Revised: August 14, 2013
Accepted: September 3, 2013
Published online: November 2, 2013
Processing time: 115 Days and 17.2 Hours
Melanoma is one of the most aggressive cancers and its high metastatic potential has a large impact on the number of melanoma deaths. The pathological diagnosis is still the gold standard for melanoma and immunohistochemistry plays an important role in discriminating between melanomas. Recently, emerging molecular knowledge may lead to further identification of clinically relevant biomarkers, such as S100B, MIA, TA-90IC, 5-S-CD, SPARC, CSPG4, HSP105, IMP3, KIF2A, miR-221, YKL-40, some cancer stem cells (CD133, Nestin, CD166, CD20, CD271) and some monoclonal antibodies (KBA62, PNL2), for malignant melanoma detection, risk stratification and prediction/prognosis. However, all of the current main markers have some shortcomings. For example, all markers have limitations in sensitivity and specificity, even the first-line marker, S100 protein. So, sometimes, many of the classification criteria that have been proposed show considerable overlap, making it difficult to categorize cases reproducibly, based on histopathological criteria alone. Besides that, the increased expression of some proteins in melanomas suggests that there are abnormal proteins synthesized due to the genetic pathway. Therefore, we expect that there will be more instrumental breakthroughs in the abnormal gene field, especially with respect to gene mutation. Ultimately, novel melanoma biomarkers could be found and gradually become targeted treatment strategies for a poor prognosis in advanced melanoma in the near future.
Core tip: Melanoma is one of the most common cancers and its high metastatic potential has a large impact on the number of melanoma deaths. Emerging molecular knowledge may lead to further identification of clinically relevant biomarkers, such as S100B, MIA, TA-90IC, 5-S-CD, SPARC, CSPG4, HSP105, IMP3, KIF2A, miR-221, YKL-40, some cancer stem cells (CD133, Nestin, CD166, CD20, CD271) and some monoclonal antibodies (KBA62, PNL2), for malignant melanoma detection, risk stratification and prediction/prognosis. However, all current markers have some shortcomings and thus we expect there will be more novel melanoma biomarkers discovered as supplementary diagnostic criteria in the near future.
- Citation: Wang YN, Yamamoto Y, Furukawa F. Potential biomarkers for malignant melanoma. World J Dermatol 2013; 2(4): 44-50
- URL: https://www.wjgnet.com/2218-6190/full/v2/i4/44.htm
- DOI: https://dx.doi.org/10.5314/wjd.v2.i4.44
Melanoma is a malignancy characterized by a high potential to metastasize at a relatively small size of the primary tumor[1]. A nationwide survey of Japanese patients with malignant skin tumors from 1987 to 2001 showed that the most prevalent skin tumor was basal cell carcinoma, which increased year by year, followed by squamous cell carcinoma, and then malignant melanoma[2]. Furthermore, the number of patients with malignant skin tumors has increased year by year. The prognosis of patients with advanced malignant melanoma remains extremely poor but that of patients in stage III has shown some improvement[2].
A pathological diagnosis is still the gold standard for melanoma. Immunohistochemistry plays an important role in discriminating between melanomas, but all markers have limitations in sensitivity and specificity; even the first-line marker, S100 protein, is not expressed in all melanomas[3]. In the quest to reduce cancer mortality and morbidity, there is a continued effort to identify novel biomarkers to aid in the early detection and accurate prediction of tumor behavior[4]. The following discussion reviews some potentially novel and adjuvant biomarkers to help to diagnose melanoma.
The S100 proteins are a multi-gene calcium-binding family comprising of 20 known human members, each coded by a separate gene[4]. S100B is a homodimer of the S100b chain originally identified as “S100” by Moore et al[5] in 1965. Serum levels of S100B have been evaluated in patients with melanoma at different stages and have been shown to increase in a stage-dependent manner[6]. S100B expression levels are the highest in grade VI melanoma[6-8] and have been associated with the presence of metastases[9,10]. Increased expression levels of serum S100B also correlate with reduced survival[8,11], have been shown to reflect the tumor load, stage and prognosis[7,12-14], and are an independent prognostic factor for a poor outcome in melanoma[11].
This allows S100B to be used as a diagnostic marker and in the staging of malignant melanomas in a clinical setting. S100B has also proven to be a valuable marker in assessing a patient’s response to treatment[15]. Decreased levels of S100B following treatment correlates with a good response to therapy and with increased survival[10].
Melanoma inhibitory activity (MIA) is an 11 kDa protein secreted by malignant melanomas, is strongly expressed in melanoma cells but not in melanocytes, and is likely to represent a key molecule regulating melanoma progression[16]. Purified MIA causes a significant alteration of cell morphology as melanoma cells round up[17]. MIA protein is a clinically-valuable marker in patients with malignant melanomas as elevated values can diagnose metastatic melanoma stages III and IV[18]. MIA expression in vivo correlates with progressive malignancy and is now widely-recognized as a novel serum marker for the malignant progression of metastasizing melanoma[19,20].
Recently, an immunohistochemical marker, insulin-like growth factor-II messenger RNA (mRNA)-binding protein-3 (IMP3), has been reported to differentiate between melanomas and benign nevi[21]. IMP3, also known as K homology domain-containing protein overexpressed in cancer or L523S, promotes tumor cell proliferation by enhancing insulin-like growth factor-II protein expression[22].
In the research from Pryor et al[21], fifty-six melanocytic neoplasms from 48 subjects were immunohistochemically studied using a monoclonal antibody against L523S/IMP-3. Their study demonstrated that IMP-3 is expressed in malignant melanomas but not in benign nevi, even when dysplastic features are present. Furthermore, IMP-3 is expressed in a significantly higher proportion of melanomas than Spitz nevi and IMP-3 is expressed in metastatic melanomas significantly more than in thin melanomas[21].
In conclusion, IMP-3 appears to be involved in the progression of malignant melanomas and may play an important role in the regulation of the biological behavior of these tumors. Additionally, IMP-3 may have diagnostic utility in distinguishing melanoma cells from benign nevic cells, dysplastic nevi and Spitz nevi[21].
Kinesin family member 20A (KIF20A) belongs to a large family of proteins that share a conserved motor domain which binds to microtubules and couples ATP hydrolysis to generate mechanical force[23].
In research by Yamashita et al[24], KIF20A expression was detected in 59% of melanomas and 12% of nevi by immunohistochemistry, and 64% of melanomas and 60% of nevi by quantitative reverse transcript PCR. The primary melanomas that were immunopositive for KIF20A showed a significantly greater thickness than those that were immunonegative and patients with KIF20A+ melanomas tended to develop recurrence earlier. These results suggest that immunotherapy with KIF20A may be a novel treatment option for advanced melanoma[24].
KBA62 recognizes an unknown determinant expressed in melanoma cells[25] and is a monoclonal antibody raised to a melanoma cell line recognizing yet another unidentified epitope[26]. PNL2 monoclonal antibody was raised against the somatostatin receptor but was found to be nonreactive to the intended target protein[3].
In the study by Aung et al[3], KBA62 and PNL2 were sensitive markers for metastatic melanomas and were expressed in a great majority of cases (86% and 89%, respectively, for KBA62 and PNL2) by examining a large number of metastatic melanomas and other melanocytic neoplasms and their mimics. Moreover, KBA62 and PNL2 also recognized S100 protein-negative metastatic melanomas (KBA62, 4 out of 7 and PNL2, 6 out of 7), indicating that these new markers are useful diagnostic complements for this rare subgroup (2% of all metastatic melanomas)[3].
Heat shock protein-105 (HSP105), identified by the serological identification of antigens by recombinant expression cloning (SEREX), is overexpressed in a variety of human cancers. The amino acid sequences and expression patterns of HSP105 are also very similar in humans and mice.
Miyazaki et al[27] found that HSP105 was highly immunogenic in mice and that HSP105 DNA vaccination induced antitumor immunity without causing autoimmunity. Therefore, HSP105 is an ideal tumor antigen that could be useful for immunotherapy or the prevention of various human tumors that overexpress HSP105, including colorectal cancer and melanoma. Whether HSP105 is an ideal target for immunotherapy in human cancers, which are at a high risk of melanoma, will continue to be investigated in their laboratory[27].
5-S-cysteinyldopa (5-S-CD) has been used as a biochemical marker of melanoma progression. In one study by Wakamatsu et al[28], serum levels of 5-S-CD were assayed in 2648 samples taken from 218 patients in order to evaluate the usefulness of this parameter in following melanoma progression and prognosis.
5-S-CD levels were significantly elevated above the upper limit of the normal range (10 nmol/L) in stage IV melanoma patients. The sensitivity of the elevated serum 5-S-CD levels in detecting distant metastasis was 73%, whereas the specificity was 98% and the positive predictive value 94%. Patients without metastases had elevated 5-S-CD values in 5% of the 1480 serum samples. In 33% of the patients, an elevation in serum 5-S-CD levels preceded the clinical detection of visceral metastases and in 37%, an elevation of 5-S-CD levels occurred at the same time as visceral metastasis. Patients with elevated 5-S-CD levels before or after surgical treatment had significantly shorter survival times than those with normal levels[28].
These results show that the serum level of 5-S-CD is a sensitive and specific marker for predicting distant metastases. Elevated serum levels of 5-S-CD, before or after surgical treatment, are associated with a poor prognosis[28].
However, the serum levels of 5-S-CD, which is a common tumor marker for malignant melanoma, sometimes remain within the normal limit, especially in the early stages. Therefore, it is inadequate to use 5-S-CD for the early detection of malignant melanoma[29,30].
Urine tumor-associated antigen (U-TAA) is a high molecular-weight glycoprotein identified in the urine of patients with metastatic melanoma[31]. It comprises of multiple subunits, including an immunogenic 90-kD subunit designated TA-90[32]. TA-90 is expressed by 71% of melanoma cell lines and tumor biopsies and up to 70% of breast, colon and lung carcinomas and soft tissue sarcomas[33,34]. The study of Kelley et al[34] showed that an enzyme-linked immunosorbent assay for TA-90 in circulating immune complexes (TA90-IC) can detect subclinical metastasis before the surgical treatment of early-stage melanoma and thus they assayed the TA90-IC levels in the postoperative sera from patients with melanomas and evaluated their relationship to recurrence and survival.
Secreted protein acidic and rich in cysteine (SPARC), also called osteonectin or BM-40, is a glycoprotein that modulates cellular interactions with the extracellular matrix during tissue remodeling[35]. SPARC was overexpressed in primary and metastatic melanomas and an overexpression of SPARC by melanoma cells was associated with an invasive phenotype in vivo[36,37].
Ikuta et al[30] recently identified glypican-3 (GPC3) as a novel tumor marker but it could only diagnose 40% of melanomas. Therefore, we focused our attention on SPARC overexpressed in melanomas as another candidate tumor marker. Secreted SPARC protein was quantified using ELISA in the sera from 109 melanoma patients, five patients with large congenital melanocytic nevus, 61 age-matched healthy donors and 13 disease-free patients after undergoing surgical removal. Surprisingly, 19 of the 36 patients showing increased SPARC levels were in stages 0 to II. The serum SPARC levels decreased below the cutoff level in 10 out of 13 patients after surgical removal. Using SPARC plus GPC3 in combination enabled us to diagnose 47 out of 75 (66.2%) melanoma patients at an early stage (0-II). Thus, SPARC or its combination with GPC3 is considered a potentially useful tumor marker, especially for melanoma at an early stage[30].
Chondroitin sulphate proteoglycan 4 (CSPG4) consists of a N-linked glycoprotein of 280 kDa and a proteoglycan component of about 450 kDa[38] and plays an important role in melanoma cell proliferation, migration and metastasis[39].
This transmembrane proteoglycan was originally identified as a highly immunogenic tumor antigen on the surface of melanoma cells and is associated with melanoma tumor formation and a poor prognosis in certain melanomas and several other tumor types. CSPG4 is essential to the growth of melanoma tumors through its modulation of integrin function and enhanced growth factor receptor-regulated pathways, including the sustained activation of ERK 1, 2. CSPG4 expression has further been correlated to the resistance of melanomas against conventional chemotherapeutics[40].
YKL-40, a 40-kDa secreted glycoprotein, is produced by cancer cells and inflammatory cells and plays a role in inflammation, cell proliferation, differentiation, protection against apoptosis, the stimulation of angiogenesis and the regulation of extracellular tissue remodeling[41].
Elevated plasma YKL-40 levels are an independent prognostic biomarker of shortened survival. There is still insufficient evidence to support its value outside of clinical trials as a screening tool, prognosticator of survival, predictor of treatment response and as a monitoring tool in the routine management of individual patients with cancer or diseases characterized by inflammation. Large, prospective, longitudinal clinical cancer studies are needed to determine if plasma YKL-40 levels represent a new cancer biomarker or are mainly a biomarker of inflammation[42].
Cancer stem cells, derived from the clonal expansion of atypical cells, exist in a wide array of tumors and are becoming increasingly important to the understanding of the molecular mechanisms that regulate self-renewal, differentiation and progression of metastasis[43,44]. The identification of cancer stem cells can potentially help to refine the classification, diagnosis and treatment of cancers, including malignant melanomas[45]. Recently, cancer stem cell markers, which are also expressed on melanocytes, have been described[45].
CD133 (human prominin-1/AC133) is a transmembrane glycoprotein that is expressed on hematopoietic stem cells, endothelial progenitors and dermal-derived stem cells capable of differentiating into neural cells[46,47]. CD133 (Prominin-1) is considered the most important cancer stem cell associated marker identified thus far and its increased expression is observed in the cancer stem cell fraction of a large variety of human malignancies, including malignant melanoma[48].
Some studies by Al Dhaybi et al[49] have shown that CD133+ cancer stem cell expression in childhood malignant melanoma might correlate with lymph node metastasis and, in fact, some studies showed that all malignant melanoma patients who had associated metastases had a positive expression of CD133. Moreover, they found stronger CD133 expression in metastatic/or visceral metastases[49].
CD133 expression might be associated with an increased risk of metastasis and a worse outcome in childhood malignant melanoma. However, it is sometimes difficult to distinguish a Spitz nevus from a malignant melanoma and many investigators are searching for tools and techniques that may help enhance diagnostic accuracy[50]. CD133 expression might be a useful tool to suggest malignant behavior[49].
Nestin is an intermediate filament expressed in the cytoplasm of neuroepithelial stem cells[51,52]. Its expression has also been found in metastatic melanomas[53]. In Klein’s[45] research, nestin expression was identified in 35/64 banal nevi, 44/63 primary melanomas and 69/80 metastatic melanomas and thus its expression seems to correlate with the high proliferative and migrational activity of these tumors[45].
Activated leukocyte adhesion molecule (CD166) is a member of the immunoglobulin super family and is a type 1 transmembrane protein. CD166 is expressed on the surface of mesenchymal stem cells and has been found on human melanoma cell lines[54]. In addition, its expression correlates with tumor thickness in primary melanomas[55].
In analogy with other groups, Fang et al[56] used in vitro sphere culture conditions to enrich for cells with stem cell features. Surprisingly, cells within the spheres expressed the B cell marker CD20 (also known as MS4A1). Extending their CD20 experiments into the clinical setting, they used rituximab (an anti-CD20 antibody) treatment in a group of nine patients with metastatic melanomas at clinical stage IV. After a treatment period of 2 years and a subsequent median follow-up time of 42 mo, two thirds of the patients included in this study were recurrence-free, without any signs of major side effects or toxicity[57].
The low-affinity neurotrophin receptor p75 (p75NTR, CD271) has recently been identified as a surface marker for tumor-initiating cells in melanomas[58,59]. As shown by Boiko et al[58], CD271 (p75NTR)-expressing melanoma cells had a higher tumor-initiation capacity than CD271-negative cells and, moreover, gave rise to metastases upon transplantation, unlike CD271-negative cells.
Circulating melanoma cells are thought to be valuable for improving prognostic measures in melanoma patients. Research by Freeman et al[60] demonstrated that a combination of markers should be targeted for the optimal isolation of circulating melanoma cells. In addition, there are significantly more circulating melanoma cells in metastatic patients compared with non-metastatic patients and therefore the quantification of circulating melanoma cells may prove to be a useful marker of disease progression[60].
Growing evidence has supported the use of micro-RNAs (miRNAs) expression profiles to clearly distinguish between normal and neoplastic tissues, thus leading to the identification of new diagnostic and/or prognostic markers[61].
For example, miR-221, encoded on the X chromosome, is one of these miRNAs[61] and microRNA-221 (miR-221) is known to be abnormally expressed in malignant melanoma cells. To evaluate the possibility that serum miR-221 levels can be a marker for malignant melanoma, serum samples were obtained by Kanemaru et al[61] from 94 malignant melanoma patients and 20 healthy controls. MicroRNAs were purified from the serum and the miR-221 levels were measured by quantitative real-time PCR. Malignant melanoma patients had significantly higher miR-221 levels than healthy controls. Among the malignant melanoma patients, the miR-221 levels were significantly increased in patients with stages I-IV malignant melanoma compared to those with malignant melanoma in situ and the levels were correlated with tumor thickness. Moreover, a longitudinal study revealed a tendency for the miR-221 levels to decrease after the surgical removal of the primary tumor and to increase again at recurrence. The serum levels of miR-221 were significantly increased and were correlated with the tumor thickness in malignant melanoma patients; thus, it may be useful not only for the diagnosis and prognosis of malignant melanomas, but also for differentiating in situ from stage I-IV malignant melanomas and for evaluating tumor progression and monitoring patients[61].
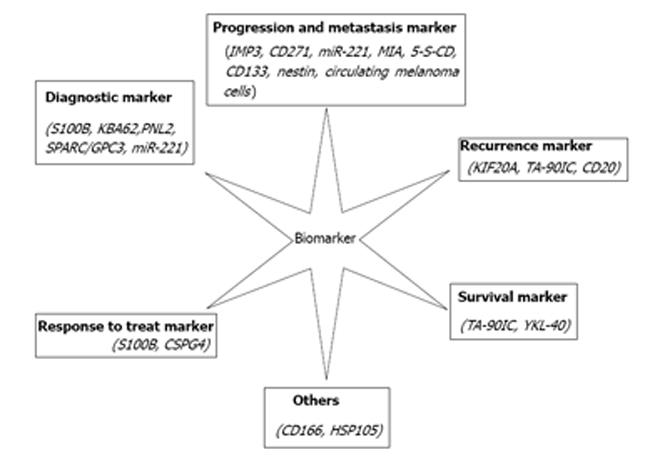
In the end, the increased expression of some proteins in melanomas suggests that there are abnormal proteins synthesized due to the genetic pathway. Moreover, all of the current main markers have some shortcomings. Therefore, we expect that there will be more instrumental breakthroughs in the abnormal gene field, especially with respect to gene mutation. Ultimately, potential melanoma biomarkers (Figure 1) could be found and gradually become targeted treatment strategies for a poor prognosis in advanced melanoma.
P- Reviewers Aksoy B, Negosanti L S- Editor Song XX L- Editor Roemmele A E- Editor Liu XM
| 1. | McArthur GA, Ribas A. Targeting oncogenic drivers and the immune system in melanoma. J Clin Oncol. 2013;31:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Ishihara K, Saida T, Otsuka F, Yamazaki N. Statistical profiles of malignant melanoma and other skin cancers in Japan: 2007 update. Int J Clin Oncol. 2008;13:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Aung PP, Sarlomo-Rikala M, Lasota J, Lai JP, Wang ZF, Miettinen M. KBA62 and PNL2: 2 new melanoma markers-immunohistochemical analysis of 1563 tumors including metastatic, desmoplastic, and mucosal melanomas and their mimics. Am J Surg Pathol. 2012;36:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Salama I, Malone PS, Mihaimeed F, Jones JL. A review of the S100 proteins in cancer. Eur J Surg Oncol. 2008;34:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 343] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 5. | Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965;19:739-744. [PubMed] |
| 6. | Bonfrer JM, Korse CM, Nieweg OE, Rankin EM. The luminescence immunoassay S-100: a sensitive test to measure circulating S-100B: its prognostic value in malignant melanoma. Br J Cancer. 1998;77:2210-2214. [PubMed] |
| 7. | Andrés R, Mayordomo JI, Zaballos P, Rodino J, Isla D, Escudero P, Elosegui L, Filipovich E, Saenz A, Polo E. Prognostic value of serum S-100B in malignant melanoma. Tumori. 2004;90:607-610. [PubMed] |
| 8. | Heizmann CW. S100B protein in clinical diagnostics: assay specificity. Clin Chem. 2004;50:249-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Zissimopoulos A, Karpouzis A, Karaitianos I, Baziotis N, Tselios I, Koutis C. Serum levels of S-100b protein after four years follow-up of patients with melanoma. Hell J Nucl Med. 2006;9:204-207. [PubMed] |
| 10. | Schlagenhauff B, Schittek B, Ellwanger U, Stroebel W, Blum A, Schwarz M, Rassner G, Garbe C. Significance of serum protein S100 levels in screening for melanoma metastasis: does protein S100 enable early detection of melanoma recurrence? Melanoma Res. 2000;10:451-459. [PubMed] |
| 11. | Harpio R, Einarsson R. S100 proteins as cancer biomarkers with focus on S100B in malignant melanoma. Clin Biochem. 2004;37:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Hauschild A, Engel G, Brenner W, Gläser R, Mönig H, Henze E, Christophers E. Predictive value of serum S100B for monitoring patients with metastatic melanoma during chemotherapy and/or immunotherapy. Br J Dermatol. 1999;140:1065-1071. [PubMed] |
| 13. | Hauschild A, Michaelsen J, Brenner W, Rudolph P, Gläser R, Henze E, Christophers E. Prognostic significance of serum S100B detection compared with routine blood parameters in advanced metastatic melanoma patients. Melanoma Res. 1999;9:155-161. [PubMed] |
| 14. | Schultz ES, Diepgen TL, Von Den Driesch P. Clinical and prognostic relevance of serum S-100 beta protein in malignant melanoma. Br J Dermatol. 1998;138:426-430. [PubMed] |
| 15. | Hamberg AP, Korse CM, Bonfrer JM, de Gast GC. Serum S100B is suitable for prediction and monitoring of response to chemoimmunotherapy in metastatic malignant melanoma. Melanoma Res. 2003;13:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Jachimczak P, Apfel R, Bosserhoff AK, Fabel K, Hau P, Tschertner I, Wise P, Schlingensiepen KH, Schuler-Thurner B, Bogdahn U. Inhibition of immunosuppressive effects of melanoma-inhibiting activity (MIA) by antisense techniques. Int J Cancer. 2005;113:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Blesch A, Bosserhoff AK, Apfel R, Behl C, Hessdoerfer B, Schmitt A, Jachimczak P, Lottspeich F, Buettner R, Bogdahn U. Cloning of a novel malignant melanoma-derived growth-regulatory protein, MIA. Cancer Res. 1994;54:5695-5701. [PubMed] |
| 18. | Stoll R, Renner C, Zweckstetter M, Brüggert M, Ambrosius D, Palme S, Engh RA, Golob M, Breibach I, Buettner R. The extracellular human melanoma inhibitory activity (MIA) protein adopts an SH3 domain-like fold. EMBO J. 2001;20:340-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Bosserhoff AK, Lederer M, Kaufmann M, Hein R, Stolz W, Apfel R, Bogdahn U, Buettner R. MIA, a novel serum marker for progression of malignant melanoma. Anticancer Res. 1999;19:2691-2693. [PubMed] |
| 20. | Bosserhoff AK, Hauschild A, Hein R, Schadendorf D, Stockfleth E, Bogenrieder T, Landthaler M, Buettner R, Stolz W. Elevated MIA serum levels are of relevance for management of metastasized malignant melanomas: results of a German multicenter study. J Invest Dermatol. 2000;114:395-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Pryor JG, Bourne PA, Yang Q, Spaulding BO, Scott GA, Xu H. IMP-3 is a novel progression marker in malignant melanoma. Mod Pathol. 2008;21:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Liao B, Hu Y, Herrick DJ, Brewer G. The RNA-binding protein IMP-3 is a translational activator of insulin-like growth factor II leader-3 mRNA during proliferation of human K562 leukemia cells. J Biol Chem. 2005;280:18517-18524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 170] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Hirokawa N, Noda Y, Okada Y. Kinesin and dynein superfamily proteins in organelle transport and cell division. Curr Opin Cell Biol. 1998;10:60-73. [PubMed] |
| 24. | Yamashita J, Fukushima S, Jinnin M, Honda N, Makino K, Sakai K, Masuguchi S, Inoue Y, Ihn H. Kinesin family member 20A is a novel melanoma-associated antigen. Acta Derm Venereol. 2012;92:593-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Pagès C, Rochaix P, al Saati T, Valmary-Degano S, Boulinguez S, Launay F, Carle P, Lauwers F, Payoux P, Le Guellec S. KBA.62: a useful marker for primary and metastatic melanomas. Hum Pathol. 2008;39:1136-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Cohen-Knafo E, al Saati T, Aziza J, Ralfkiaer E, Selves J, Gorguet B, Delsol G. Production and characterisation of an antimelanoma monoclonal antibody KBA.62 using a new melanoma cell line reactive on paraffin wax embedded sections. J Clin Pathol. 1995;48:826-831. [PubMed] |
| 27. | Miyazaki M, Nakatsura T, Yokomine K, Senju S, Monji M, Hosaka S, Komori H, Yoshitake Y, Motomura Y, Minohara M. DNA vaccination of HSP105 leads to tumor rejection of colorectal cancer and melanoma in mice through activation of both CD4 T cells and CD8 T cells. Cancer Sci. 2005;96:695-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Wakamatsu K, Kageshita T, Furue M, Hatta N, Kiyohara Y, Nakayama J, Ono T, Saida T, Takata M, Tsuchida T. Evaluation of 5-S-cysteinyldopa as a marker of melanoma progression: 10 years’ experience. Melanoma Res. 2002;12:245-253. [PubMed] |
| 29. | Mouawad R, Spano JP, Khayat D. Old and new serological biomarkers in melanoma: where we are in 2009. Melanoma Res. 2010;20:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Ikuta Y, Nakatsura T, Kageshita T, Fukushima S, Ito S, Wakamatsu K, Baba H, Nishimura Y. Highly sensitive detection of melanoma at an early stage based on the increased serum secreted protein acidic and rich in cysteine and glypican-3 levels. Clin Cancer Res. 2005;11:8079-8088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Bosserhoff AK, Kaufmann M, Kaluza B, Bartke I, Zirngibl H, Hein R, Stolz W, Buettner R. Melanoma-inhibiting activity, a novel serum marker for progression of malignant melanoma. Cancer Res. 1997;57:3149-3153. [PubMed] |
| 32. | Euhus DM, Gupta RK, Morton DL. Characterization of a 90-100 kDa tumor-associated antigen in the sera of melanoma patients. Int J Cancer. 1990;45:1065-1070. [PubMed] |
| 33. | Stahlecker J, Gauger A, Bosserhoff A, Büttner R, Ring J, Hein R. MIA as a reliable tumor marker in the serum of patients with malignant melanoma. Anticancer Res. 2000;20:5041-5044. [PubMed] |
| 34. | Kelley MC, Gupta RK, Hsueh EC, Yee R, Stern S, Morton DL. Tumor-associated antigen TA90 immune complex assay predicts recurrence and survival after surgical treatment of stage I-III melanoma. J Clin Oncol. 2001;19:1176-1182. [PubMed] |
| 35. | Lane TF, Sage EH. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 1994;8:163-173. [PubMed] |
| 36. | Ledda F, Bravo AI, Adris S, Bover L, Mordoh J, Podhajcer OL. The expression of the secreted protein acidic and rich in cysteine (SPARC) is associated with the neoplastic progression of human melanoma. J Invest Dermatol. 1997;108:210-214. [PubMed] |
| 37. | Ledda MF, Adris S, Bravo AI, Kairiyama C, Bover L, Chernajovsky Y, Mordoh J, Podhajcer OL. Suppression of SPARC expression by antisense RNA abrogates the tumorigenicity of human melanoma cells. Nat Med. 1997;3:171-176. [PubMed] |
| 38. | Wilson BS, Imai K, Natali PG, Ferrone S. Distribution and molecular characterization of a cell-surface and a cytoplasmic antigen detectable in human melanoma cells with monoclonal antibodies. Int J Cancer. 1981;28:293-300. [PubMed] |
| 39. | Chang CC, Campoli M, Luo W, Zhao W, Zaenker KS, Ferrone S. Immunotherapy of melanoma targeting human high molecular weight melanoma-associated antigen: potential role of nonimmunological mechanisms. Ann N Y Acad Sci. 2004;1028:340-350. [PubMed] |
| 40. | Price MA, Colvin Wanshura LE, Yang J, Carlson J, Xiang B, Li G, Ferrone S, Dudek AZ, Turley EA, McCarthy JB. CSPG4, a potential therapeutic target, facilitates malignant progression of melanoma. Pigment Cell Melanoma Res. 2011;24:1148-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 41. | Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, Urist M, McMasters KM, Ross MI, Kirkwood JM. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622-3634. [PubMed] |
| 42. | Kazakova MH, Sarafian VS. YKL-40--a novel biomarker in clinical practice? Folia Med (Plovdiv). 2009;51:5-14. [PubMed] |
| 43. | Tirino V, Desiderio V, d’Aquino R, De Francesco F, Pirozzi G, Graziano A, Galderisi U, Cavaliere C, De Rosa A, Papaccio G. Detection and characterization of CD133+ cancer stem cells in human solid tumours. PLoS One. 2008;3:e3469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 44. | Zabierowski SE, Herlyn M. Melanoma stem cells: the dark seed of melanoma. J Clin Oncol. 2008;26:2890-2894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 45. | Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJ, Tahan SR. Increased expression of stem cell markers in malignant melanoma. Mod Pathol. 2007;20:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 229] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 46. | Belicchi M, Pisati F, Lopa R, Porretti L, Fortunato F, Sironi M, Scalamogna M, Parati EA, Bresolin N, Torrente Y. Human skin-derived stem cells migrate throughout forebrain and differentiate into astrocytes after injection into adult mouse brain. J Neurosci Res. 2004;77:475-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 47. | Shmelkov SV, St Clair R, Lyden D, Rafii S. AC133/CD133/Prominin-1. Int J Biochem Cell Biol. 2005;37:715-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 276] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 48. | Rappa G, Fodstad O, Lorico A. The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem Cells. 2008;26:3008-3017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 49. | Al Dhaybi R, Sartelet H, Powell J, Kokta V. Expression of CD133+ cancer stem cells in childhood malignant melanoma and its correlation with metastasis. Mod Pathol. 2010;23:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Sulit DJ, Guardiano RA, Krivda S. Classic and atypical Spitz nevi: review of the literature. Cutis. 2007;79:141-146. [PubMed] |
| 51. | Dahlstrand J, Zimmerman LB, McKay RD, Lendahl U. Characterization of the human nestin gene reveals a close evolutionary relationship to neurofilaments. J Cell Sci. 1992;103:589-597. [PubMed] |
| 52. | Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585-595. [PubMed] |
| 53. | Flørenes VA, Holm R, Myklebost O, Lendahl U, Fodstad O. Expression of the neuroectodermal intermediate filament nestin in human melanomas. Cancer Res. 1994;54:354-356. [PubMed] |
| 54. | Swart GW, Lunter PC, Kilsdonk JW, Kempen LC. Activated leukocyte cell adhesion molecule (ALCAM/CD166): signaling at the divide of melanoma cell clustering and cell migration? Cancer Metastasis Rev. 2005;24:223-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 55. | van Kempen LC, van den Oord JJ, van Muijen GN, Weidle UH, Bloemers HP, Swart GW. Activated leukocyte cell adhesion molecule/CD166, a marker of tumor progression in primary malignant melanoma of the skin. Am J Pathol. 2000;156:769-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 162] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328-9337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 923] [Cited by in RCA: 932] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 57. | Pinc A, Somasundaram R, Wagner C, Hörmann M, Karanikas G, Jalili A, Bauer W, Brunner P, Grabmeier-Pfistershammer K, Gschaider M. Targeting CD20 in melanoma patients at high risk of disease recurrence. Mol Ther. 2012;20:1056-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 58. | Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 601] [Cited by in RCA: 556] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 59. | Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, Belloni B, Seifert B, Moch H, Dummer R, van den Broek M. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71:3098-3109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 60. | Freeman JB, Gray ES, Millward M, Pearce R, Ziman M. Evaluation of a multi-marker immunomagnetic enrichment assay for the quantification of circulating melanoma cells. J Transl Med. 2012;10:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Kanemaru H, Fukushima S, Yamashita J, Honda N, Oyama R, Kakimoto A, Masuguchi S, Ishihara T, Inoue Y, Jinnin M. The circulating microRNA-221 level in patients with malignant melanoma as a new tumor marker. J Dermatol Sci. 2011;61:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |