Published online Sep 2, 2022. doi: 10.5314/wjd.v10.i2.10
Peer-review started: March 6, 2022
First decision: April 13, 2022
Revised: April 22, 2022
Accepted: July 26, 2022
Article in press: July 26, 2022
Published online: September 2, 2022
Processing time: 178 Days and 6.6 Hours
Montelukast or Singulair is a leukotriene receptor antagonist that reduces inflammation and relaxes the smooth muscles. It is known to be a safe and tolerable drug; nevertheless, it might be associated with several mild to severe adverse effects, one of which is dermatomyositis. Dermatomyositis is a rare acquired autoimmune myositis of unknown cause affecting adults and children. The literature has infrequently reported the association between dermatomyositis and montelukast use.
The current study reports a case of a 48-year-old black woman with a previous history of bronchial asthma and allergic rhinosinusitis who presented with typical signs and symptoms of dermatomyositis which were confirmed by investigations. Before developing dermatomyositis, the patient was prescribed montelukast for atopy and consumed the drug for five months. After administration of prednisolone, the patient had a significant improvement and is still being followed up.
Even though montelukast is widely used and believed to be a safe drug for managing several conditions, the present case report highlights the possibility of adverse effects of montelukast. Therefore, future studies with advanced study designs are highly recommended to investigate the association between dermatomyositis and montelukast use.
Core Tip: Even though montelukast is widely used and believed to be a safe drug for managing several conditions, the present case report brings to light the possibility of adverse effects of montelukast. Especially in such a rare and serious complication of montelukast, physicians must be aware of the presentation of dermatomyositis, the diagnostic modalities, and the best treatment options for the patients, as the prompt diagnosis will prevent further complications progression of the condition.
- Citation: Aly MH, Alshehri AA, Mohammed A, Almaghrabi MA, Alharbi MM. Connection between dermatomyositis and montelukast sodium use: A case report. World J Dermatol 2022; 10(2): 10-16
- URL: https://www.wjgnet.com/2218-6190/full/v10/i2/10.htm
- DOI: https://dx.doi.org/10.5314/wjd.v10.i2.10
Dermatomyositis is a rare acquired autoimmune myositis with unknown etiology that affects adults and children. It is an inflammatory myopathy characterized by a striated muscle mononuclear inflammatory infiltrate. Genetic susceptibility is no longer in doubt since predisposing human leukocyte antigen systems have been identified. Complement activation results in the deposition of the membrane attack complex in the blood vessel wall that leads to microangiopathy and inflammatory response. Because some infectious organisms may play a role in self-tolerance and generation of the autoimmune response, the disorder appears to be seasonal[1]. In addition to infectious microorganisms, medications can be a trigger to initiate these responses[2]. Females are more affected than males. The overall age and gender-adjusted incidence of dermatomyositis, including all subtypes, was 9.63 per 1000000, peaking at ages 45-60 years in adults and ages 5-15 years in children[1].
Dermatomyositis is characterized by distinct skin changes and symmetric proximal skeletal muscle weakness. It can also affect other organ systems, such as the cardiovascular, pulmonary, and gastrointestinal systems. A significant proportion of patients with dermatomyositis have an underlying malignancy that affects the case’s prognosis[1,2]. In addition to corticosteroids and immunosuppressive therapy, several European and American national guidelines for treating dermatomyositis recommend intravenous immunoglobulin as adjuvant therapy[3].
A 48-year-old black woman comes into our clinic complaining of an erythematous skin rash and proximal weakness.
The patient’s symptoms began three months before. The patient had an erythematous skin rash with scaling on the dorsum of her hands, a heliotrope rash around her eyelids, and bilateral facial hyperpigmentation. It was associated with proximal weakness in both the upper and lower limbs. In addition, the patient experienced intermittent fever, night sweats, and constipation. The rest of the constitutional symptoms were negative. There has been no recent travel history or contact with sick individuals.
The patient had a history of hypertension, allergic rhinosinusitis, nasal polyp, wheezing, and bronchial asthma suggestive of atopy and had been prescribed montelukast sodium for five months. Bude
The patient had no relevant family history.
A neurological examination revealed proximal weakness in both the upper and lower limbs, with a power grade of 2/5 in both. The investigation also revealed signs of inflammation, which were supported by laboratory results.
Laboratory findings indicated that leukocytosis was 14.99 (normal range 4.00-11.00 × 104/μL), neutrophilia was 98.40% (normal range 37%-80%), eosinophilia was 17.2% (normal range 0.60%-7.30%), and erythrocyte sedimentation rate was found to be 22 mm/h (normal 0-12 mm/h). Table 1 shows the detailed results of the hematological parameters. Table 2 illustrates the various biochemical parameters, which revealed an elevated serum creatinine kinase (CK) level of 16180 U/L (normal range 20-180 U/L). The renal function test and liver function test were normal.
| Parameter | Result | Reference range (units) |
| Hemoglobin | 13.8 | 12.0-15.0 (g/dL) |
| Red blood cells | 4.89 | 4.20-5.40 (107/μL) |
| NRBC count | 0.0 | 0.0-0.1 (109/L) |
| Platelet count | 406 | 150-450 (104/μL) |
| Total leukocyte count | 14.99 | 4.00-11.00 (104/μL) |
| Neutrophils | 98.40 | 37.00-80.00 (%) |
| Lymphocytes | 22.00 | 10.00-50.00 (%) |
| Monocytes | 4.60 | 0.0-12.00 (%) |
| Eosinophils | 17.2 | 0.60-7.30 (%) |
| Basophils | 1.40 | 0.0-1.70 (%) |
| Erythrocyte sedimentation rate | 22 | 0.0-12.0 (mm/h) |
| Parameter | Result | Reference range (units) |
| Random blood sugar | 139 | 70.2-140.5 (mg/dL) |
| Renal function test | ||
| Urea | 34 | 17.0-50.0 (mg/dL) |
| Creatinine | 0.66 | 0.50-0.90 (mg/dL) |
| Sodium | 139.3 | 136.0-145 (mmol/L) |
| Potassium | 4.23 | 3.40-5.10 (mmol/L) |
| Magnesium | 0.87 | 0.66-1.07 (mmol/L) |
| Liver function test | ||
| Bilirubin, total | 3.80 | 3.40-20.50 (μmol/L) |
| Bilirubin, conjugated | 1.90 | 0.00-9.00 (μmol/L) |
| Alanine aminotransferase | 10 | 5-55 (U/L) |
| Aspartate aminotransferase | 12 | 5-34 (U/L) |
| Total protein | 7.21 | 6.60-8.70 (g/dL) |
| Albumin | 4.40 | 3.50-5.20 (g/dL) |
| Alkaline phosphatase | 69 | 40-150 (U/L) |
| Lactate dehydrogenase | 190 | 125-220 (U/L) |
| Creatinine kinase | 16.180 | 20-180 (U/L) |
| Vitamin D (25-OH) | 50 | > 30.0-70.0 (ng/mL) |
| Calcium | 9.74 | 8.40-10.20 (mg/dL) |
Jo-1 and dsDNA autoantibodies were negative, while anti-Mi-2 antibodies were positive. The nerve conduction study revealed myopathic changes, and the electromyography showed low activity with non-specific changes.
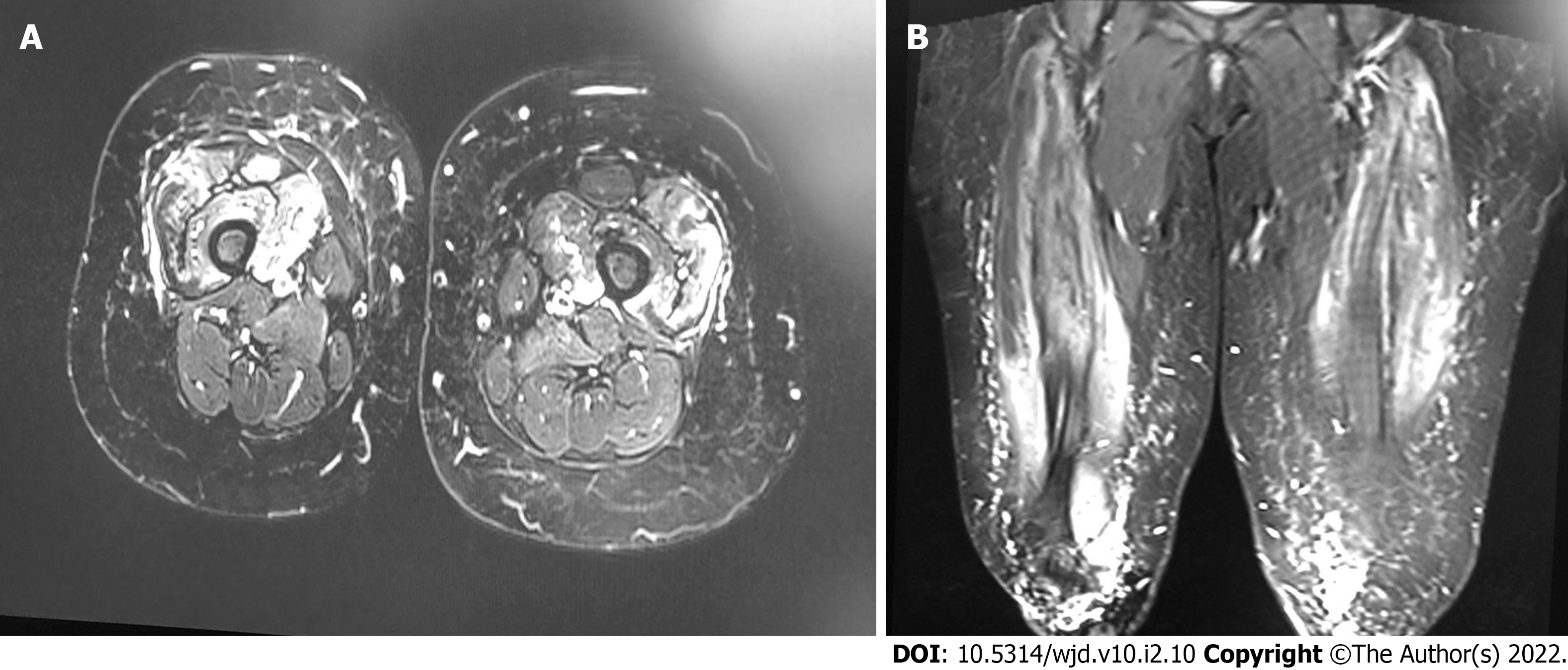
The magnetic resonance imaging of the patient’s muscles revealed bilateral asymmetrical (right > left) quadriceps muscle affection with active/acute inflammation features in the context of long-standing/chronic atrophic changes. Therefore, the advanced potential target for muscle biopsy is the right vastus medialis muscle (middle third), as shown in Figure 1. Furthermore, the patient was tested negative for malignancy workup (mammogram and pan computer tomography), antineutrophil cytoplasmic antibodies (ANCA), and coronavirus disease 2019.
The patient was diagnosed with dermatomyositis based on the findings mentioned above. Nonetheless, muscle biopsy is the gold standard for diagnosing dermatomyositis, and the patient was scheduled for one, but unfortunately, she declined.
Montelukast was withdrawn, and the patient was administered 60 mg of prednisolone every day for 45 d.
After two weeks of using prednisolone, the patient’s muscle power improved significantly (grade 4/5 in both upper and lower limbs), and her CK level returned to normal. She is currently on a tapering dose of orally disintegrating tablet formulation of prednisolone. The dose was reduced by 10 mg every two weeks until it reached 20 mg. And she is still being followed up.
Montelukast (Singulair) is a leukotriene receptor antagonist that inhibits the binding of leukotriene D4 to its receptor[4]. It was approved for medical use in 1998[5]. The pathophysiological influence reduces the inflammation process that contributes to asthma symptoms and relaxes the smooth muscles[6,7]. The medication is frequently used in the maintenance therapy of asthma but not in acute asthma attacks[4,6]. Other indications include prevention of exercise-induced bronchoconstriction and symptomatic relief of seasonal or perennial allergic rhinitis[7].
Montelukast is a safe and tolerable drug in adults, children, and pregnant women[4,5]. However, it may cause mild adverse effects, such as headache, fatigue, gastrointestinal disturbance, pharyngitis, upper respiratory tract infection, cough, and sore throat[6,8]. Furthermore, the serious adverse effects may include depression, hallucinations, suicidal ideation, insomnia, anxiety, changes in behavior, tremors, moderate acute hepatitis, and acute pancreatitis[8-11]. Shear and Litt[12] (2004) identified dermatological reactions associated with montelukast, with the most common adverse effect being an unspecified rash with or without blistering. In addition to skin ulcers, erythema nodosum, ecchymosis, urticaria, vasculitis, angioedema, and rarely, nodules.
Previous studies had evaluated the montelukast-induced ANCA-associated vasculitis, also known as Churg-Strauss syndrome (CSS), which has been widely reported in the literature and is a significant concern. CSS is an uncommon autoimmune disease that causes vasculitis in patients with asthma or allergic rhinitis[13,14]. Several studies have suggested the association between montelukast use and CSS development. A case-crossover previous survey illustrated that the drug was associated with a 4.5-fold increased risk of CSS onset within three months[13]. However, a prior case series study in the United States on six asthmatic patients receiving montelukast demonstrates no association between montelukast and CSS development[14]. Furthermore, a recent study published by Pandey et al[13] in June 2021 reported that montelukast appeared to be a confounding factor not associated with the development of the syndrome. In contrast, steroid withdrawal is the primary factor contributing to CSS development.
In contrast to our patient, a recent clinical study was conducted by the United States Food and Drug Administration (FDA) and eHealthMe to analyze the side effects of montelukast based on the patient reports[15]. Phase IV of the clinical study concluded on August 16, 2021, that among 97983 patients who reported having side effects while taking montelukast, twenty patients (0.02%) had dermatomyositis. The study also found that the most common characteristics among those patients were female gender, age between 20-29 years, and use of the medication for 1 to 6 mo[15]. Therefore, an early diagnosis of dermatomyositis is critical to start treatment before conditional progression[1]. If dermatomyositis is not treated, it can progress to critical conditions affecting the heart, swallowing, and breathing, which can be fatal[1].
Although dermatomyositis was suspected in our patient, a confirmatory muscle biopsy was not performed, which is considered the gold standard to diagnose dermatomyositis. As a result, the patient improved significantly after discontinuing montelukast and receiving a short-term steroid, indicating that montelukast may play a role in this condition. This potential association was also proposed by the FDA and eHealthMe study[15].
Because montelukast was previously thought to be the leading cause of CSS, the present study raises concerns about the association between montelukast and dermatomyositis. Future studies with advanced study designs are strongly advised to investigate the possible link between dermatomyositis and montelukast use.
Even though montelukast is thought to be a safe drug, the present case highlights the possibility of adverse effects of montelukast. Physicians must be aware of the presentation of dermatomyositis, the diagnostic modalities, and the best treatment options for the patients, especially in such a rare and severe montelukast complication. Future studies with advanced study designs are strongly recommended to investigate the potential link between dermatomyositis and montelukast use.
The authors would like to acknowledge our superhero Dr. Almalki AM, College of Medicine, at Umm Al-Qura University, for the unlimited support and the incredible efforts he paid on this research project. Warmest congratulations on his graduation, and best wishes for him on the next adventure.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dermatology
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fazilat-Panah D, Iran; Ghimire R, Nepal S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Gara S, Jamil RT, Muse ME, Litaiem N. Juvenile Dermatomyositis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan 25. [PubMed] |
| 2. | Shen C, Che G. Dermatomyositis as an antecedent sign of lung cancer in an eldly patient: a case report. J Thorac Dis. 2014;6:E15-E18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 3. | Aggarwal R, Charles-Schoeman C, Schessl J, Dimachkie MM, Beckmann I, Levine T. Prospective, double-blind, randomized, placebo-controlled phase III study evaluating efficacy and safety of octagam 10% in patients with dermatomyositis ("ProDERM Study"). Medicine (Baltimore). 2021;100:e23677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Amirav I. Real-life effectiveness of Singulair (montelukast) in 506 children with mild to moderate asthma. Isr Med Assoc J. 2008;10:287-291. [PubMed] [DOI] [Full Text] |
| 5. | Sarkar M, Koren G, Kalra S, Ying A, Smorlesi C, De Santis M, Diav-Citrin O, Avgil M, Lavigne SV, Berkovich M, Einarson A. Montelukast use during pregnancy: a multicentre, prospective, comparative study of infant outcomes. Eur J Clin Pharmacol. 2009;65:1259-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Meltzer EO, Lockey RF, Friedman BF, Kalberg C, Goode-Sellers S, Srebro S, Edwards L, Rickard K; Fluticasone Propionate Clinical Research Study Group. Efficacy and safety of low-dose fluticasone propionate compared with montelukast for maintenance treatment of persistent asthma. Mayo Clin Proc. 2002;77:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Nayak A, Langdon RB. Montelukast in the treatment of allergic rhinitis: an evidence-based review. Drugs. 2007;67:887-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Aypak C, Türedi Ö, Solmaz N, Yıkılkan H, Görpelioğlu S. A rare adverse effect of montelukast treatment: ecchymosis. Respir Care. 2013;58:e104-e106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Romero-Gómez M, Sánchez-Muñoz D, Castilla L, Castro M. [Acute hepatitis due to montelukast]. Med Clin (Barc). 2003;120:239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | GOV. UK. Montelukast (Singulair): reminder of the risk of neuropsychiatric reactions. Sep 19, 2019. [cited 5 March 2022]. Available from: https://www.gov.uk/drug-safety-update/montelukast-singulair-reminder-of-the-risk-of-neuropsychiatric-reactions. |
| 11. | Das S, Mondal S, Dey JK, Bandyopadhyay S, Saha I, Tripathi SK. A case of montelukast induced hypercholesterolemia, severe hypertriglyceridemia and pancreatitis. J Young Pharm. 2013;5:64-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Shear N, Litt JZ. Litt's drug eruption reference manual including drug interactions. 10th ed. London: CRC Press, 2004. |
| 13. | Pandey L, Sawale G, Punmiya A. Research article a review on toxicological study of montelukast. Int J Recent Adv Multidiscip Res. 2021;8:6975-6982. |
| 14. | Wechsler ME, Finn D, Gunawardena D, Westlake R, Barker A, Haranath SP, Pauwels RA, Kips JC, Drazen JM. Churg-Strauss syndrome in patients receiving montelukast as treatment for asthma. Chest. 2000;117:708-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 138] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | eHealthMe. Singulair and Dermatomyositis - a phase IV clinical study of FDA data. [cited 5 March 2022]. Available from: https://www.ehealthme.com/ds/singulair/dermatomyositis/. |