Revised: May 28, 2014
Accepted: June 27, 2014
Published online: August 2, 2014
Processing time: 187 Days and 12.4 Hours
AIM: To establish whether d-limonene can protect against induction of cyclobutane pyrimidine dimers (CPDs) and sunburn in ultraviolet irradiation (UVR) irradiated mouse skin.
METHODS: The d-limonene was given in 4 daily oral 20 μL aliquots at different concentrations as follows: 100%, 10% or 1% in liponate and 100% liponate as control. One day after the final d-limonene treatment, the mice were anesthetized with i.p. sodium pentobarbital and placed in boxes to allow a rectangular (2 cm × 4 cm) region of dorsal skin to be irradiated with a single, ultraviolet radiation dose of 1.5 kJ/m2. Skin samples from UVR irradiated area were obtained at 5 min after UVR exposure for CPD detection, at 6 d after UVR exposure, skin samples were obtained for in situ analysis for N-myc downstream regulating gene 1 (NDRG1) (a stress response gene), proliferating cell nuclear antigen (PCNA) (an S-phase marker) and filaggrin (a barrier integrity gene). Based on immunohistochemistry staining, the number of CPD, NDRG1 and PCNA positive cells, as well as unstained cells was counted in 3 different individually selected areas and percentage of positive cells was established.
RESULTS: CPD reduction occurred as follows: liponate only-none; 1% d-limonene-54.3% reduction of CPDs; 10% d-limonene-73.4% reduction of CPDs; 100% d-limonene-86.1% reduction of CPDs, the latter equivalent to a UV dose of only 0.21 kJ/m2. Sunburn was also dose-dependently reduced by d-limonene. The NDRG1 protein was strongly induced by UVR (70.0% ± 10.4% positive cells), but 1% d-limonene reduced the response to 64.6% ± 9.2%, 10% d-limonene reduced the response to 16.2% ± 3.4% and 100% d-limonene reduced the response to 6.3% ± 1.7%. Similarly, PCNA was 52.4% ± 9.9% positive in UVR exposed skin, and 1% d-limonene reduced it to 42.9% ± 8.1%, 10% d-limonene reduced it to 36.2% ± 6.7% and 100% d-limonene reduce it to 13.8% ± 3.4%. NDRG1 and PCNA were increased by d-limonene or UVR separately, but combined they produced less than either agent separately owing to the protective effect of pre-exposure to d-limonene.
CONCLUSION: Overall d-limonene acted to protect against ultraviolet B-induced DNA photodamage and sunburn in UVR exposed skin.
Core tip: Skh-1 hairless mice were given 4 daily 20 μL aliquots of different concentrations of d-limonene, and then irradiated to a single ultraviolet irradiation. Skin samples from the ultraviolet-exposed area of mice showed that ultraviolet irradiation induced cyclobutane pyrimidine dimers formation, N-myc downstream regulating gene 1 and proliferating cell nuclear antigen expression, pretreatment of d-limonene significantly reduced these responses. Pure d-limonene also induced the expression of epidermal barrier protein filaggrin. In conclusion, d-limonene protected the mice skin from UV-induced DNA photodamage and sunburn in mice skin.
-
Citation: Uddin AN, Wu F, Labuda I, Tchou-Wong KM, Burns FJ.
d -limonene prevents ultraviolet irradiation: Induced cyclobutane pyrimidine dimers in Skh1 mouse skin. World J Dermatol 2014; 3(3): 64-72 - URL: https://www.wjgnet.com/2218-6190/full/v3/i3/64.htm
- DOI: https://dx.doi.org/10.5314/wjd.v3.i3.64
Understanding how aroma terpenes prevent sunburn and/or skin cancer in mice could lead to more effective and safer ways of blocking sun damage to human skin. As one of the most common terpenes in nature, d-limonene is listed in the Code of Federal Regulations as generally recognized as safe (GRAS) as a flavoring agent (http://www.cfsan.fda.gov/-Ird/fcf182.html) and is found in common foods, such as, fruit juices, soft drinks, baked goods, ice cream, and pudding at low concentrations[1]. Limonene has well-established chemopreventive activity against many cancer types in animal models[2,3], including mammary[4], skin[5], liver[6], lung[7] and forestomach cancers[8].
Previously, we showed that β-damascenone, an aroma terpene, protected against sunburn by activating both keratinocyte and sebaceous gland pathways that fortified and thickened the cornified barrier layers of mouse epidermis[9]. Our immunohistological and gene expression results of keratin, caspase 14, filaggrin or loricrin were consistent with the idea that fortification of the cornified envelope by the activation of new sebum secretion was the key mechanism of β-damascenone-induced sunburn protection by absorbing ultraviolet irradiation (UVR) and reducing its penetration into the skin[9].
In the current study, the effect of d-limonene on the protection of mouse skin against UVR-induced sunburn and DNA photodamages was quantified in UVR-irradiated mice skin. The stress-related N-myc downstream regulating gene 1 (NDRG1) gene protein, proliferation [as assessed by proliferating cell nuclear antigen (PCNA)] and expression of skin barrier function gene, filaggrin, were detected by immunohistochemistry. Results indicated that d-limonene protected against DNA photodamages and sunburn of the mice skin. The protection was likely associated with activation by the d-limonene of keratinocyte and sebaceous gland proliferation and induction of endogenous UV absorbents in the outer cornified envelop of skin, thereby increasing UVR absorption and protecting underlying cutaneous tissues.
Albino, female Skh-1 mice of 6 wk old used in all experiments were purchased from Charles River Laboratories (Wilmington, MA).
The test terpene, d-limonene, was obtained as a 99.99% pure compound from Biokeys for Flavors( Norwood, NJ, United States). For 10% or 1.0% compounds, pure d-limonene was diluted with the triglyceride, liponate. d-Limonene was administered orally, sometimes topically.
Thirty mice were grouped as follows: (1) control (3 mice); (2) UVB alone (3 mice); (3) 100% d-limonene (6 mice, 3 topical, 3 oral); (4) 100% d-limonene + UVR (6 mice = 3 topical d-limonene + 3 oral d-limonene); (5) 10% d-limonene + UVR (6 mice = 3 topical d-limonene + 3 oral d-limonene); and (6) 1.0% d-limonene + UVR (6 mice = 3 topical d-limonene + 3 oral d-limonene). d-Limonene was given topically or orally. For topical exposure, 20 μL d-limonene (100% or diluted) was applied on the dorsum of the mice in the same amounts as was administered by feeding tube.
One day after the final d-limonene application, mice were exposed to a single dose of 1.5 kJ/m2 of UVR (7.5 min duration). For positional restraint during UVR exposures, the mice were anesthetized with i.p. sodium pentobarbital (Nembutal) at a dose of 35 mg/kg body weight and placed in boxes configured to allow a rectangular (2 cm × 4 cm) region of dorsal skin to be exposed to UVR. The UVR was generated by of a bank of four parallel Westinghouse fluorescent sun lamps (FS-20, Westinghouse, Bloomfield, NJ, United States). The lamps were positioned 24 cm above the skin surface. The dose rate of UV was 0.20 kJ/m2 per minute as measured with a digital radiometer/ photometer (1400A, International Light Inc., Wilmington, MA, United States). Sunburn was evaluated and photographed at a peak response generally 4 to 6 d after UV exposure. A minimal erythema dose (MED) experiment was performed in the dose range of 0.25 kJ/m2 to 2.0 kJ/m2. All experiments were conducted according to protocols approved by the Institutional Animal Care and Use Committee of New York University School of Medicine.
For detection of cyclobutane pyrimidine dimer (CPD) photoproducts, a separate group of 27 Skh1 mice were used as control, UVR alone, UVR + 100/10/1.0% d-limonene for oral applications. Four daily d-limonene doses and a single UVR exposure was applied as described in sunburn experiments. Skin samples from the dorsal area of mice exposed to UVR were obtained at 5 min after each UVR exposure for CDP detection. For NDRG1, PCNA and filaggrin detection, skin samples were obtained at 6 d after UVR exposure.
CPDs were detected with conventional streptavidin-biotin methods. Formalin-fixed paraffin-embedded sections were deparaffinized in xylene and absolute ethyl alcohol. The DNA was denatured by incubating slides in 2N HCl for 10 min and then neutralizing by incubating in 50 mmol/L Tris-base for 5 min at room temperature. After blocking with non-immune serum, slides were incubated overnight at 4 °C with mouse monoclonal anti-CPD antibody (Cosmo Bio Co., Tokyo, Japan) at a dilution of 1:300 in PBS. Slides were then incubated for 15 min with biotinylated antibody and then with the streptavidin-biotin peroxidase system Ultra Streptavidin Detection System kit (Covance Research Products, Dedham, MA, United States).
Immunostaining of NDRG1, PCNA and filaggrin were performed on paraffin sections of skin samples. The sections were incubated overnight at 4 °C with different antibodies as follows: a rabbit polyclonal anti-NDRG1 antibody (Abcam, Cambridge, MA, United States) at a dilution of 1:300 in PBS, a mouse monoclonal anti-PCNA antibody (Covance Research Products) at a dilution of 1:100 in PBS, a purified polyclonal anti-filaggrin antibody (Covance Research Products) at a dilution of 1:300 in PBS. Then immunostaining was performed according to the protocol of with the streptavidin-biotin peroxidase system Ultra Streptavidin Detection System kit (Covance Research Products).
Stained cutaneous tissues were observed under a light microscope and images were created by using a Canon Powershot SD500 digital camera with a Scopetronix microscope adapter. The number of CPD, NDRG1 and PCNA positive cells as well as unstained cells was counted in 3 different randomly selected areas of equal length for each sample and the percentage of positive cells was calculated. Values are expressed as mean ± SE.
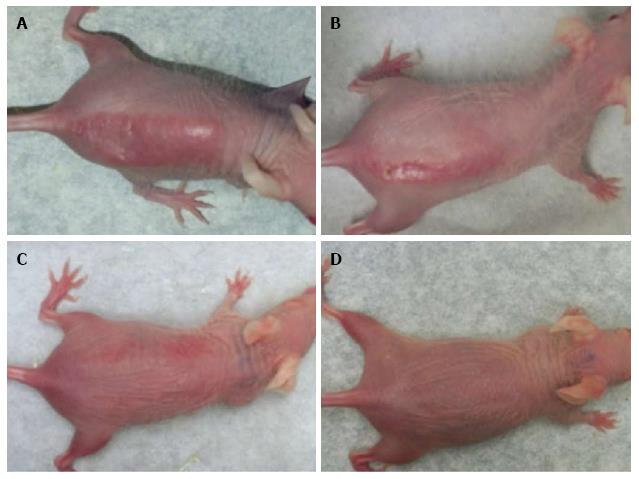
Administration of 100% d-limonene orally significantly prevented DNA photodamage as well as sunburn/erythema in Skh-1 mice exposed to 1.5 kJ/m2 of UVR. Four doses of 20 μL each (0.95 μg/g body weight) of 100% d-limonene provided complete protection from UVR-induced sunburn. Topical d-limonene prevented UVR-induced sunburn slightly more effectively than oral d-limonene. Sunburn protection was gradually reduced with lower concentrations such as 10%, 1% or 0% d-limonene in a dose-dependent fashion (Figure 1). The sunburn protection was less but still significant at 10% d-limonene but was absent at 1% d-limonene. A minimal erythemal dose (MED) for these mice was established at 0.75 kJ/m2.
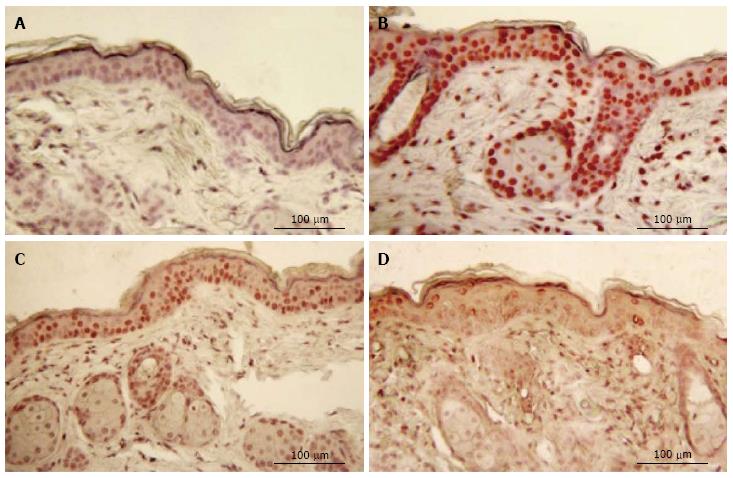
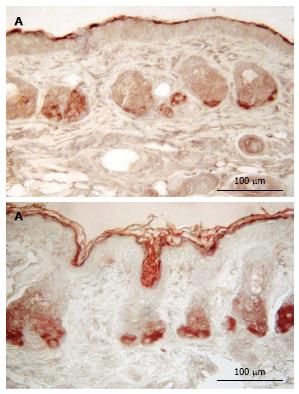
No staining for CPDs was observed in epidermis that had not been exposed to UV (Figure 2). Five min after UVR exposure, CPD-stained cells were observed among the epithelial cells superficial dermal fibroblasts. UV irradiation significantly increased the CPD staining as shown in Figure 2B. Mice given oral 100% d-limonene before UVR exposure exhibited only 15% of the UVR-induced CPDs in compared to mice not treated with d-limonene (Figure 2D). The photoproducts were reduced for 10% or 1% d-limonene in a dose-dependent fashion as follows and as shown in Table 1. The percentage of CPD positive cells was 94.7% ± 11.1% in UV exposed, 45.7 ± 8.3% (52% reduction) in 1% d-limonene pretreated, 26.6 ± 5.8% (72% reduction) in 10% d-limonene pretreated, and 13.9 ± 4.5% (85% reduction) in 100% d-limonene pretreated mice skin epidermis.
| Groups | CPD-positive cells (%) | NDRG1-positive cells (%) | PCNA-positive cells (%) |
| Control | 0.0 | 6.0 ± 1.4 | 7.1 ± 1.7 |
| UVR only | 94.7 ± 11.1 | 70.0 ± 10.4 | 52.4 ± 9.9 |
| UVR + 1.0% d-limonene | 45.7 ± 8.3 | 64.6 ± 9.2 | 42.9 ± 8.1 |
| UVR + 10% d-limonene | 26.6 ± 5.8 | 16.2 ± 3.4 | 36.2 ± 6.7 |
| UVR + 100% d-limonene | 13.9 ± 4.5 | 6.3 ± 1.7 | 13.8 ± 3.4 |
| 100% d-limonene alone | 0.0 | 28.1 ± 5.2 | 74.6 ± 11.1 |
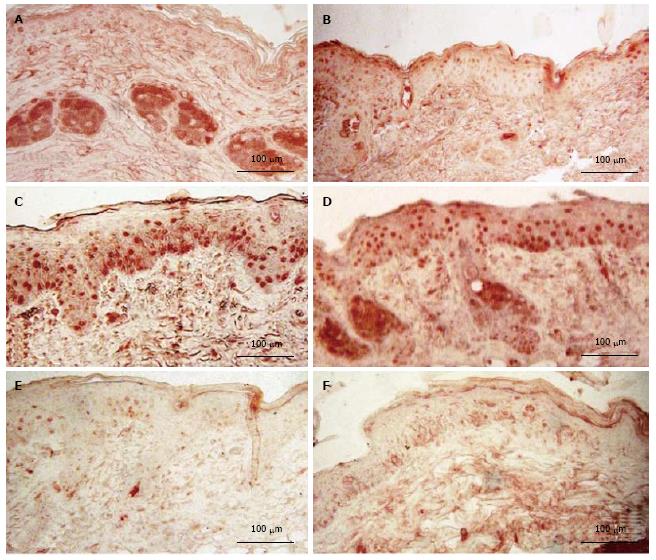
Mice exposed to UVR strongly expressed NDRG1 (Figure 3C), however, mice exposed to UVR following treatment with d-limonene, showed reduced NDRG1 protein in epidermis (Figure 3D-F), even though d-limonene alone produced an increased level of NDRG1 (28.1% ± 5.2%, Figure 3B). In control skin, little or no NDRG1 was observed in epidermis but low levels were detected in sebaceous glands. The NDRG1 protein, a cytoplasmic protein involved in stress response, indicated that 70.0% ± 10.4% of keratinocytes were affected by the DNA damaging effect of the UVR. The UVR-induced NDRG1 index remained at 64.6% ± 9.2 % at 1.0% d-limonene, but was significantly reduced at 10% d-limonene to 16.2% ± 3.4% and at 100% d-limonene to less than 6.3% ± 1.7% (Table 1) ; the latter indicating a nearly complete elimination of cellular damage or response to damage; a finding that is consistent with sunburn prevention and CPD inhibition.
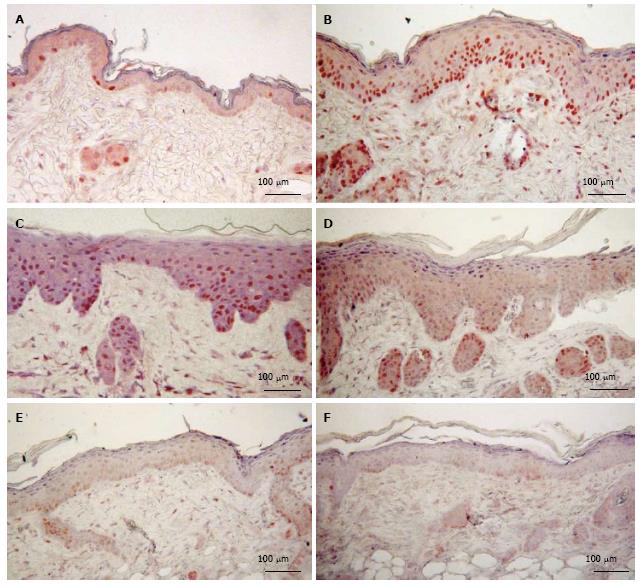
As shown in Figure 4 both d-limonene and UVR induced proliferative stimulation of in the epidermis, the hair follicles (not shown) and the sebaceous glands (not shown). However, prior treatment of mice with d-limonene exposure significantly reduced the PCNA-positive cells in skin compared to that of UVR alone mice. The percentage of PCNA-positive nuclei (S-phase nuclei) in epidermis of d-limonene-treated mice was 74.6% ± 11.1%, vs 7.1% ± 1.7% in controls; a 10.5 folds increase (Table 1). The increased proliferation of keratinocytes was apparently balanced by an increased rate of differentiation, because the total nucleated keratinocyte count was unaffected. The percentage of PCNA positive cells was 52.4% ± 9.9% in UV exposed skin, but 1% d-limonene reduced the number to 42.9% ± 8.1%, 10% d-limonene reduced the number to 36.2% ± 6.7% and 100% d-limonene reduced the number to 13.8% ± 3.4%.
As shown in Figure 5, d-limonene elevated the filaggrin level in the epidermis and sebaceous glands in comparison to control, similar to what was observed in our β-damascenone study[3]. d-Limonene increased the thickness of the cornified envelope layer of epidermis for a prolonged period up to 12 d.
DNA damage induced by UVR is thought to play an important role in the pathogenesis of skin cancers[10,11]. The major types of DNA damage induced by UVR are cyclobutane pyrimidine dimers (CPDs) and (6-4) photoproducts (6-4PPs). Approximately 75 % of UVR-induced DNA damage is CPDs and the remaining is 6-4PPs and the Dewar isomer of 6-4PPs[12]. These types of DNA lesions are repaired by nucleotide excision repair system in normal cells[13,14]. The formation and repair of DNA photoproducts appears to be crucial for cancer induction as cells from xeroderma pigmentosum (XP) patients, who are highly susceptible to UVR-induced skin cancer as a result of a mutation, cannot remove these photoproducts[15-17].
The results that either oral or topical d-limonene blocked UVB-induced sunburn in Skh1 mouse skin (Figure 1) are consistent with our previous observations that β-damascenone, also an aroma terpene, acted as a sunburn protective agent similar to current observations[9]. The preventive mechanism of d-limonene exhibited two components, reducing the formation of DNA photodamages (Figure 2) and lowering of stress, proliferation genes, including NDRG1and PCNA (Figures 3, 4). It is noteworthy that while pure d-limonene induced NDRG1, PCNA, it eliminated the response of these same genes to UVR exposure. This can be explained by invoking d-limonene’s stimulation of proliferation and differentiation leading to a thickened outer cornified barrier prior to UVR as a protective, absorptive effect. Pure d-limonene is not a cutaneous sensitizer, but it is reported to be irritating at high doses. Applications of 25% or 40% of d-limonene solutions failed to cause long-term irritation in the ears of rabbits, although application of undiluted d-limonene caused skin redness and irritation[18]. Present results are consistent with these earlier observations.
It is of utmost importance that mice given pure d-limonene followed by UVR exposure exhibited reduced UVR-induced CPD photoproducts in the epidermal DNA in a dose-dependent fashion. While the cellular response to d-limonene is still to be elucidated, the possibility of direct biochemical reaction between d-limonene and cellular DNA is unlikely as most of the oral d-limonene is rapidly excreted from the body after metabolized in liver to form perillyl alcohol and carveols which distributes in the blood to other parts of the body[19]. Possibly, d-limonene’s ability to activate proliferation and differentiation of epidermis reflects an underlying, most likely vestigial, UVR protection system in mouse skin.
Another possibility is that d-limonene has triggered a gene cluster associated with the strengthening and thickening of UVR-absorbing cutaneous envelopes, possibly including the activation of NDRG1. In situ analysis showed an accumulation of NDRG1 in the suprabasal layers of the skin, as well as in the more differentiated areas of mouse skin papillomas[20]. Although NDRG1 protein is up-regulated during a variety of cell stresses[21,22], including DNA damage[23], nickel[24] and hypoxia[25], etc., its exact function remains unknown.
Prior treatment of mice with d-limonene followed by UVR exposure significantly reduced the NDRG1 expression in skin compared to that of UVR and d-limonene alone. UVR is known to initiate ROS and/or inflammatory stress responses[26,27], but d-limonene was ineffective when administered after the UVB (data not shown), which implies that the anti-oxidative or inflammatory activity of the d-limonene was not a likely basis for its UVB protective effect. In vivo studies with d-limonene have shown its efficacy against hepatocellular carcinoma, and inhibition of the overexpression of c-myc and c-jun proteins as one of the mechanisms by which d-limonene exhibits its anticarcinogenic effect[6]. NDRG1, as the downstream of target of N/c-myc may also involve the activity of the d-limonene.
Like β-damascenone, d-limonene increased the number and size of the sebaceous glands and also induced a higher level of the filaggrin protein in sebaceous glands and in follicular lumens near the skin surface. This result suggests that terpene-induced sebum formation may represent a secondary but inducible pathway whereby filaggrin is made available for strengthening and repairing the cornified envelop layer of epidermis, beyond the well-known filaggrin origin in keratinocytes. The latter filaggrin originates from the degradation of the large and insoluble polyprotein profilaggrin and is further proteolyzed to amino acids that serves as building blocks for epidermal structure, hydration and barrier function[28,29]. Filaggrin knockdown study in a human skin model showed increased skin sensitivity to UVR and significant decreased amount of urocanic acid, a product from fliaggrin degradation acts as an UV absorbent within the stratum corneum[30]. More study will be needed to resolve the question whether treatment with the d-limonene increased this endogenous UVB protective chromophore.
Taking together current d-limonene data and previously reported β-damascenone results, suggest that d-limonene protected against UVR-induced DNA damage, sunburn by activating stress response and proliferation and gene NDRG1, PCNA and filaggrin which strengthen the skin barrier against UV rays. Even though pure d-limonene alone seemingly had some adverse effect, it will protect the skin from UV damage by optimizing the dosage.
The authors acknowledge Salamia Lasano for expert help in producing histological slides.
d-Limonene, an aromatic terpene, has been reported to protect against many cancer types, including mammary, skin, liver, lung and forestomach. Skin cancer caused by the ultraviolet component of sunlight is a major risk in human populations lacking the protection of melanin.
The research explores the possibility of using very low concentrations of a safe, natural substance taken orally to provide effective and long-lasting protection against ultraviolet radiation-induced sunburn and, if DNA damage is prevented, skin cancer.
Whether taken orally or applied directly to the mouse skin surface, d-limonene prevented UVB-induced DNA damage in the form of cyclobutane pyrimidine dimers (CPDs) and sunburn. Elevated ultraviolet irradiation (UVR) absorption was associated with a thicker and stronger skin barrier. d-Limonene also counteracted the UVR-induced expression of proliferating cell nuclear antigen and N-myc downstream regulating gene 1. These results show that aromatic terpenes prevent sunburn and DNA damage in mouse skin and are a new and innovative approach to protecting skin against ultraviolet radiation induced skin damage.
The ultraviolet radiation protection persisted for about 2 wk and might persist even longer in human skin because of its longer turnover time. The interesting discovery was that oral d-limonene was about equally effective as a topical application for activating what spears to be a previously unknown natural UVR protective system contained in mouse and human skin.
Aromatic terpenes are non-nutritive substances found in citrus and other fruits and herbs. They contribute to the distinctive fragrance of plants, and are generally considered to be safe for consumption by humans and animals.
This manuscript described an interesting finding that administration of d-Limonene is able to prevent UV-induced sunburn and CPD formation in mouse skin.
P- Reviewer: Cimpean AM, Wang QE S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Kim YW, Kim MJ, Chung BY, Bang du Y, Lim SK, Choi SM, Lim DS, Cho MC, Yoon K, Kim HS. Safety evaluation and risk assessment of d-Limonene. J Toxicol Environ Health B Crit Rev. 2013;16:17-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 2. | Crowell PL. Prevention and therapy of cancer by dietary monoterpenes. J Nutr. 1999;129:775S-778S. [PubMed] |
| 3. | Kuttan G, Pratheeshkumar P, Manu KA, Kuttan R. Inhibition of tumor progression by naturally occurring terpenoids. Pharm Biol. 2011;49:995-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Elegbede JA, Elson CE, Qureshi A, Tanner MA, Gould MN. Inhibition of DMBA-induced mammary cancer by the monoterpene d-limonene. Carcinogenesis. 1984;5:661-664. [PubMed] |
| 5. | Chaudhary SC, Siddiqui MS, Athar M, Alam MS. D-Limonene modulates inflammation, oxidative stress and Ras-ERK pathway to inhibit murine skin tumorigenesis. Hum Exp Toxicol. 2012;31:798-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Giri RK, Parija T, Das BR. d-limonene chemoprevention of hepatocarcinogenesis in AKR mice: inhibition of c-jun and c-myc. Oncol Rep. 1999;6:1123-1127. [PubMed] |
| 7. | Wattenberg LW, Coccia JB. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone carcinogenesis in mice by D-limonene and citrus fruit oils. Carcinogenesis. 1991;12:115-117. [PubMed] |
| 8. | Wattenberg LW, Sparnins VL, Barany G. Inhibition of N-nitrosodiethylamine carcinogenesis in mice by naturally occurring organosulfur compounds and monoterpenes. Cancer Res. 1989;49:2689-2692. [PubMed] |
| 9. | Uddin AN, Labuda I, Burns FJ. A novel mechanism of filaggrin induction and sunburn prevention by β-damascenone in Skh-1 mice. Toxicol Appl Pharmacol. 2012;265:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Marrot L, Meunier JR. Skin DNA photodamage and its biological consequences. J Am Acad Dermatol. 2008;58:S139-S148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 224] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Molho-Pessach V, Lotem M. Ultraviolet radiation and cutaneous carcinogenesis. Curr Probl Dermatol. 2007;35:14-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Mori T, Nakane M, Hattori T, Matsunaga T, Ihara M, Nikaido O. Simultaneous establishment of monoclonal antibodies specific for either cyclobutane pyrimidine dimer or (6-4)photoproduct from the same mouse immunized with ultraviolet-irradiated DNA. Photochem Photobiol. 1991;54:225-232. [PubMed] |
| 13. | de Lima-Bessa KM, Armelini MG, Chiganças V, Jacysyn JF, Amarante-Mendes GP, Sarasin A, Menck CF. CPDs and 6-4PPs play different roles in UV-induced cell death in normal and NER-deficient human cells. DNA Repair (Amst). 2008;7:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Sinha RP, Häder DP. UV-induced DNA damage and repair: a review. Photochem Photobiol Sci. 2002;1:225-236. [PubMed] |
| 15. | Moriwaki S. Hereditary Disorders with Defective Repair of UV-Induced DNA Damage. Jpn Clin Med. 2013;4:29-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Hiramoto T, Matsunaga T, Ichihashi M, Nikaido O, Fujiwara Y, Mishima Y. Repair of 254 nm ultraviolet-induced (6-4) photoproducts: monoclonal antibody recognition and differential defects in xeroderma pigmentosum complementation groups A, D, and variant. J Invest Dermatol. 1989;93:703-706. [PubMed] |
| 17. | Wang HT, Choi B, Tang MS. Melanocytes are deficient in repair of oxidative DNA damage and UV-induced photoproducts. Proc Natl Acad Sci USA. 2010;107:12180-12185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Lacy MJ, Kent CR, Voss EW. d-Limonene: an effective vasodilator for use in collecting rabbit blood. Lab Anim Sci. 1987;37:485-487. [PubMed] |
| 19. | Shimada T, Shindo M, Miyazawa M. Species differences in the metabolism of (+)- and (-)-limonenes and their metabolites, carveols and carvones, by cytochrome P450 enzymes in liver microsomes of mice, rats, guinea pigs, rabbits, dogs, monkeys, and humans. Drug Metab Pharmacokinet. 2002;17:507-515. [PubMed] |
| 20. | Gómez-Casero E, Navarro M, Rodríguez-Puebla ML, Larcher F, Paramio JM, Conti CJ, Jorcano JL. Regulation of the differentiation-related gene Drg-1 during mouse skin carcinogenesis. Mol Carcinog. 2001;32:100-109. [PubMed] |
| 21. | Kitowska A, Pawełczyk T. N-myc downstream regulated 1 gene and its place in the cellular machinery. Acta Biochim Pol. 2010;57:15-21. [PubMed] |
| 22. | Fang BA, Kovačević Ž, Park KC, Kalinowski DS, Jansson PJ, Lane DJ, Sahni S, Richardson DR. Molecular functions of the iron-regulated metastasis suppressor, NDRG1, and its potential as a molecular target for cancer therapy. Biochim Biophys Acta. 2014;1845:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Kurdistani SK, Arizti P, Reimer CL, Sugrue MM, Aaronson SA, Lee SW. Inhibition of tumor cell growth by RTP/rit42 and its responsiveness to p53 and DNA damage. Cancer Res. 1998;58:4439-4444. [PubMed] |
| 24. | Salnikow K, Blagosklonny MV, Ryan H, Johnson R, Costa M. Carcinogenic nickel induces genes involved with hypoxic stress. Cancer Res. 2000;60:38-41. [PubMed] |
| 25. | Park H, Adams MA, Lachat P, Bosman F, Pang SC, Graham CH. Hypoxia induces the expression of a 43-kDa protein (PROXY-1) in normal and malignant cells. Biochem Biophys Res Commun. 2000;276:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Svobodová A, Vostálová J. Solar radiation induced skin damage: review of protective and preventive options. Int J Radiat Biol. 2010;86:999-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Svobodova A, Walterova D, Vostalova J. Ultraviolet light induced alteration to the skin. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:25-38. [PubMed] |
| 28. | Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol. 2012;132:751-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 394] [Cited by in RCA: 372] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 29. | Scharschmidt TC, Man MQ, Hatano Y, Crumrine D, Gunathilake R, Sundberg JP, Silva KA, Mauro TM, Hupe M, Cho S. Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. J Allergy Clin Immunol. 2009;124:496-506, 506.e1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 30. | Mildner M, Jin J, Eckhart L, Kezic S, Gruber F, Barresi C, Stremnitzer C, Buchberger M, Mlitz V, Ballaun C. Knockdown of filaggrin impairs diffusion barrier function and increases UV sensitivity in a human skin model. J Invest Dermatol. 2010;130:2286-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 207] [Article Influence: 13.8] [Reference Citation Analysis (0)] |