative staphylococci can secrete peptidases and lipid hydrolases[163] which may further erode the skin barrier. Indeed it has been shown that elevated levels of the enzyme ceramidase (which catalyses the degradation of ceramide to sphingosine and FFAs) are secreted by the bacterial flora of AD patient skin relative to healthy controls[164]. Finally, psychological stress (PS) may precipitate atopic diseases[165] by disturbing the permeability barrier[166,167], SC integrity[166] and antimicrobial defences[168] in the skin of mouse models, via a mechanism thought to be mediated by increased production of endogenous glucocorticoids[167-169].
The mechanistic link between AD and subsequent phenotypes in the atopic march remains unclear and has been the subject of intensive research in recent years. Generally, current data favour a model in which the downstream systemic effects of allergen penetration through the impaired skin barrier cause immune cells to mount an exaggerated inflammatory response at any allergen-exposed epithelial surface[136,154,170]. This theory fits with several observations: (1) AD is usually the first manifestation of atopy[18]; (2) FLG is not expressed in bronchial airways[171] nor the oesophageal epithelium beyond the oro-pharyngeal mucosa[172], suggesting that filaggrin does not directly influence permeability of these epithelia; and (3) allergic sensitization induced by epicutaneous exposure to peanut allergen inhibits subsequent oral tolerance in mice[173].
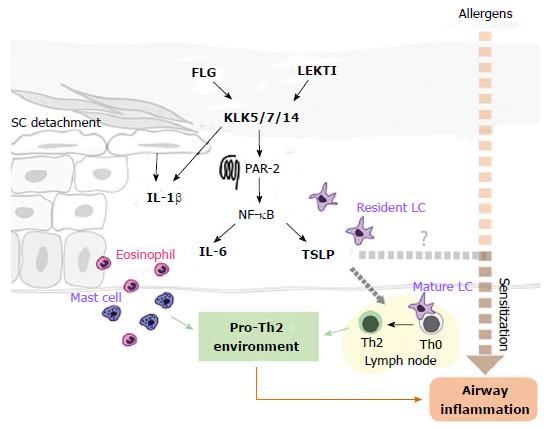
Central to the uncertainty over the atopic march is the strength of the connection between early allergic sensitization in AD and the risk of allergic airway disease, with the epidemiological data being somewhat inconsistent[14]. Functional studies on the atopic march have identified a prominent role for the cytokine TSLP as a promoter of the Th2 response in AD and a trigger linking epicutaneous sensitization to subsequent asthma[174,175]. TSLP is expressed primarily in lung and skin epithelia[176] where it is recognised as a “master switch” from epithelial barrier disruption to Th2 inflammation[177,178]. Accordingly, TSLP expression is up-regulated in the SC of AD patients compared with healthy subjects[179]. Notably, a recent study using mice in which TSLP is selectively and inducibly ablated in epidermal keratinocytes suggests that skin-derived TSLP is essential for skin allergic inflammation and epicutaneous sensitization, which in turn leads to allergic asthma[180]. This study, in contrast to previous findings[181,182], indicated that keratinocyte-derived TSLP acts as an essential “adjuvant” to the Th2 response induced by topical allergen treatment, but that skin expression of TSLP caused by barrier disruption alone (i.e., without allergen) is not sufficient to promote the full inflammatory phenotype. It is also noteworthy that in this model sensitization was achieved through barrier-defective skin (as opposed to intraperitoneal or intradermal injection of allergen[181,183]), followed by airway challenge, conditions representative of those in human AD. Airway inflammation appears to require an antigen-specific memory CD4+ T cell response[180,183], but occurs independently of TSLP presence in the lung[175,180,183] and circulating TSLP[183], suggesting that skin-derived TSLP is both necessary and sufficient for manifestation of asthma symptoms.
The relative importance of TSLP in the human atopic march remains to be clarified. A recent study in an American paediatric AD cohort identified the TSLP variant rs1898671 (which produces attenuated TSLP) as protective against the development of persistent AD[184] however no association with comorbid asthma was identified[184]. It has further been demonstrated that risk of childhood asthma is influenced by epistasis between SPINK5 and TSLP[185]. The authors postulate that TSLP and SPINK5 function in a common pathway in which LEKTI deficiency ultimately leads to TSLP production, an exaggerated Th2 response and allergic lung inflammation[185]. Although the analysed cohort comprised both asthmatic patients with and without concomitant AD, these findings support the view that the systemic consequences of an epidermal pathway are sufficient to induce inflammation at remote epithelia in the human atopic march (Figure 3). Thus it will be interesting to see if evidence for a similar pathway in patients with AD-associated asthma emerges in the coming years. Together, the above findings reinforce the attractive idea that early and aggressive intervention directed towards the skin barrier may impede the progression from AD to subsequent airway inflammation in atopic patients.
The pathogenic mechanisms described above create a strong case for prioritising protection and restoration of the skin barrier in atopic individuals. The current foundations of general AD management include the avoidance of triggering factors and optimal skin care[165], with efforts to address epidermal barrier defects centred on the regular use of emollients. Emollients help to hydrate the skin and soothe pruritus[186]; when applied liberally, they can provide a short-term artificial barrier to reduce TEWL and protect against the penetration of allergens and irritants. The benefits of emollient therapy in controlling the cutaneous symptoms of AD are accepted on the basis of clinical experience[186]. A recent feasibility study of early emollient therapy for AD prevention has shown promising preliminary results[187], but further trials are warranted before the efficacy of this approach can be confirmed. Thus far, emollient monotherapy has rarely proved sufficient for disease resolution in moderate-to-severe AD, in which the use of anti-inflammatory agents is often necessary for exacerbation management[165].
However, corticosteroids and topical calcineurin inhibitors are associated with a spectrum of cutaneous and systemic side effects[165,188] including the impairment of both permeability and antimicrobial barrier functions in AD skin[189-191]. Interestingly, selected emollients have also been reported to disrupt the skin barrier. The majority of over-the-counter (OTC) moisturisers contain non-physiological ingredients (e.g., petrolatum and lanolin) which function by undefined biological mechanisms and in certain cases have been found to compromise SC integrity, permeability barrier function[192] and epidermal differentiation[193]. The above findings, together with recent advances in our understanding of skin pathophysiology, have shifted interest towards novel “barrier replacement strategies”. Such therapies aim to correct underlying biochemical abnormalities; they are based upon physiological components and may therefore minimise the likelihood of an unfavourable response[194]. For instance, prescription barrier repair creams (BRCs) are based on SC lipids and differ from their non-physiological counterparts in that they are taken up by keratinocytes, packaged into LBs and ultimately secreted to form lamellar bilayers[195]. In accordance with the lipid deficits in AD skin, a number of “designer” ceramide-dominant and triple-lipid-based barrier repair formulations have now been tested in AD patients[196]. Trials of one ceramide-dominant BRC demonstrated significant reductions in disease severity[197,198] and marked restoration of the epidermal barrier when used as adjunct to topical anti-inflammatories[197]. Moreover, improvements in severity, pruritus and sleep were comparable to the effects of the TCS fluticasone[198], suggesting that BRCs hold potential steroid-sparing effects. However small study size, variation in study design, commercial pressures and the possibility of publication bias make such data difficult to interpret. Furthermore, it is worth noting that the direct comparison of OTC emollients with BRCs has demonstrated equal efficacy for the treatment of mild-to-moderate AD[199], with a notably significant cost disparity between the two treatments. Thus the prescription of BRCs over cheaper and simpler OTC alternatives remains contentious. Large-scale randomized control trials will be necessary to determine whether BRCs are indeed superior for long-term management of AD.
The identification of filaggrin deficiency as a strong predisposing factor for atopic disorders has opened the prospect of filaggrin or NMF restoration as another barrier repair strategy. Topically applied recombinant filaggrin peptide has been shown to penetrate to the SG of reconstructed human epidermis, and is internalised and processed to restore epidermal structure in maft mice[200]. Furthermore, a recent study has identified a candidate drug that promotes filaggrin mRNA and protein expression in vitro, and suppresses the development of skin inflammation when administered orally in the NC/Nga mouse model of AD[201]. These findings, whilst preliminary, hold promise for filaggrin restoration as part of future AD therapy. Clarification of the relative importance of the functions of profilaggrin, filaggrin and filaggrin degradation products will be useful in directing research in this area[202].
Thus the new appreciation of skin barrier pathophy-siology should encourage greater clinical emphasis on the optimization of skin care and avoidance of barrier-breaching products in neonates and children. Yet a number of important questions remain. For instance, can restoration of the permeability barrier alone help to normalise epidermal gene expression? Will barrier-based interventions also protect against inflammation and the development of comorbid atopic disorders in patients with AD? Long-term follow-up studies with examination of treatment efficacy at the molecular level will be required. Finally, additional potential treatment avenues (e.g., selective inhibition of SPs and TSLP) have yet to be explored and it is expected that further elucidation of the mechanisms of skin barrier dysfunction in the coming years will identify practicable therapeutic targets.
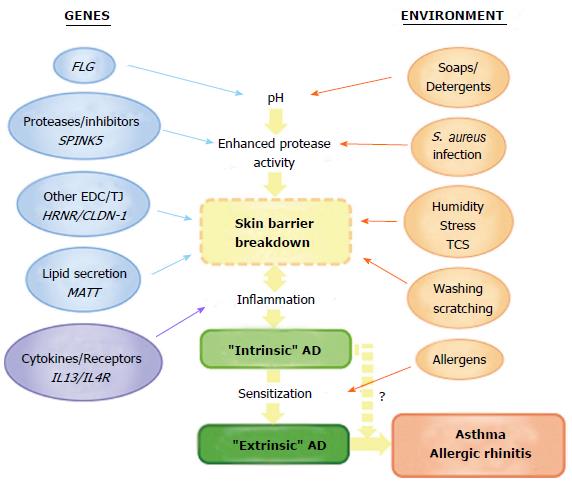
The discovery of loss-of-function mutations in FLG has led to a new appreciation of skin barrier dysfunction as primary pathogenic mechanism in atopic disease. Undoubtedly one of the greatest challenges for future research is the dissection of the complex interactions between multiple genetic, environmental and immunologic factors which influence disease pathogenesis (Figure 4). Some interactions with FLG have already been identified. Of note, the combined occurrence of mutations in FLG, an epidermal gene, and in the genes encoding IL-10 and IL-13, mediators of acquired immunity, have been shown to have a multiplicative effect on AD risk[203] and inter-regulation by SC and SG skin barriers at the mRNA and protein levels has been reported[101,204]. These interactions, whilst yet to be demonstrated in human patients, may present further difficulties in determining the relative importance of individual barrier components in AD pathogenesis. Progress in the molecular genetics of atopic disease has been accelerated by advances in next-generation sequencing techniques, but the additional complexity of multiple gene-gene and gene-environment interactions requires further development of bioinformatics analysis. The integration of genetic, transcriptomic and proteomic analyses is also computationally demanding. However, a recent transcriptomic analysis of AD skin used FLG genotype to stratify data and has offered insight into novel pathways and predicted functional networks[205]. The expanding understanding of epigenetic variation is also predicted to contribute further novel mechanistic insights in the coming years.
Understanding the interplay between atopic genes and environmental factors will be vital to explaining (and perhaps controlling) the rising prevalence of atopic diseases. This will require long-term epidemiological studies in which early genetic profiling of FLG and other disease genes is coupled with careful monitoring of patient environment. Such studies may help to explain how specific gene variants act in the context of different external insults to induce a range of related yet distinct atopic phenotypes. Evaluation of FLG genetic status is not commonplace in pharmacogenetic studies or current clinical management of AD. However careful clinical examination can identify FLG null genotype[206] and as the multiple influences of filaggrin on atopic disease trajectory are clarified, this will benefit clinical care and prognostic predictions. More detailed knowledge of genotypes associated with atopic phenotypes could in the future help to direct the prescription of personalised therapeutic regimes, including focused instructions for disease prevention and targeted treatment. Thus early control of skin barrier function in high-risk patients may in future prevent allergic sensitization and what had previously been considered the inevitable path through the atopic march.
P- Reviewer: Aksoy B, Hu SCS, Kaliyadan F S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Galassi C, De Sario M, Biggeri A, Bisanti L, Chellini E, Ciccone G, Petronio MG, Piffer S, Sestini P, Rusconi F. Changes in prevalence of asthma and allergies among children and adolescents in Italy: 1994-2002. Pediatrics. 2006;117:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Deckers IA, McLean S, Linssen S, Mommers M, van Schayck CP, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS One. 2012;7:e39803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 432] [Cited by in RCA: 379] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 3. | Kjaer HF, Eller E, Høst A, Andersen KE, Bindslev-Jensen C. The prevalence of allergic diseases in an unselected group of 6-year-old children. The DARC birth cohort study. Pediatr Allergy Immunol. 2008;19:737-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Schernhammer ES, Vutuc C, Waldhör T, Haidinger G. Time trends of the prevalence of asthma and allergic disease in Austrian children. Pediatr Allergy Immunol. 2008;19:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | McNeill G, Tagiyeva N, Aucott L, Russell G, Helms PJ. Changes in the prevalence of asthma, eczema and hay fever in pre-pubertal children: a 40-year perspective. Paediatr Perinat Epidemiol. 2009;23:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251-1258.e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 656] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 7. | Brown SJ, Relton CL, Liao H, Zhao Y, Sandilands A, Wilson IJ, Burn J, Reynolds NJ, McLean WH, Cordell HJ. Filaggrin null mutations and childhood atopic eczema: a population-based case-control study. J Allergy Clin Immunol. 2008;121:940-946.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Casolaro V, Georas SN, Song Z, Ono SJ. Biology and genetics of atopic disease. Curr Opin Immunol. 1996;8:796-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Lewis-Jones S. Quality of life and childhood atopic derma-titis: the misery of living with childhood eczema. Int J Clin Pract. 2006;60:984-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 308] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 10. | Williams HC. Clinical practice. Atopic dermatitis. N Engl J Med. 2005;352:2314-2324. [PubMed] |
| 11. | Stone KD. Atopic diseases of childhood. Curr Opin Pediatr. 2003;15:495-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | MacLean JA, Eidelman FJ. The genetics of atopy and atopic eczema. Arch Dermatol. 2001;137:1474-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel-Koomen C, Dreborg S, Haahtela T, Kowalski ML, Mygind N, Ring J, van Cauwenberge P. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56:813-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1176] [Cited by in RCA: 1049] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 14. | Flohr C, Johansson SG, Wahlgren CF, Williams H. How atopic is atopic dermatitis? J Allergy Clin Immunol. 2004;114:150-158. [PubMed] |
| 15. | Novak N, Bieber T. Allergic and nonallergic forms of atopic diseases. J Allergy Clin Immunol. 2003;112:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 269] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 16. | Grammatikos AP. The genetic and environmental basis of atopic diseases. Ann Med. 2008;40:482-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Hahn EL, Bacharier LB. The atopic march: the pattern of allergic disease development in childhood. Immunol Allergy Clin North Am. 2005;25:231-246, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112:S118-S127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 816] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 19. | Gustafsson D, Sjöberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis--a prospective follow-up to 7 years of age. Allergy. 2000;55:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 284] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 20. | Spergel JM. From atopic dermatitis to asthma: the atopic march. Ann Allergy Asthma Immunol. 2010;105:99-106; quiz 107-109, 117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 327] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 21. | Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 449] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 22. | Brandt EB, Sivaprasad U. Th2 Cytokines and Atopic Derm-atitis. Journal of clinical & cellular immunology. 2011;2:110. [RCA] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 446] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 23. | Elias PM, Steinhoff M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J Invest Dermatol. 2008;128:1067-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 24. | Madison KC. Barrier function of the skin: “la raison d’être” of the epidermis. J Invest Dermatol. 2003;121:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 722] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 25. | Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17:1063-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1064] [Cited by in RCA: 1170] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 26. | Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 1261] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 27. | Feingold KR, Elias PM. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim Biophys Acta. 2014;1841:280-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 269] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 28. | Jakasa I, Verberk MM, Esposito M, Bos JD, Kezic S. Altered penetration of polyethylene glycols into uninvolved skin of atopic dermatitis patients. J Invest Dermatol. 2007;127:129-134. [PubMed] |
| 29. | Gupta J, Grube E, Ericksen MB, Stevenson MD, Lucky AW, Sheth AP, Assa’ad AH, Khurana Hershey GK. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121:725-730.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Linde YW. Dry skin in atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1992;177:9-13. [PubMed] |
| 31. | Watanabe M, Tagami H, Horii I, Takahashi M, Kligman AM. Functional analyses of the superficial stratum corneum in atopic xerosis. Arch Dermatol. 1991;127:1689-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Jensen JM, Pfeiffer S, Witt M, Bräutigam M, Neumann C, Weichenthal M, Schwarz T, Fölster-Holst R, Proksch E. Different effects of pimecrolimus and betamethasone on the skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol. 2009;124:R19-R28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Ishikawa J, Narita H, Kondo N, Hotta M, Takagi Y, Masukawa Y, Kitahara T, Takema Y, Koyano S, Yamazaki S. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130:2511-2514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 274] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 34. | Janssens M, van Smeden J, Gooris GS, Bras W, Portale G, Caspers PJ, Vreeken RJ, Hankemeier T, Kezic S, Wolterbeek R. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res. 2012;53:2755-2766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 336] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 35. | Jungersted JM, Scheer H, Mempel M, Baurecht H, Cifuentes L, Høgh JK, Hellgren LI, Jemec GB, Agner T, Weidinger S. Stratum corneum lipids, skin barrier function and filaggrin mutations in patients with atopic eczema. Allergy. 2010;65:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 234] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 36. | Breuer K, HAussler S, Kapp A, Werfel T. Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br J Dermatol. 2002;147:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 216] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 37. | David TJ, Cambridge GC. Bacterial infection and atopic eczema. Arch Dis Child. 1986;61:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 581] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Bibel DJ, Miller SJ, Brown BE, Pandey BB, Elias PM, Shinefield HR, Aly R. Antimicrobial activity of stratum corneum lipids from normal and essential fatty acid-deficient mice. J Invest Dermatol. 1989;92:632-638. [PubMed] |
| 40. | Baker BS. The role of microorganisms in atopic dermatitis. Clin Exp Immunol. 2006;144:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 41. | Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1464] [Cited by in RCA: 1396] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 42. | Arikawa J, Ishibashi M, Kawashima M, Takagi Y, Ichikawa Y, Imokawa G. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J Invest Dermatol. 2002;119:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 182] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 43. | Cho SH, Strickland I, Tomkinson A, Fehringer AP, Gelfand EW, Leung DY. Preferential binding of Staphylococcus aureus to skin sites of Th2-mediated inflammation in a murine model. J Invest Dermatol. 2001;116:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 136] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 44. | Correale CE, Walker C, Murphy L, Craig TJ. Atopic dermatitis: a review of diagnosis and treatment. Am Fam Physician. 1999;60:1191-1198, 1109-1210. [PubMed] |
| 45. | Yosipovitch G, Papoiu AD. What causes itch in atopic dermatitis? Curr Allergy Asthma Rep. 2008;8:306-311. [PubMed] |
| 46. | Altrichter S, Kriehuber E, Moser J, Valenta R, Kopp T, Stingl G. Serum IgE autoantibodies target keratinocytes in patients with atopic dermatitis. J Invest Dermatol. 2008;128:2232-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJ. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2365] [Cited by in RCA: 2034] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 48. | Smith FJ, Irvine AD, Terron-Kwiatkowski A, Sandilands A, Campbell LE, Zhao Y, Liao H, Evans AT, Goudie DR, Lewis-Jones S. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 687] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 49. | Rodríguez E, Baurecht H, Herberich E, Wagenpfeil S, Brown SJ, Cordell HJ, Irvine AD, Weidinger S. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J Allergy Clin Immunol. 2009;123:1361-70.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 296] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 50. | Chen H, Common JE, Haines RL, Balakrishnan A, Brown SJ, Goh CS, Cordell HJ, Sandilands A, Campbell LE, Kroboth K. Wide spectrum of filaggrin-null mutations in atopic dermatitis highlights differences between Singaporean Chinese and European populations. Br J Dermatol. 2011;165:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 51. | Enomoto H, Hirata K, Otsuka K, Kawai T, Takahashi T, Hirota T, Suzuki Y, Tamari M, Otsuka F, Fujieda S. Filaggrin null mutations are associated with atopic dermatitis and elevated levels of IgE in the Japanese population: a family and case-control study. J Hum Genet. 2008;53:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Nemoto-Hasebe I, Akiyama M, Nomura T, Sandilands A, McLean WH, Shimizu H. FLG mutation p.Lys4021X in the C-terminal imperfect filaggrin repeat in Japanese patients with atopic eczema. Br J Dermatol. 2009;161:1387-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Nomura T, Akiyama M, Sandilands A, Nemoto-Hasebe I, Sakai K, Nagasaki A, Ota M, Hata H, Evans AT, Palmer CN. Specific filaggrin mutations cause ichthyosis vulgaris and are significantly associated with atopic dermatitis in Japan. J Invest Dermatol. 2008;128:1436-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 54. | Nomura T, Sandilands A, Akiyama M, Liao H, Evans AT, Sakai K, Ota M, Sugiura H, Yamamoto K, Sato H. Unique mutations in the filaggrin gene in Japanese patients with ichthyosis vulgaris and atopic dermatitis. J Allergy Clin Immunol. 2007;119:434-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 178] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 55. | Osawa R, Konno S, Akiyama M, Nemoto-Hasebe I, Nomura T, Nomura Y, Abe R, Sandilands A, McLean WH, Hizawa N. Japanese-specific filaggrin gene mutations in Japanese patients suffering from atopic eczema and asthma. J Invest Dermatol. 2010;130:2834-2836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Zhang H, Guo Y, Wang W, Yu X, Yao Z. Associations of FLG mutations between ichthyosis vulgaris and atopic dermatitis in Han Chinese. Allergy. 2011;66:1253-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 57. | Barker JN, Palmer CN, Zhao Y, Liao H, Hull PR, Lee SP, Allen MH, Meggitt SJ, Reynolds NJ, Trembath RC. Null mutations in the filaggrin gene (FLG) determine major susceptibility to early-onset atopic dermatitis that persists into adulthood. J Invest Dermatol. 2007;127:564-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 241] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 58. | Brown SJ, Sandilands A, Zhao Y, Liao H, Relton CL, Meggitt SJ, Trembath RC, Barker JN, Reynolds NJ, Cordell HJ. Prevalent and low-frequency null mutations in the filaggrin gene are associated with early-onset and persistent atopic eczema. J Invest Dermatol. 2008;128:1591-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 59. | Margolis DJ, Apter AJ, Gupta J, Hoffstad O, Papadopoulos M, Campbell LE, Sandilands A, McLean WH, Rebbeck TR, Mitra N. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 60. | van den Oord RA, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ. 2009;339:b2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 325] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 61. | Gao PS, Rafaels NM, Hand T, Murray T, Boguniewicz M, Hata T, Schneider L, Hanifin JM, Gallo RL, Gao L. Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. J Allergy Clin Immunol. 2009;124:507-513, 513.e1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 62. | Cai SC, Chen H, Koh WP, Common JE, van Bever HP, McLean WH, Lane EB, Giam YC, Tang MB. Filaggrin mutations are associated with recurrent skin infection in Singaporean Chinese patients with atopic dermatitis. Br J Dermatol. 2012;166:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Henderson J, Northstone K, Lee SP, Liao H, Zhao Y, Pembrey M, Mukhopadhyay S, Smith GD, Palmer CN, McLean WH. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol. 2008;121:872-877.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 64. | Marenholz I, Nickel R, Rüschendorf F, Schulz F, Esparza-Gordillo J, Kerscher T, Grüber C, Lau S, Worm M, Keil T. Filaggrin loss-of-function mutations predispose to phenotypes involved in the atopic march. J Allergy Clin Immunol. 2006;118:866-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 256] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 65. | Weidinger S, Illig T, Baurecht H, Irvine AD, Rodriguez E, Diaz-Lacava A, Klopp N, Wagenpfeil S, Zhao Y, Liao H. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006;118:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 421] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 66. | Weidinger S, O’Sullivan M, Illig T, Baurecht H, Depner M, Rodriguez E, Ruether A, Klopp N, Vogelberg C, Weiland SK. Filaggrin mutations, atopic eczema, hay fever, and asthma in children. J Allergy Clin Immunol. 2008;121:1203-1209.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 291] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 67. | Weidinger S, Rodríguez E, Stahl C, Wagenpfeil S, Klopp N, Illig T, Novak N. Filaggrin mutations strongly predispose to early-onset and extrinsic atopic dermatitis. J Invest Dermatol. 2007;127:724-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 68. | Schuttelaar ML, Kerkhof M, Jonkman MF, Koppelman GH, Brunekreef B, de Jongste JC, Wijga A, McLean WH, Postma DS. Filaggrin mutations in the onset of eczema, sensitization, asthma, hay fever and the interaction with cat exposure. Allergy. 2009;64:1758-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 69. | Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, Northstone K, Henderson J, Alizadehfar R, Ben-Shoshan M. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011;127:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 329] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 70. | Meng L, Wang L, Tang H, Tang X, Jiang X, Zhao J, Gao J, Li B, Fu X, Chen Y. Filaggrin gene mutation c.3321delA is associated with various clinical features of atopic dermatitis in the Chinese Han population. PLoS One. 2014;9:e98235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 71. | Sandilands A, Sutherland C, Irvine AD, McLean WH. Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci. 2009;122:1285-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 579] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 72. | Manabe M, Sanchez M, Sun TT, Dale BA. Interaction of filaggrin with keratin filaments during advanced stages of normal human epidermal differentiation and in ichthyosis vulgaris. Differentiation. 1991;48:43-50. [PubMed] |
| 73. | Scott IR, Harding CR. Filaggrin breakdown to water binding compounds during development of the rat stratum corneum is controlled by the water activity of the environment. Dev Biol. 1986;115:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 237] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 74. | Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004;17 Suppl 1:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 525] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 75. | Elias PM, Choi EH. Interactions among stratum corneum defensive functions. Exp Dermatol. 2005;14:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 76. | Gilmour JW, Vestey JP, George S, Norval M. Effect of phototherapy and urocanic acid isomers on natural killer cell function. J Invest Dermatol. 1993;101:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Jaksic A, Finlay-Jones JJ, Watson CJ, Spencer LK, Santucci I, Hart PH. Cis-urocanic acid synergizes with histamine for increased PGE2 production by human keratinocytes: link to indomethacin-inhibitable UVB-induced immunosuppression. Photochem Photobiol. 1995;61:303-309. [PubMed] |
| 78. | Brattsand M, Stefansson K, Lundh C, Haasum Y, Egelrud T. A proteolytic cascade of kallikreins in the stratum corneum. J Invest Dermatol. 2005;124:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 224] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 79. | Fluhr JW, Elias PM, Man MQ, Hupe M, Selden C, Sundberg JP, Tschachler E, Eckhart L, Mauro TM, Feingold KR. Is the filaggrin-histidine-urocanic acid pathway essential for stratum corneum acidification? J Invest Dermatol. 2010;130:2141-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Hachem JP, Man MQ, Crumrine D, Uchida Y, Brown BE, Rogiers V, Roseeuw D, Feingold KR, Elias PM. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol. 2005;125:510-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 81. | Miajlovic H, Fallon PG, Irvine AD, Foster TJ. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J Allergy Clin Immunol. 2010;126:1184-1190.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 82. | Sandilands A, Terron-Kwiatkowski A, Hull PR, O’Regan GM, Clayton TH, Watson RM, Carrick T, Evans AT, Liao H, Zhao Y. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet. 2007;39:650-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 474] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 83. | Thyssen JP, Godoy-Gijon E, Elias PM. Ichthyosis vulgaris: the filaggrin mutation disease. Br J Dermatol. 2013;168:1155-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 84. | Gruber R, Elias PM, Crumrine D, Lin TK, Brandner JM, Hachem JP, Presland RB, Fleckman P, Janecke AR, Sandilands A. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am J Pathol. 2011;178:2252-2263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 85. | Angelova-Fischer I, Mannheimer AC, Hinder A, Ruether A, Franke A, Neubert RH, Fischer TW, Zillikens D. Distinct barrier integrity phenotypes in filaggrin-related atopic eczema following sequential tape stripping and lipid profiling. Exp Dermatol. 2011;20:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 86. | Kezic S, Kemperman PM, Koster ES, de Jongh CM, Thio HB, Campbell LE, Irvine AD, McLean WH, Puppels GJ, Caspers PJ. Loss-of-function mutations in the filaggrin gene lead to reduced level of natural moisturizing factor in the stratum corneum. J Invest Dermatol. 2008;128:2117-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 220] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 87. | Kezic S, O’Regan GM, Lutter R, Jakasa I, Koster ES, Saunders S, Caspers P, Kemperman PM, Puppels GJ, Sandilands A. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J Allergy Clin Immunol. 2012;129:1031-1039.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 88. | O’Regan GM, Kemperman PM, Sandilands A, Chen H, Campbell LE, Kroboth K, Watson R, Rowland M, Puppels GJ, McLean WH. Raman profiles of the stratum corneum define 3 filaggrin genotype-determined atopic dermatitis endophenotypes. J Allergy Clin Immunol. 2010;126:574-580.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 89. | Winge MC, Hoppe T, Berne B, Vahlquist A, Nordenskjöld M, Bradley M, Törmä H. Filaggrin genotype determines functional and molecular alterations in skin of patients with atopic dermatitis and ichthyosis vulgaris. PLoS One. 2011;6:e28254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 90. | Brown SJ, Kroboth K, Sandilands A, Campbell LE, Pohler E, Kezic S, Cordell HJ, McLean WH, Irvine AD. Intragenic copy number variation within filaggrin contributes to the risk of atopic dermatitis with a dose-dependent effect. J Invest Dermatol. 2012;132:98-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 91. | Ginger RS, Blachford S, Rowland J, Rowson M, Harding CR. Filaggrin repeat number polymorphism is associated with a dry skin phenotype. Arch Dermatol Res. 2005;297:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Kezic S, O’Regan GM, Yau N, Sandilands A, Chen H, Campbell LE, Kroboth K, Watson R, Rowland M, McLean WH. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy. 2011;66:934-940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 219] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 93. | Pendaries V, Malaisse J, Pellerin L, Le Lamer M, Nachat R, Kezic S, Schmitt AM, Paul C, Poumay Y, Serre G. Knockdown of filaggrin in a three-dimensional reconstructed human epidermis impairs keratinocyte differentiation. J Invest Dermatol. 2014;134:2938-2946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 94. | Brauweiler AM, Bin L, Kim BE, Oyoshi MK, Geha RS, Goleva E, Leung DY. Filaggrin-dependent secretion of sphingomyelinase protects against staphylococcal α-toxin-induced keratinocyte death. J Allergy Clin Immunol. 2013;131:421-7.e1-421-7.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 95. | Ziyab AH, Karmaus W, Holloway JW, Zhang H, Ewart S, Arshad SH. DNA methylation of the filaggrin gene adds to the risk of eczema associated with loss-of-function variants. J Eur Acad Dermatol Venereol. 2013;27:e420-e423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 96. | Pellerin L, Henry J, Hsu CY, Balica S, Jean-Decoster C, Méchin MC, Hansmann B, Rodriguez E, Weindinger S, Schmitt AM. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J Allergy Clin Immunol. 2013;131:1094-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 97. | Henry J, Toulza E, Hsu CY, Pellerin L, Balica S, Mazereeuw-Hautier J, Paul C, Serre G, Jonca N, Simon M. Update on the epidermal differentiation complex. Front Biosci (Landmark Ed). 2012;17:1517-1532. [PubMed] |
| 98. | Esparza-Gordillo J, Weidinger S, Fölster-Holst R, Bauerfeind A, Ruschendorf F, Patone G, Rohde K, Marenholz I, Schulz F, Kerscher T. A common variant on chromosome 11q13 is associated with atopic dermatitis. Nat Genet. 2009;41:596-601. [PubMed] |
| 99. | Marenholz I, Rivera VA, Esparza-Gordillo J, Bauerfeind A, Lee-Kirsch MA, Ciechanowicz A, Kurek M, Piskackova T, Macek M, Lee YA. Association screening in the Epidermal Differentiation Complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 100. | Margolis DJ, Gupta J, Apter AJ, Ganguly T, Hoffstad O, Papadopoulos M, Rebbeck TR, Mitra N. Filaggrin-2 variation is associated with more persistent atopic dermatitis in African American subjects. J Allergy Clin Immunol. 2014;133:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 101. | Kirschner N, Rosenthal R, Furuse M, Moll I, Fromm M, Brandner JM. Contribution of tight junction proteins to ion, macromolecule, and water barrier in keratinocytes. J Invest Dermatol. 2013;133:1161-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 102. | De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, Berger AE, Zhang K, Vidyasagar S, Yoshida T. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127:773-786.e1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 575] [Cited by in RCA: 513] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 103. | Khnykin D, Rønnevig J, Johnsson M, Sitek JC, Blaas HG, Hausser I, Johansen FE, Jahnsen FL. Ichthyosis prematurity syndrome: clinical evaluation of 17 families with a rare disorder of lipid metabolism. J Am Acad Dermatol. 2012;66:606-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 104. | Sasaki T, Shiohama A, Kubo A, Kawasaki H, Ishida-Yamamoto A, Yamada T, Hachiya T, Shimizu A, Okano H, Kudoh J. A homozygous nonsense mutation in the gene for Tmem79, a component for the lamellar granule secretory system, produces spontaneous eczema in an experimental model of atopic dermatitis. J Allergy Clin Immunol. 2013;132:1111-1120.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 105. | Saunders SP, Goh CS, Brown SJ, Palmer CN, Porter RM, Cole C, Campbell LE, Gierlinski M, Barton GJ, Schneider G. Tmem79/Matt is the matted mouse gene and is a predisposing gene for atopic dermatitis in human subjects. J Allergy Clin Immunol. 2013;132:1121-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 106. | Kawasaki H, Nagao K, Kubo A, Hata T, Shimizu A, Mizuno H, Yamada T, Amagai M. Altered stratum corneum barrier and enhanced percutaneous immune responses in filaggrin-null mice. J Allergy Clin Immunol. 2012;129:1538-46.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 107. | Chavanas S, Bodemer C, Rochat A, Hamel-Teillac D, Ali M, Irvine AD, Bonafé JL, Wilkinson J, Taïeb A, Barrandon Y. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141-142. [PubMed] |
| 108. | Tartaglia-Polcini A, Bonnart C, Micheloni A, Cianfarani F, Andrè A, Zambruno G, Hovnanian A, D’Alessio M. SPINK5, the defective gene in netherton syndrome, encodes multiple LEKTI isoforms derived from alternative pre-mRNA processing. J Invest Dermatol. 2006;126:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Descargues P, Deraison C, Prost C, Fraitag S, Mazereeuw-Hautier J, D’Alessio M, Ishida-Yamamoto A, Bodemer C, Zambruno G, Hovnanian A. Corneodesmosomal cadherins are preferential targets of stratum corneum trypsin- and chymotrypsin-like hyperactivity in Netherton syndrome. J Invest Dermatol. 2006;126:1622-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 110. | Bonnart C, Deraison C, Lacroix M, Uchida Y, Besson C, Robin A, Briot A, Gonthier M, Lamant L, Dubus P. Elastase 2 is expressed in human and mouse epidermis and impairs skin barrier function in Netherton syndrome through filaggrin and lipid misprocessing. J Clin Invest. 2010;120:871-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 111. | Hachem JP, Wagberg F, Schmuth M, Crumrine D, Lissens W, Jayakumar A, Houben E, Mauro TM, Leonardsson G, Brattsand M. Serine protease activity and residual LEKTI expression determine phenotype in Netherton syndrome. J Invest Dermatol. 2006;126:1609-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 112. | Demerjian M, Hachem JP, Tschachler E, Denecker G, Declercq W, Vandenabeele P, Mauro T, Hupe M, Crumrine D, Roelandt T. Acute modulations in permeability barrier function regulate epidermal cornification: role of caspase-14 and the protease-activated receptor type 2. Am J Pathol. 2008;172:86-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 113. | Hachem JP, Houben E, Crumrine D, Man MQ, Schurer N, Roelandt T, Choi EH, Uchida Y, Brown BE, Feingold KR. Serine protease signaling of epidermal permeability barrier homeostasis. J Invest Dermatol. 2006;126:2074-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 114. | Stefansson K, Brattsand M, Roosterman D, Kempkes C, Bocheva G, Steinhoff M, Egelrud T. Activation of proteinase-activated receptor-2 by human kallikrein-related peptidases. J Invest Dermatol. 2008;128:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 115. | Kato A, Fukai K, Oiso N, Hosomi N, Murakami T, Ishii M. Association of SPINK5 gene polymorphisms with atopic dermatitis in the Japanese population. Br J Dermatol. 2003;148:665-669. [PubMed] |
| 116. | Kusunoki T, Okafuji I, Yoshioka T, Saito M, Nishikomori R, Heike T, Sugai M, Shimizu A, Nakahata T. SPINK5 polymorphism is associated with disease severity and food allergy in children with atopic dermatitis. J Allergy Clin Immunol. 2005;115:636-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 117. | Nishio Y, Noguchi E, Shibasaki M, Kamioka M, Ichikawa E, Ichikawa K, Umebayashi Y, Otsuka F, Arinami T. Association between polymorphisms in the SPINK5 gene and atopic dermatitis in the Japanese. Genes Immun. 2003;4:515-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 118. | Walley AJ, Chavanas S, Moffatt MF, Esnouf RM, Ubhi B, Lawrence R, Wong K, Abecasis GR, Jones EY, Harper JI. Gene polymorphism in Netherton and common atopic disease. Nat Genet. 2001;29:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 260] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 119. | Zhao LP, Di Z, Zhang L, Wang L, Ma L, Lv Y, Hong Y, Wei H, Chen HD, Gao XH. Association of SPINK5 gene polymorphisms with atopic dermatitis in Northeast China. J Eur Acad Dermatol Venereol. 2012;26:572-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 120. | Kabesch M, Carr D, Weiland SK, von Mutius E. Association between polymorphisms in serine protease inhibitor, kazal type 5 and asthma phenotypes in a large German population sample. Clin Exp Allergy. 2004;34:340-345. [PubMed] |
| 121. | Fortugno P, Furio L, Teson M, Berretti M, El Hachem M, Zambruno G, Hovnanian A, D’Alessio M. The 420K LEKTI variant alters LEKTI proteolytic activation and results in protease deregulation: implications for atopic dermatitis. Hum Mol Genet. 2012;21:4187-4200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 122. | Vasilopoulos Y, Cork MJ, Teare D, Marinou I, Ward SJ, Duff GW, Tazi-Ahnini R. A nonsynonymous substitution of cystatin A, a cysteine protease inhibitor of house dust mite protease, leads to decreased mRNA stability and shows a significant association with atopic dermatitis. Allergy. 2007;62:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 123. | Hubiche T, Ged C, Benard A, Léauté-Labrèze C, McElreavey K, de Verneuil H, Taïeb A, Boralevi F. Analysis of SPINK 5, KLK 7 and FLG genotypes in a French atopic dermatitis cohort. Acta Derm Venereol. 2007;87:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 124. | Weidinger S, Baurecht H, Wagenpfeil S, Henderson J, Novak N, Sandilands A, Chen H, Rodriguez E, O’Regan GM, Watson R. Analysis of the individual and aggregate genetic contributions of previously identified serine peptidase inhibitor Kazal type 5 (SPINK5), kallikrein-related peptidase 7 (KLK7), and filaggrin (FLG) polymorphisms to eczema risk. J Allergy Clin Immunol. 2008;122:560-568.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 125. | Moniaga CS, Kabashima K. Filaggrin in atopic dermatitis: flaky tail mice as a novel model for developing drug targets in atopic dermatitis. Inflamm Allergy Drug Targets. 2011;10:477-485. [PubMed] |
| 126. | Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, Callanan JJ, Kawasaki H, Shiohama A, Kubo A. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41:602-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 357] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 127. | Visser MJ, Landeck L, Campbell LE, McLean WH, Weidinger S, Calkoen F, John SM, Kezic S. Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol. 2013;168:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 128. | McPherson T, Sherman VJ, Aslam A, Crack L, Chan H, Lloyd-Lavery A, Jones L, Ardern-Jones M, Ogg G. Filaggrin null mutations associate with increased frequencies of allergen-specific CD4+ T-helper 2 cells in patients with atopic eczema. Br J Dermatol. 2010;163:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 129. | Nylander-Lundqvist E, Bäck O, Egelrud T. IL-1 beta activation in human epidermis. J Immunol. 1996;157:1699-1704. [PubMed] |
| 130. | Stehlik C. Multiple interleukin-1beta-converting enzymes contribute to inflammatory arthritis. Arthritis Rheum. 2009;60:3524-3530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 131. | Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, Besson C, Dubus P, Hovnanian A. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135-1147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 371] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 132. | Moniaga CS, Jeong SK, Egawa G, Nakajima S, Hara-Chikuma M, Jeon JE, Lee SH, Hibino T, Miyachi Y, Kabashima K. Protease activity enhances production of thymic stromal lymphopoietin and basophil accumulation in flaky tail mice. Am J Pathol. 2013;182:841-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 133. | Kypriotou M, Boéchat C, Huber M, Hohl D. Spontaneous atopic dermatitis-like symptoms in a/a ma ft/ma ft/J flaky tail mice appear early after birth. PLoS One. 2013;8:e67869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 134. | Lee KH, Cho KA, Kim JY, Kim JY, Baek JH, Woo SY, Kim JW. Filaggrin knockdown and Toll-like receptor 3 (TLR3) stimulation enhanced the production of thymic stromal lymphopoietin (TSLP) from epidermal layers. Exp Dermatol. 2011;20:149-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 135. | Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 2014;134:792-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 267] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 137. | Irvine AD, McLean WH. Breaking the (un)sound barrier: filaggrin is a major gene for atopic dermatitis. J Invest Dermatol. 2006;126:1200-1202. [PubMed] |
| 138. | McLean WH, Hull PR. Breach delivery: increased solute uptake points to a defective skin barrier in atopic dermatitis. J Invest Dermatol. 2007;127:8-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 139. | Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 140. | Gutowska-Owsiak D, Schaupp AL, Salimi M, Taylor S, Ogg GS. Interleukin-22 downregulates filaggrin expression and affects expression of profilaggrin processing enzymes. Br J Dermatol. 2011;165:492-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 141. | Hvid M, Johansen C, Deleuran B, Kemp K, Deleuran M, Vestergaard C. Regulation of caspase 14 expression in keratinocytes by inflammatory cytokines--a possible link between reduced skin barrier function and inflammation? Exp Dermatol. 2011;20:633-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 142. | Howell MD, Boguniewicz M, Pastore S, Novak N, Bieber T, Girolomoni G, Leung DY. Mechanism of HBD-3 deficiency in atopic dermatitis. Clin Immunol. 2006;121:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 143. | Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, Leung DY. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 259] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 144. | Kisich KO, Carspecken CW, Fiéve S, Boguniewicz M, Leung DY. Defective killing of Staphylococcus aureus in atopic dermatitis is associated with reduced mobilization of human beta-defensin-3. J Allergy Clin Immunol. 2008;122:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 145. | Hatano Y, Katagiri K, Arakawa S, Fujiwara S. Interleukin-4 depresses levels of transcripts for acid-sphingomyelinase and glucocerebrosidase and the amount of ceramide in acetone-wounded epidermis, as demonstrated in a living skin equivalent. J Dermatol Sci. 2007;47:45-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 146. | Hatano Y, Terashi H, Arakawa S, Katagiri K. Interleukin-4 suppresses the enhancement of ceramide synthesis and cutaneous permeability barrier functions induced by tumor necrosis factor-alpha and interferon-gamma in human epidermis. J Invest Dermatol. 2005;124:786-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 147. | Danso MO, van Drongelen V, Mulder A, van Esch J, Scott H, van Smeden J, El Ghalbzouri A, Bouwstra JA. TNF-α and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J Invest Dermatol. 2014;134:1941-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 247] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 148. | Morizane S, Yamasaki K, Kajita A, Ikeda K, Zhan M, Aoyama Y, Gallo RL, Iwatsuki K. TH2 cytokines increase kallikrein 7 expression and function in patients with atopic dermatitis. J Allergy Clin Immunol. 2012;130:259-261.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 149. | Hatano Y, Adachi Y, Elias PM, Crumrine D, Sakai T, Kurahashi R, Katagiri K, Fujiwara S. The Th2 cytokine, interleukin-4, abrogates the cohesion of normal stratum corneum in mice: implications for pathogenesis of atopic dermatitis. Exp Dermatol. 2013;22:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 150. | Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126:332-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 440] [Cited by in RCA: 406] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 151. | Kobayashi J, Inai T, Morita K, Moroi Y, Urabe K, Shibata Y, Furue M. Reciprocal regulation of permeability through a cultured keratinocyte sheet by IFN-gamma and IL-4. Cytokine. 2004;28:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 152. | Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, Alenius H, Dieu-Nosjean MC, Meller S, Rieker J. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 670] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 153. | Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 715] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 154. | Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, Duff GW, Ward SJ, Tazi-Ahnini R. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J Allergy Clin Immunol. 2006;118:3-21; quiz 22-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 329] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 155. | Nakamura T, Hirasawa Y, Takai T, Mitsuishi K, Okuda M, Kato T, Okumura K, Ikeda S, Ogawa H. Reduction of skin barrier function by proteolytic activity of a recombinant house dust mite major allergen Der f 1. J Invest Dermatol. 2006;126:2719-2723. [PubMed] |
| 156. | Roelandt T, Heughebaert C, Hachem JP. Proteolytically active allergens cause barrier breakdown. J Invest Dermatol. 2008;128:1878-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 157. | Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, Stewart GA, Taylor GW, Garrod DR, Cannell MB. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 528] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 158. | Wan H, Winton HL, Soeller C, Taylor GW, Gruenert DC, Thompson PJ, Cannell MB, Stewart GA, Garrod DR, Robinson C. The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clin Exp Allergy. 2001;31:279-294. [PubMed] |
| 159. | Jeong SK, Kim HJ, Youm JK, Ahn SK, Choi EH, Sohn MH, Kim KE, Hong JH, Shin DM, Lee SH. Mite and cockroach allergens activate protease-activated receptor 2 and delay epidermal permeability barrier recovery. J Invest Dermatol. 2008;128:1930-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 160. | Kato T, Takai T, Fujimura T, Matsuoka H, Ogawa T, Murayama K, Ishii A, Ikeda S, Okumura K, Ogawa H. Mite serine protease activates protease-activated receptor-2 and induces cytokine release in human keratinocytes. Allergy. 2009;64:1366-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 161. | Landheer J, Giovannone B, Mattson JD, Tjabringa S, Bruijnzeel-Koomen CA, McClanahan T, de Waal Malefyt R, Knol E, Hijnen D. Epicutaneous application of house dust mite induces thymic stromal lymphopoietin in nonlesional skin of patients with atopic dermatitis. J Allergy Clin Immunol. 2013;132:1252-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 162. | Clarke SR, Mohamed R, Bian L, Routh AF, Kokai-Kun JF, Mond JJ, Tarkowski A, Foster SJ. The Staphylococcus aureus surface protein IsdA mediates resistance to innate defenses of human skin. Cell Host Microbe. 2007;1:199-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 163. | Otto M. Virulence factors of the coagulase-negative staphy-lococci. Front Biosci. 2004;9:841-863. [PubMed] |
| 164. | Ohnishi Y, Okino N, Ito M, Imayama S. Ceramidase activity in bacterial skin flora as a possible cause of ceramide deficiency in atopic dermatitis. Clin Diagn Lab Immunol. 1999;6:101-104. [PubMed] |
| 165. | Akdis CA, Akdis M, Bieber T, Bindslev-Jensen C, Boguniewicz M, Eigenmann P, Hamid Q, Kapp A, Leung DY, Lipozencic J. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Report. Allergy. 2006;61:969-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 235] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 166. | Choi EH, Brown BE, Crumrine D, Chang S, Man MQ, Elias PM, Feingold KR. Mechanisms by which psychologic stress alters cutaneous permeability barrier homeostasis and stratum corneum integrity. J Invest Dermatol. 2005;124:587-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 167. | Denda M, Tsuchiya T, Elias PM, Feingold KR. Stress alters cutaneous permeability barrier homeostasis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R367-R372. [PubMed] |
| 168. | Aberg KM, Radek KA, Choi EH, Kim DK, Demerjian M, Hupe M, Kerbleski J, Gallo RL, Ganz T, Mauro T. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007;117:3339-3349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 169. | Choi EH, Demerjian M, Crumrine D, Brown BE, Mauro T, Elias PM, Feingold KR. Glucocorticoid blockade reverses psychological stress-induced abnormalities in epidermal structure and function. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1657-R1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 170. | Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 478] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 171. | Ying S, Meng Q, Corrigan CJ, Lee TH. Lack of filaggrin expression in the human bronchial mucosa. J Allergy Clin Immunol. 2006;118:1386-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 172. | De Benedetto A, Qualia CM, Baroody FM, Beck LA. Filaggrin expression in oral, nasal, and esophageal mucosa. J Invest Dermatol. 2008;128:1594-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 173. | Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clin Exp Allergy. 2005;35:757-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 169] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 174. | Ziegler SF. Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol. 2012;130:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 175. | Demehri S, Morimoto M, Holtzman MJ, Kopan R. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 176. | Reche PA, Soumelis V, Gorman DM, Clifford T, Liu Mr M, Zurawski SM, Johnston J, Liu YJ, Spits H, de Waal Malefyt R. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336-343. [PubMed] |
| 177. | Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 437] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 178. | Soumelis V, Liu YJ. Human thymic stromal lymphopoietin: a novel epithelial cell-derived cytokine and a potential key player in the induction of allergic inflammation. Springer Semin Immunopathol. 2004;25:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 179. | Sano Y, Masuda K, Tamagawa-Mineoka R, Matsunaka H, Murakami Y, Yamashita R, Morita E, Katoh N. Thymic stromal lymphopoietin expression is increased in the horny layer of patients with atopic dermatitis. Clin Exp Immunol. 2013;171:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 180. | Leyva-Castillo JM, Hener P, Jiang H, Li M. TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J Invest Dermatol. 2013;133:154-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 181. | Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, Comeau MR, Campbell DJ, Ziegler SF. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 451] [Cited by in RCA: 460] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 182. | Li M, Messaddeq N, Teletin M, Pasquali JL, Metzger D, Chambon P. Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proc Natl Acad Sci USA. 2005;102:14795-14800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 183. | Han H, Xu W, Headley MB, Jessup HK, Lee KS, Omori M, Comeau MR, Marshak-Rothstein A, Ziegler SF. Thymic stromal lymphopoietin (TSLP)-mediated dermal inflammation aggravates experimental asthma. Mucosal Immunol. 2012;5:342-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 184. | Margolis DJ, Kim B, Apter AJ, Gupta J, Hoffstad O, Papadopoulos M, Mitra N. Thymic stromal lymphopoietin variation, filaggrin loss of function, and the persistence of atopic dermatitis. JAMA Dermatol. 2014;150:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 185. | Biagini Myers JM, Martin LJ, Kovacic MB, Mersha TB, He H, Pilipenko V, Lindsey MA, Ericksen MB, Bernstein DI, LeMasters GK. Epistasis between serine protease inhibitor Kazal-type 5 (SPINK5) and thymic stromal lymphopoietin (TSLP) genes contributes to childhood asthma. J Allergy Clin Immunol. 2014;134:891-899.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 186. | Catherine Mack Correa M, Nebus J. Management of patients with atopic dermatitis: the role of emollient therapy. Dermatol Res Pract. 2012;2012:836931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 187. | Simpson EL, Berry TM, Brown PA, Hanifin JM. A pilot study of emollient therapy for the primary prevention of atopic dermatitis. J Am Acad Dermatol. 2010;63:587-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 188. | Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54:1-15; quiz 16-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 637] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 189. | Kim M, Jung M, Hong SP, Jeon H, Kim MJ, Cho MY, Lee SH, Man MQ, Elias PM, Choi EH. Topical calcineurin inhibitors compromise stratum corneum integrity, epidermal permeability and antimicrobial barrier function. Exp Dermatol. 2010;19:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 190. | Kao JS, Fluhr JW, Man MQ, Fowler AJ, Hachem JP, Crumrine D, Ahn SK, Brown BE, Elias PM, Feingold KR. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 244] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 191. | Danby SG, Chittock J, Brown K, Albenali LH, Cork MJ. The effect of tacrolimus compared with betamethasone valerate on the skin barrier in volunteers with quiescent atopic dermatitis. Br J Dermatol. 2014;170:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 192. | Angelova-Fischer I, Dapic I, Hoek AK, Jakasa I, Fischer TW, Zillikens D, Kezic S. Skin barrier integrity and natural moisturising factor levels after cumulative dermal exposure to alkaline agents in atopic dermatitis. Acta Derm Venereol. 2014;94:640-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 193. | Törmä H, Lindberg M, Berne B. Skin barrier disruption by sodium lauryl sulfate-exposure alters the expressions of involucrin, transglutaminase 1, profilaggrin, and kallikreins during the repair phase in human skin in vivo. J Invest Dermatol. 2008;128:1212-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 194. | Hon KL, Leung AK. Use of ceramides and related products for childhood-onset eczema. Recent Pat Inflamm Allergy Drug Discov. 2013;7:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 195. | Valdman-Grinshpoun Y, Ben-Amitai D, Zvulunov A. Barrier-restoring therapies in atopic dermatitis: current approaches and future perspectives. Dermatol Res Pract. 2012;2012:923134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 196. | Kircik LH, Del Rosso JQ, Aversa D. Evaluating Clinical Use of a Ceramide-dominant, Physiologic Lipid-based Topical Emulsion for Atopic Dermatitis. J Clin Aesthet Dermatol. 2011;4:34-40. [PubMed] |
| 197. | Chamlin SL, Kao J, Frieden IJ, Sheu MY, Fowler AJ, Fluhr JW, Williams ML, Elias PM. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier function provide a sensitive indicator of disease activity. J Am Acad Dermatol. 2002;47:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 282] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 198. | Sugarman JL, Parish LC. Efficacy of a lipid-based barrier repair formulation in moderate-to-severe pediatric atopic dermatitis. J Drugs Dermatol. 2009;8:1106-1111. [PubMed] |
| 199. | Miller DW, Koch SB, Yentzer BA, Clark AR, O’Neill JR, Fountain J, Weber TM, Fleischer AB. An over-the-counter moisturizer is as clinically effective as, and more cost-effective than, prescription barrier creams in the treatment of children with mild-to-moderate atopic dermatitis: a randomized, controlled trial. J Drugs Dermatol. 2011;10:531-537. [PubMed] |
| 200. | Stout TE, McFarland T, Mitchell JC, Appukuttan B, Stout JT. Recombinant filaggrin is internalized and processed to correct filaggrin deficiency. J Invest Dermatol. 2014;134:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 201. | Otsuka A, Doi H, Egawa G, Maekawa A, Fujita T, Nakamizo S, Nakashima C, Nakajima S, Watanabe T, Miyachi Y. Possible new therapeutic strategy to regulate atopic dermatitis through upregulating filaggrin expression. J Allergy Clin Immunol. 2014;133:139-146.e1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 202. | Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol. 2012;132:751-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 394] [Cited by in RCA: 371] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 203. | Lesiak A, Kuna P, Zakrzewski M, van Geel M, Bladergroen RS, Przybylowska K, Stelmach I, Majak P, Hawro T, Sysa-Jedrzejowska A. Combined occurrence of filaggrin mutations and IL-10 or IL-13 polymorphisms predisposes to atopic dermatitis. Exp Dermatol. 2011;20:491-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 204. | Nakai K, Yoneda K, Hosokawa Y, Moriue T, Presland RB, Fallon PG, Kabashima K, Kosaka H, Kubota Y. Reduced expression of epidermal growth factor receptor, E-cadherin, and occludin in the skin of flaky tail mice is due to filaggrin and loricrin deficiencies. Am J Pathol. 2012;181:969-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 205. | Cole C, Kroboth K, Schurch NJ, Sandilands A, Sherstnev A, O’Regan GM, Watson RM, Irwin McLean WH, Barton GJ, Irvine AD. Filaggrin-stratified transcriptomic analysis of pediatric skin identifies mechanistic pathways in patients with atopic dermatitis. J Allergy Clin Immunol. 2014;134:82-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 206. | Brown SJ, Relton CL, Liao H, Zhao Y, Sandilands A, McLean WH, Cordell HJ, Reynolds NJ. Filaggrin haploinsufficiency is highly penetrant and is associated with increased severity of eczema: further delineation of the skin phenotype in a prospective epidemiological study of 792 school children. Br J Dermatol. 2009;161:884-889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |