Revised: August 3, 2012
Accepted: August 10, 2012
Published online: October 2, 2012
Management of difficult wounds can be a complex, challenging and expensive task, especially for wounds showing a slow healing process. Topical negative pressure (TNP) therapy has greatly improved difficult wounds treatment. It allows to treat patient on an outpatient management, to reduce the complication rate with shorter hospital stay, to avoid frequent dressings with expensive advanced materials and allow a lower commitment of health professionals. Vacuum Assisted Closure® (VAC®) system is a therapeutic device based on the administration of a controlled TNP introduced by Morykwas and Argenta in 1997. It is indicated in different kinds of wound, but clinical evidences are present only for few of them. In this work we summarize indications and recommendations for VAC® therapy and we analyze the actual better choice of treatment based on evidences and personal experience in order to stimulate further studies. Finally we introduce recent applications of VAC® system such as Prevena®, VAC Instill® and VAC Via®. Prevena® is a system based on TNP indicated in the management of closed wounds that present risk factors for dehiscence. VAC Instill® is a system that allows to associate TNP and topical administration of solutions, such as antibiotics or disinfectants, to treat specific type of wounds. VAC Via® is a device based on TNP, characterized by little dimension and a preset system that allow the treatment of little wounds for 7 d, with no impairment for the patient. The aim of our paper is to describe a report of VAC® therapy use in order to stimulate further studies and to define the level of evidence of VAC® therapy.
- Citation: Negosanti L, Pinto V, Sgarzani R. Clinical evidences, personal experiences, recent applications. World J Dermatol 2012; 1(3): 13-23
- URL: https://www.wjgnet.com/2218-6190/full/v1/i3/13.htm
- DOI: https://dx.doi.org/10.5314/wjd.v1.i3.13
Management of difficult wounds can be a complex, expensive and time consuming task.
The increasing age of general population and life style changes (sedentary life, metabolic syndrome) can led to higher incidence of chronic wounds and lesions in patients with poor general conditions (vascular disease, diabetes).
Direct consequences are a higher number of patients in relative good general status affected by chronic wounds requiring higher costs for health care system due to hospitalization, outpatient management, frequent dressings and commitment of health professionals.
Topical negative pressure (TNP) therapy has greatly improved difficult wounds treatment. It allows to treat patients on an outpatient base, reduce the complication rate with lower hospital stay, avoid frequent dressings with expensive advanced matherials and so there is a lower commitment of health professionals. The possibility to manage the therapy with a relative comfortable instrument allows the patient to carry out daily activities.
The use of negative pressure in clinical practice date back to ancient times. It was described in Chinese medicine in combination to ago puncture[1] thanks to its hyperaemic effect. In 1841, Junod used heated glass cup applied on the skin to create vacuum and stimulate circulation[2]. Different techniques of TNP application were later described. In the 1980s, in the Russian literature, the use of TNP in combination with aggressive debridement was reported to significantly reduce bacterial counts in suppurative wounds[3]. In 1989, Chariker et al[3] applied TNP therapy in patients with incisional or cutaneous fistulas using moist gauze placed over the wound surface and a flat drain placed over the gauze covered by a bio-occlusive dressing. Fleischmann et al[4] in 1993 described the application of TNP trough a foam dressing observing the formation of granulation tissue and wound cleaning.
Morykwas et al[5] elaborated a system composed by a polyurethane (PU) foam covered by a semi occlusive dressing and connected to a vacuum source to induce TNP on wound in animals. This work was the start point for the production of the Vacuum Assisted Closure® (VAC®) system by Kinetic Concepts Inc. (San Antonio, United States).
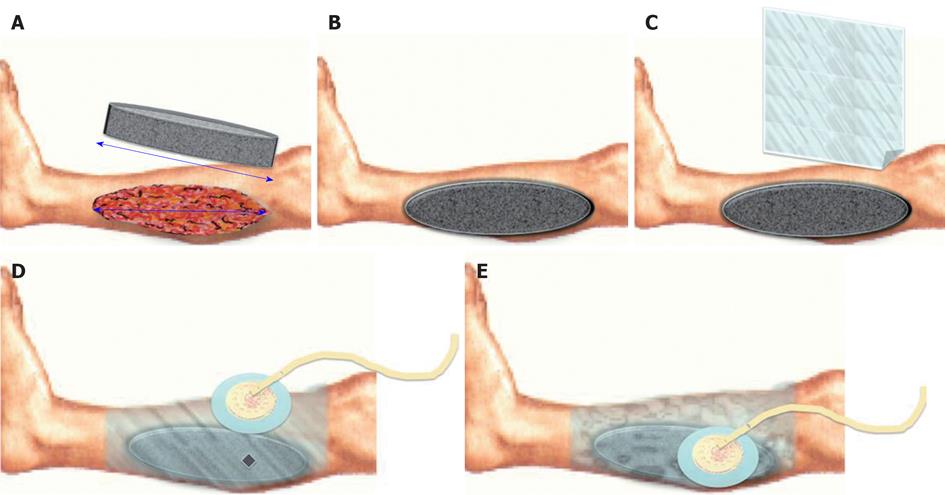
VAC® device is made up by (Figure 1): (1) Vacuum source; (2) Foam dressing: it is the interface between the vacuum source and the wound. Its essential role consists in the uniform administration of TNP on the whole wound surface, even in difficult anatomical sites (e.g., groin). Other systems use a moist gauze fill in the wound as interface; (3) The foam can be made by PU (black) that is hydrophobic and presents big holes (400-600 μm), polyvinyl alchool (white) that is hydrophilic and with small holes or PU combined with Silver (gray) with an improved bacteriostatic effect; (4) Foam choice depends on wound characteristics and supposed treatment’s goal; (5) Semi occlusive transparent adhesive drape fixed on heal skin, all around the wound to isolate it from the external environment; (6) An adhesive disk (Pad) positioned on an hole created in the drape and connected to the vacuum source trough a suction drain; (7) A reservoir connected to the drain; and (8) A processor that elaborate signals from the different components of the device and that shows dysfunction (e.g., air leakage). In particular KCI developed the Therapeutic Regulated Accurate Care (TRAC®) pad system that controls continuously the pressure at the wound bed and not only the one derived from the vacuum source; it allows the clinician to diversify the intensity of the negative pressure and provides a even setter distribution of negative pressure at the wound bed. The SensaTRAC® system allows to monitor and maintain the desired pressure at the wound area for a constant administration of the therapy, it allows to reduce clogging of pipes and false alarms using an advanced computational fluid dynamics and to use Smart Alarms® for maximum patient safety. All these possibilities led to an easy and effective application of VAC® Therapy and improved patient comfort.
The direct effect of VAC® is to create a wet environment with a sterile and close dressing.
TNP administer by VAC® Therapy creates forces that, applied to the wound bed, are able to develop an environment that promotes wound healing. These forces can be distinguished in macro-deformations and micro-deformations.
Macro-deformations consist in a visible stretching that occurs when the negative pressure shrinks the foam. The direct effect is to close the wound edges, to allow an uniform distribution of pressure at the whole wound bed and to remove the exudates and infectious materials.
Micro-deformations are the modifications present at the cellular level. The direct effect is to reduce oedema, to promote perfusion, to increase cells proliferation and migration and to promote granulation tissue formation.
The final effects of VAC® Therapy leading to promote wound healing could be so resumed in: (1) Remove infectious materials; (2) Provide an adequate protection against infection; (3) Remove exudates; (4) Reduce oedema; (5) Provide a moist environment; (6) Increase blood flow promoting perfusion; and (7) Promote cell migration and proliferation during granulation tissue formation.
The reported physiological effects were described in clinical or animal studies. Morykwas et al[5] demonstrated that a negative pressure of 125 mmHg increase the vascularisation at 4 times, while higher negative pressure induce capillary collapse with blood flow reduction. Fluid suctions led to oedema reduction with a consequent lower external pressure on capillary and blood flow increasing. This effects is particularly evident with PU foam thanks to bigger holes.
Another aspect studied by Morykwas et al[5] was granulation tissue formation induced by TNP; it resulted increased of 63% with continuous TNP and of 103% with intermittent regimen, compared to classical dressings. This result was explained by cells adaptation to continuous physical forces. Other favourable effects of intermittent TNP are blood flow increase due to deactivation of the auto regulatory capillary system, and the number of mitosis, due to the “relax” period in which cells are able to produce new structural components. The disadvantage of intermittent therapy is higher pain than the one experienced by patients with the continuous regimen.
Some Authors proposed to start VAC® with continuous -125 mmHg for 48 h and then switch to an intermittent regimen.
Cellular proliferation induced by mechanical forces is a well known concept in plastic surgery (e.g., tissue expansion, osteogenic distraction)[6,7]. TNP induces tissue deformity with a mechanical stress that stimulate angiogenesis and cellular proliferation.
Other described effects induced by VAC® are the reduction of substances present in the exudates that hinder wound healing, bacterial grow reduction due to the closed system that on one hand prevent external contamination and on the other hand enable an amplification of physiological antimicrobial agents thanks to blood flow increasing[8-10]. In addiction foam contraction during treatment reduces the perimeter of the lesion, accelerating the healing[1].
Contraindications[11] to the use of VAC® in acute wounds are presence of necrotic tissue, massive invasive infection, exposed cortical bone, untreated ostemyelitis, malignancy and active bleeding at the wound site. Necrotic tissue must be removed before VAC® therapy application with a careful debridement; infection must be treated with systemic antibiotics and the use of Granufoam Silver® which can help in the treatment of local infection, a frequent wound check with culture from wound bed must be done and VAC® therapy must be immediately stopped if the result remains poor. Sensitivity to Silver is a contraindication to the use of GranuFoam Silver®. Exposed cortical bone represents a limit to granulation tissue formation onto wound bed. Malignancy is an absolute contraindication due to the risk of cancer cells proliferation. Active bleeding from the wound site and the presence of exposed vessels or organs can led to hemorrhage during VAC® therapy. In case of small exposure a non-adherent dressing positioned between the wound bed and the foam can help to reduce this risk.
Other contraindication to VAC® therapy is the presence of non-enteric or unexplored fistulas.
VAC® device must not be placed directly in contact with exposed blood vessels, anastomotic sites, organs or nerves; in these cases the use of a non-adherent layer between deep structures and foam can be useful to prevent any damage to the underlying anatomical structures.
Complications encountered during VAC® therapy are pain and hemorrhage, as just reported.
Pain is often associated with dressing changes[12] and in this cases instillation of anesthetic agent via the suction tube into the foam before dressing change may be of great benefit. Pain associated with the treatment itself is often linked to intermittent regimen; in this cases a continuous TNP regimen can help to reduce pain.
Hemorrhage is linked to the suction of active bleedings at the wound. The presence of bleeding is a contraindication to TNP and in presence of a high risk for bleeding it’s important to monitor continuously the canister. If active bleeding develops suddenly or in large amount during treatment, or if bright red blood is seen in the tube or in the canister, immediately stop VAC®.
An important aspect to consider is the coast load; at a first sight it is possible to think that high costs of VAC® device and dressing’s materials can be a limitation when compared to other dressings. Otherwise we have to consider that dressing’s costs are only a singular parameter that play a role in wound’s management costs. Other important aspects we should take into consideration when evaluating from the budget perspective are costs related to nursery assistance, hospitalization and complications’ management[13]. Health economy should evaluate treatment coasts also in relation to benefits achieved through it. In wound treatment costs depends on many factors such as frequency of dressing’s change, time of necessary nursing assistance, rate and time of wound healing, effects on hospital stay duration and complications[1]. Armstrong et al[14] evaluated the use of VAC® in diabetic foot after amputation. They reported an healing percentage of 56 in VAC® group and of 39 in group treated with standard dressings. The medium time of healing was 56 d in VAC® group and 77 d in the other one. Similar results were reported in pressure ulcers[15]. Schweim et al[16] demonstrated a significant reduction in hospitalization rate in patients treated by VAC® and several studies[14,16,17] reported lower complications rate. Hiskett et al[18] reported that TNP allows to reach qualitative and economic benefits in home care. Obviously comparison between all studies performed about costs and benefits of VAC® is restricted because we have different cost of nursery care, different pattern of use of TNP consumables and wound outcome. Philbeck et al[19] reported that the shorter healing time and downgrading of required operations in patients treated by VAC® correlates to decreased overall costs of care and, in particular, wounds can be treated in the community with minimal impact on nursing care and hospitalization. All presented considerations led to consider VAC® therapy a valuable treatment in terms of health care costs. In facts it allows to treat patients at home, reducing hospitalization time and high costs’ operations.
VAC® therapy is indicated in many kinds of wounds. In some cases there are clinical evidences of its application, in others there are only case reports or small series reporting good results. In our experience we used VAC® in different wounds with different goals and can occur to stop therapy after an evident failure. It is important to determine the goal before starting and to evaluate the wound after an adequate time to decide if the intermediate result is good enough to continue the therapy or if there are signs of failure guiding us to choose alternative treatments. Wound must be prepared before VAC® therapy we need to remove eschar and devitalized tissues, to treat infection and, first of all, to make a correct diagnosis. Wounds due to malignancy or ischemia are not suitable for VAC®, as just reported. We describe the principal indications for VAC® reported in literature and correlate them with our experience and level of evidence.
Open abdominal wounds are one of the first indication introduced for VAC® therapy.
The open abdomen is the result of decompression laparotomy and dehiscence or necrotizing fasciitis. This condition is associated with high morbidity and mortality[1].
The main goals are to achieve a primary closure or to obtain granulation tissue formation to allow a skin grafting[2]. VAC® demonstrates to improves survival, decrease surgical reconstructive complexity and reduce complication rate, such as compartment syndrome[3].
VAC® induces both skin and fascial approximation, reduces bowel oedema, bacterial counts and inflammatory substances, avoiding frequent dressing changes, maintaining intact skin and improving fluid management[4].
Dressing must be changed every 48-72 h in absence of infection and exposed bowel must be covered with a non-adherent layer to prevent fistula formation and other complications. A continuous pressure of -175 mmHg prevents fascial retraction and visceral adherence and multiple dressing sheet perforations permit intra-abdominal fluid and oedema evacuation[5]. Optimal results may take 21 d or more to show off.
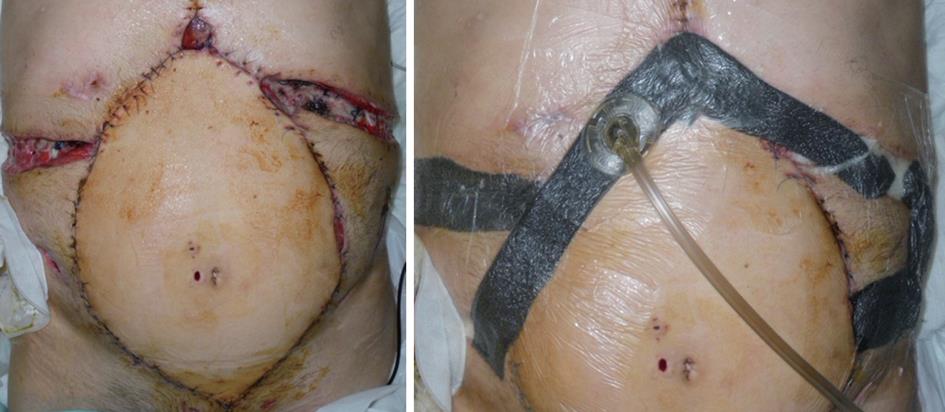
We applied VAC® in many cases of open abdominal wound as a bridge tool to defintive closure with skin graft and in one case of patient treated for dehiscence after multivisceral and abdominal wall transplantation as a definitive closure method, due to the impossibility to perform surgery (Figure 2).
Dehisced sternal wounds are an optimal and well documented indication for VAC with the aim to achieve primary closure or prepare the wound for delayed reconstruction.
Mediastinitis occurs in 1%-5% of patients following sternotomy[20] with high morbidity and mortality[21].
The positive effects of VAC® consist of stabilization and salvage of the sternum, drainage of anterior mediastinum, early mobilization and reduction of mortality rate. Treatment starts after debridement. A non-adherent layer is interposed between deep structures and foam to protect mediastinal structures against direct contact with TNP[22,23]. Full-thickness perforations allow transmission of negative pressure trough the dressing to anterior mediastinum. A double layer dressing enables optimal thoracic stabilization (sternal layer) as well as a good distribution of negative pressure over the entire wound surface (subcutaneous layer)[24]. Dressing must be changed every 48 h to evaluate the wound and perform bacterial swab at the wound bed. Also serum C-reactive protein (CRP) level can be useful to guide therapy[25]. TNP must be applied in continuous fashion at-125 mmHg. Expertise is required to treat this kind of wounds. VAC® is contraindicated in case of active bleeding or anticoagulation beyond therapeutic range.
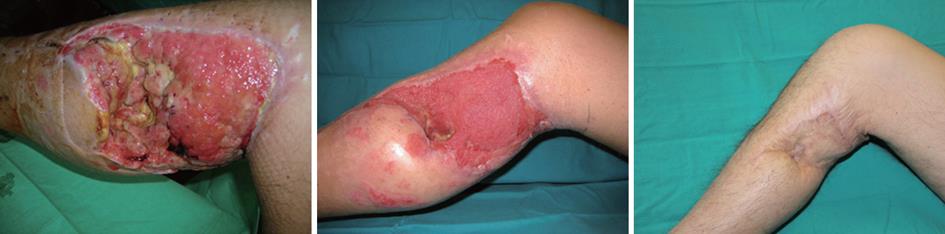
In traumatic wounds VAC® therapy can led to several benefits as stabilize soft tissue, reduce secondary damage, salvage of compromised tissue, reduce oedema, wound size and complexity (Figure 3). The goals are to promote granulation tissue formation and perfusion, to remove fluids, exudate and infected materials and to assist take of flap or skin/bioengineered tissue. The result is a reduction in complexity of reconstructive procedures, scar formation and an improved patient care and comfort due to a reduced number and frequency of dressing changes. The paramount indication is a large loss of soft tissue, but in literature several applications are described such as treatment of inflammatory wounds, open fractures management, energy trauma wounds, fasciotomy wounds, degloving injuries and burns. In all these cases VAC® therapy can help stabilization of skin graft and donor site healing[21], stabilization of energy injuries allowing safer transfer of the patient; management of open fractures reducing complexity of secondary surgery, prevent the progression of partial-thickness burns[26] and to prepare full-thickness burns to skin grafting[27].
General recommended settings consist in a continuous cycle at -125 mmHg for 48 h and then, if possible, an intermittent cycle until healing is reached. Dressing must be changed every 48-72 h, only in presence of infected wound a more frequent dressing changes should be taken in consideration. Therapy should be used after debridement and vital structures must be covered with non-adherent dressing. The presence of orthopedic hardware does not represent a contraindication.
Inflammatory wounds occur in scleroderma, systemic lupus erythematosus, hypercoagulation disorders, rheumatoid arthritis and vasculitic conditions. The goal of VAC® treatment is to enhance wound bed preparation for surgical closure or delayed secondary heal. In this cases the evaluation should be done after 1-3 d.
Complex resistant ulcers often present with abundant exudates or difficult anatomical sites and poor-wound bed. In this cases VAC® could be an ideal option up to 2 wk, then you should check the result by evaluating granulation tissue formation.
In open fractures VAC® therapy could be considered when primary closure is not possible providing a temporary cover. Delayed surgical closure of open fractures is characterized by a high risk of infection and impairment of the bone synthesis[28]. VAC® therapy protects wound from infection, reduces oedema and increase the rate of viable tissue over the bone, with a simpler delayed reconstructive procedure, that has to be performed as early as possible.
Fasciotomy incisions often present oedema, skin retraction and skin edge necrosis[1] that limit primary closure after compartment syndrome resolution. VAC® reduces oedema and splint wound edges allowing primary closure in shorter time in comparison to conventional dressing[29,30].
In energy trauma, TNP is most suitable for complex soft tissue injuries in the absence of exposed bone, as loss of tissue from the foot, exposed tendons, tissue loss in gunshot wounds and degloving injuries[31-35]. Treatment should start with continuous negative pressure between -50 mmHg to -125 mmHg, although, when possible, intermittent therapy should start in the next 48 h, this may led to greater granulation tissue formation[13].
Even if coverage of exposed bone with well-vascularised tissue remains the gold standard for open fractures[11], VAC® may allow, as temporary dressing, a downstaging of the wound[36].
In burns’ treatment VAC therapy aids to reduce oedema, infectious and inflammatory materials and help to improve wound perfusion. In particular it prevents burn progression in partial-thickness burns. It is well known that in partial-thickness burns is present a zone of stasis characterized by microcirculation impairment at 12-24 h post-burn with consequent hypoxia, ischemia and cell death that led to full-thickness burn[37]. TNP applied to partial-thickness burn within 6 h after injury and for at least 48 h, helps to reduce oedema and increase blood flow, stopping the progression to full-thickness burn[38].
Skin graft and bioengineered tissue fixation require tie-over dressing for 5 d. Problems may arise when facing irregular surfaces (e.g., perineum, inguinal fold), areas prone to movement or exudative recipient beds. TNP may be used for wound bed preparation to reduce size and to assist granulation tissue formation[39]. VAC® aids to deal with serous fluid or hematoma, bolstering the graft to the bed, and increases angiogenesis and splinting of graft in difficult area[24].
The foam must be applied with an interpositional non-adherent barrier between graft and foam; TNP must be set at a range between -100 mmHg and -125 mmHg, in continuous fashion, for a period of 3-4 d[40]. Multiple holes should be performed in the graft before VAC® positioning in order to increase fluid collection. VAC® therapy is mentioned, in literature, also to improve skin graft donor site healing, showing a faster reepithelialisation with good results[41].
VAC® therapy can be applied to improve vascularization in flaps which have suffered partial necrosis after performing debridement of necrotic tissue, allowing salvage of the of the flap.
TNP improves flap survival by reducing oedema, increasing blood flow and bolstering flap placing at the wound bed. Moreover VAC® hide the flap, thus the flap monitoring is more difficult. Positive effects of TNP on venous congestion are still not well described. Morykwas et al[5] reported good results in animal studies in enhancing viability of random pattern flaps. Another useful application of VAC® therapy in flap surgery is the improvement of donor site healing. A particular indication is in radial forearm flap donor site management. VAC® therapy induces granulation tissue formation over exposed deep structures, improving skin grafting[42,43]. Recommended settings are a continuous cycle at -125 mmHg for 72 h.
In diabetic foot VAC® is indicated in uninfected and not ischemic deep complex ulcers with the goal to reduce the surface area. VAC® reduces the complexity of the subsequent surgical closure procedures[14,44]. In combination with systemic antibiotics, VAC® allows healing of underlying osteomyelitis avoiding ulcers recurrence[3]. VAC® therapy is used for 1-2 wk; after this time, the wound should be evaluated and to be continued if the wound has improved; if progress is poor an alternative treatment must be considered[45]. In post-surgery diabetic foot wounds, VAC® is indicated after open partial foot amputation[14], to aid skin graft or bioengineered tissue replacement fixation[46,47]. VAC is not recommended as a first line treatment in superficial wounds, but it can play a role as a second choice after advanced dressings failure[45]. Recommended settings are a continuous cycle at -125 mmHg in first 48 h, then, if possible, an intermittent cycle for rest of treatment. In absence of infection dressing can be changed every 48-72 h.
In pressure ulcers VAC® therapy can help to reduce volume of a large cavity wound, to promote comforts for the patient and to reduce nursery management. Goals are promoting granulation tissue formation, promoting perfusion and providing a closed, moist wound healing environment. It is not indicated for stage 2 ulcers and in case of deep tissue injuries. The best indication is stages 3 and 4 wounds, in combination with pressure redistribution, good skin care and planning of an adequate nutrition[48]. In these cases VAC® could be useful both preoperatively, to allow less complex reconstruction and post-surgery to manage dehiscence, to improve perfusion of flap or grafts fixation. The effects should be evaluated continuously for a period up to 2 wk. Recommended settings are a continuous cycle at -125 mmHg in first 48 h, then, if possible, an intermittent cycle for rest of treatment. In absence of infection dressing can be changed every 48-72 h.
In venous insufficiency[49,50] ulcers VAC® can be used to reduce oedema, to promote perfusion, to remove exudate, to promote granulation tissue formation and to provide a closed, moist wound healing environment. Recommended settings are a continuous cycle at -125 mmHg in first 48 h, then, if possible, an intermittent cycle for rest of treatment. In absence of infection dressing can be changed every 48-72 h. Wound should be adequately prepared before therapy with a correct debridement and compression garment or bandage may be placed taking care not to induce any pressure point. In presence of explored tunnels or undermining, the first choice should be VAC WhiteFoam® dressing. Foam should be placed in contact with all wound surfaces and in presence of more foam pieces a correct foam to foam contact must be ensured to achieve a better distribution of negative pressure. Superficial or retention sutures should be covered with a non-adherent material.
VAC® may help to promote healing in wounds around enteric fistula, but cannot be considered for effluent management or containment. The goal in acute fistula should be to promote closure of the fistula. Chronic fistulas are segregated from surrounding or adjacent abdominal wound, then VAC® is applied to the wound and the effluent from fistula are deviated into another containment system. General recommendation is to start with a -125 mmHg pressure and, if effluent is noted in the tubing, pressure should be raised of 25 mmHg for 20-30 min and then check effluent. If it is still present, continue to increase the pressure and observe until there is no effluent in the tubing, up to a maximum of 200 mmHg. Reduction in the amount of effluent is an early sign of initial approximation of the fistula. Otherwise, if effluent continues to flow VAC® must be stopped and other treatment should be taken into consideration.
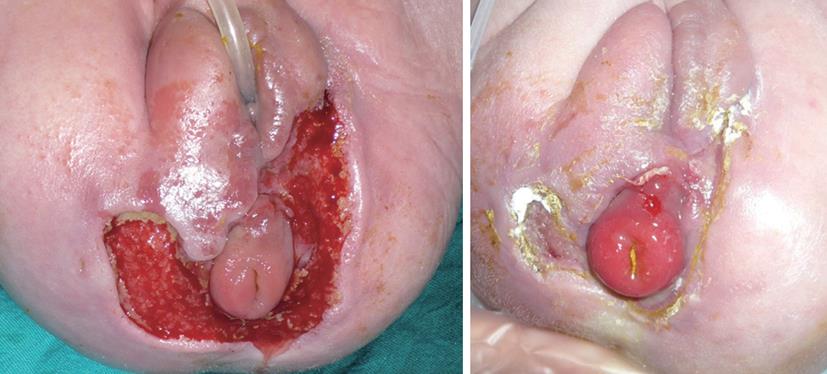
The indications for negative pressure therapy in paediatric and neonatal wounds have been discussed by Baharestani[51]. In our experience we treated a rare case simultaneous acute myeloid leukaemia and necrotizing fasciitis due to Pseudomonas aeruginosa in a newborn (Figure 4)[52]. Our patient presented with perineal erythema and haemorrhagic pustules with a rapid progression into necrotizing fasciitis and, at 23 d, was present a lesion of approximately 18 cm2 involving the superficial and deep fascial planes around labia majora, a greenish secretion, a black eschar with an erythematous halo. Swap culture revealed the presence of Pseudomonas aeruginosa, however, blood cultures were negative. The infant was treated with systemic administration of: broad-spectrum antibiotics, immunoglobulins, inotropic drugs and nutritional support. The initial treatment of the wound consisted of surgical debridement followed by the application of advanced dressings, such as silver PU foam and hydrofiber. Due to the reduced healing rate and the persistence of Pseudomonas colonies in the wound, we decided to apply VAC® device with GranuFoam Silver® dressing[53]. The dressing was changed every 48 h. During hospitalization at the Neonatal Intensive Care Unit, the newborn was continuously monitored: pain was assessed and opioid analgesia was used. The positioning of the VAC® dressing on the perineal area was challenging. The main problems were: preventing direct suction on the anal sphinter while maintaining normal sphinteric functions and avoiding pressure sores due to the suction tube. TNP was set at -50 mmHg according to McCord[53] for 6 d and then raised to -75 mmHg for 7 d. The rise in negative pressure was concurrant with an increase of the CRP value[54] (from 1.09 to 5.07 mg/dL), however, local signs of inflammation were reduced. After 13 d of negative pressure treatment, the wound was almost healed. Definitive closure was achieved in 5 d of application of collagen dressing (Condress®, Abiogen, Pisa). After these treatments there were no signs of necrotizing facitiis and the swab culture was negative for Pseudomonas Aeruginosa. In our experience, the use of TNP therapy for neonatal necrotizing facitiis allowed us to achieve rapid wound healing after debridement. Interestingly, after the increase of suctioning pressure from -50 to -75 mmHg, a higher CRP value was observed, although local signs of inflammation had reduced. This can be explained by the fact that TNP determines the local release of interleukin (IL)-6, IL-8 and vascular endothelial growth factor and IL-6 induces an increase in plasma CRP concentration[55].
An important consideration should be done about the useful application of VAC® therapy in patients with important comorbilities and contraindications to surgery, because treatment of patients with complex wound often requires surgical debridment and reconstruction with skin grafts or flaps. VAC® can be considered an alternative treatment in these patients[55].
In our experience we treated patients affected by complex wounds with conditions that contra indicates surgical procedures, as neonates, pregnant, or old patient with overall condition, such as advanced dementia or immobilization, that made difficult the management with the need for hospitalization. In all these cases the VAC® therapy has resulted in healing without the need to subject the patient to treatment achieving excellent results. The VAC® therapy is the treatment of choice when following patient at home and achieving healing quickly with satisfactory results, is needed.
No evidences are reported for recent applications introduced by KCI: Prevena, VAC® Instill and VAC® Via.
These three devices were introduced in last few years with specific indications that must be cited in a work with the aim to resume all the possible indications of VAC® therapy and the necessity of further studies to define the level of evidence of these treatments.
Prevena®
Prevena® is a new device introduced in 2010 by KCI for the treatment of closed surgical incisions with VAC® therapy.
It consists in a preformed dressing made by a foam covered by a transparent film directly connected to the vacuum system predisposed at -125 mmHg and set for 7 d of treatment. It is generally positioned on the incision at the end of surgery, in a sterile environment. The goal is to create a favourable environment for healing processes, to approach the edges of the incision up to the closing, to stimulate perfusion, to reduce side tension, oedema and acts as a barrier against external contamination. The device is small and easy to carry.
Advantages of TNP on closed incisions were reported in several studies[56-58].
The indications of Prevena® are all surgical incisions at high risk for complications, such as in patients with poor general conditions due to diabetes, obesity or poor vascular status[59-61].
In high-energy trauma wounds the rate of complications (necrosis, infections) was reported in a range from 33% to 50%[62] and in sternal wound the mortality rate in case of infection is about 33% at 1 year[63,64].
Other conditions at high risk are orthopedic procedures[56,57], lower extremities bypass[62], abdominal[65,66] and cardiothoracic procedures[67].
Stannard et al[68] purposed a good system to classify the risk of wound complications high lightening cases that are best suited for TNP. Patients found to benefit from TNP are those with one or more risk factors for infection, seroma, hematoma, and dehiscence.
Contraindications to TNP are wounds with infection, dehiscence or cellulitis and incisions with ischemia or fragile skin due to radiotherapy, steroid or patient’s age.
In literature there are studies reporting good results in the reduction of incidence of dehiscence and infection in high-energy trauma wounds[56], absence of complications in sternal wound patients at high risk for infection[57] and no infections in patients with foot and ankle trauma[58].
These considerations are the base of Prevena® system, it can be considered an useful and easy to use device in all surgical wounds at high risk for complications.
VAC Instill®
VAC Instill® system was introduced by KCI in 2003. it allows to add solutions to the wound bed, and it can be useful for wounds showing no response to conventional VAC® and as initial management in selected high risk wounds[69].
It is indicated in patients who would benefit from vacuum assisted drainage and controlled delivery of topical wound treatment solutions in case of chronic, acute, traumatic, subacute and dehisced wounds, partial thickness burns and ulcers.
Topical agents may be intended for extended tissue contact and compatible with VAC® dressing and disposable components (e.g., hypochlorous acid solutions applied at high concentrations for longer periods may damage VAC® system). The VAC GranuFoam Silver® is not indicated with instillation therapy.
The device is similar to traditional VAC® system with additional features. The foam is placed as usual and covered by the drape. Two different pads are connected to the dressing: one is the traditional suction drain connected to the vacuum source and the canister; the other is connected to an irrigation bag, containing the selected solution. Clinicians set automated infusion of fluids at presetted intervals without compromising the integrity of the occlusive dressing. It is important to consider patient’s position because instillation is driven by gravity. Instilled and drained fluid volumes must be monitored during treatment. In case of deep wound, a hole can be made deep in the foam in order to achieve a better action onto wound bed.
The treatment consists of repetitive cycles of TNP, instillation and holding time. Holding time allowed instilled solution to irrigate the whole wound and to perform its action, neither instillation nor TNP are applied during this period.
Instillation time must be enough to saturate the foam; holding time can range from 1 s to 1 h and continuous TNP can range from 1 min to 12 h.
Donalee[70] recommends 1-2 min of instillation time, 5 min of holding time and 5 h of TNP.
A test should be made after dress placement starting with a TNP cycle to check the seal and then with instillation to quantify the total amount of fluid necessary to saturate the foam. A wound culture may be obtained before starting in order to select the optimal fluid to instill[69].
The main goal is to reduce the bioburden within the wound; furthermore VAC Instill® may help in reduce pain in selected patient[69,71,72], even by using analgesic solutions.
In literature case reports about VAC Instill®[69,73,74] were reported. In all cases good results were achieved in terms of decrease in the main time to obtain a bioburden reduction, wound closure and hospital discharge.
In our experience we treated one patient affected by abdominal dehiscence with the presence of an exposed mesh. This condition often requires mesh removal, especially in case of infection of the device.
Our patient presented an infection of exposed mesh due to Klebsiella Pneumoniae sensible to Teicoplanin.
The continuous infusion was made using two solutions alternatively: 450 cc of physiologic saline + 50 cc of Betadine®; 500 cc of physiologic saline + 5 g of Teicoplanin (an injection of this solution is made every 45 min and then the TNP is set at 0 mmHg for 5 min then the TNP is set at -125 mmHg)
Instillation was performed every 45 min, then holding time was set at 5 min and a continuous TNP of 125 mmHg was set. The treatment was performed until the absence of infection of the wound, confirmed by microbiological examination, that was 7 d after starting. Then traditional VAC®
was continued to obtain the complete healing and coverage of prosthesis in 10 d.
VAC Via®
VAC Via® is a new device introduced by KCI for patients affected by wounds that can be treated at home. The device is relative small and set for a period of treatment of 7 d at -125 mmHg of pressure. The canister presents a capacity of 150 mL. According to our experience, we can consider that the device is simple to use and comfortable for the patient. The best indication is a relative small single wounds in a site that does not impair quotidian activities. In our experience in wide wounds or multiple ones, the device was not useful for the impossibility to regulate the pressure in relation to movements. In selected cases it can be very useful to treat patient at home, with minimal impairment in daily life.
Based on the International Expert Panel on Negative Pressure Wound Therapy[75] guidelines we found the grade and the level of evidence reported in Table 1. In the literature significant studies about other kinds of wounds are not reported.
| Indication | Recommendation | Grade | Evidence level |
| Soft tissue trauma | Bridge to definitive closure when primary closure is not possible | C | L2-L3 |
| Stopped when delayed surgical closure is possible | C | L2-L3 | |
| Improve the healing of fasciotomy incisions | C | L2 | |
| Downscale the complexity of closure procedures | C | L2-L3 | |
| Open fractures wounds | Bridge to definitive closure when primary closure is not possible | B | L1-L3-L4 |
| Stopped when delayed surgical closure is possible | B | L1-L3 | |
| Downscale the complexity of closure procedures | C | L2-L3 | |
| Partial thickness burns | Preventing burn wound progression | C | L2-L3 |
| Flap procedures | For flaps which have suffered partial necrosis after debridement of necrotic tissue | D | L3-L4 |
| Manage donor sites which cannot be closed primarily | D | L3 | |
| Graft procedures | Improve the rate of graft success | A | L1-L2 |
| Case at high risk of graft loss | B | L1-L2 | |
| Left undisturbed for 3-7 d post-grafting | B | L1-L2 | |
| Continuous pressure level | B | L1-L2-L3 |
Grade A of evidence is reported only for open abdominal wounds and graft procedures with the goal to improve the success rate. Grade D of evidence is reported in flap procedures with an evidence level 3 or 4. Other indications present an evidence grade between B and C. Considering these evidences, we think that VAC® therapy may be used in different kind of wounds with good results, reported by many studies in literature, even if an evidence based result has not even shown at this time. In our experience, VAC® therapy was useful in case of open abdomen, soft tissue trauma and paediatric patients, reporting good results. Further studies are necessary to define the evidence of VAC® therapy, especially in diabetic foot wounds, pressure ulcers, venous insufficiency ulcers and enteric fistulas. Obviously recent applications should be studied in the future to define the real utility in clinical practice, even if, also in our experience, VAC Instill® and VAC Via® reported good results. Many attempts were made to obtain an international consensus conference to define recommendation for VAC® use in different kind of wound and initial results were just obtained. We believe that further studies and definitive recommendations will underline the indications for VAC® therapy and asses the most useful regimen of treatment for each case.
Peer reviewer: Bruno Sarmento, Professor, Department of Pharmaceutical Sciences, Advanced Health Sciences Institute, R Central de Gandra 1317, 4585-116 Gandra, Portugal
S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Banwell P, Téot L. Topical Negative Pressure (TNP) Therapy. First international topical negative pressure (TNP) therapy focus group meeting proceedings. London: TXP Communications 2004; . |
| 2. | Usupov Y, Yepifanov M. Active wound drainage. Vestnik Khirugii. 1987;4:42-45. |
| 3. | Chariker ME, Jeter KF, Tintle TE, Bottsford JE. Effective management of incisional and cutaneous fistulae with closed suction wound drainage. Contemp Surg. 1989;34:59-63. |
| 4. | Fleischmann W, Strecker W, Bombelli M, Kinzl L. [Vacuum sealing as treatment of soft tissue damage in open fractures]. Unfallchirurg. 1993;96:488-492. [PubMed] |
| 5. | Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1526] [Cited by in RCA: 1442] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 6. | Sumpio BE, Banes AJ, Link WG, Johnson G. Enhanced collagen production by smooth muscle cells during repetitive mechanical stretching. Arch Surg. 1988;123:1233-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 127] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Ilizarov GA. Clinical application of the tension-stress effect for limb lengthening. Clin Orthop Relat Res. 1990;8-26. [PubMed] |
| 8. | Greene AK, Puder M, Roy R, Arsenault D, Kwei S, Moses MA, Orgill DP. Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg. 2006;56:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Gustafsson RI, Sjögren J, Ingemansson R. Deep sternal wound infection: a sternal-sparing technique with vacuum-assisted closure therapy. Ann Thorac Surg. 2003;76:2048-253; discussion 2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Stechmiller JK, Kilpadi DV, Childress B, Schultz GS. Effect of Vacuum-Assisted Closure Therapy on the expression of cytokines and proteases in wound fluid of adults with pressure ulcers. Wound Repair Regen. 2006;14:371-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Banwell P. V.A.C. Therapy Clinical Guidelines: A reference source for clinicians. San Antonio: Kinetic Concepts Inc 2007; . |
| 12. | Krasner DL. Managing wound pain in patients with vacuum-assisted closure devices. Ostomy Wound Manage. 2002;48:38-43. [PubMed] |
| 13. | Harding K, Cutting K, Price P. The cost-effectiveness of wound management protocols of care. Br J Nurs. 2000;9:S6, S8, S10 passim. [PubMed] |
| 14. | Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366:1704-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 573] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 15. | Smith N. The benefits of VAC therapy in the management of pressure ulcers. Br J Nurs. 2004;13:1359-1365. [PubMed] |
| 16. | Schwien T, Gilbert J, Lang C. Pressure ulcer prevalence and the role of negative pressure wound therapy in home health quality outcomes. Ostomy Wound Manage. 2005;51:47-60. [PubMed] |
| 17. | Joseph E, Hamori CA, Bergman S, Roaf E, Swann NF, Anastasi GW. A prospective randomized trial of vacuum-assisted closure versus standard therapy of chronic non-healing wounds. Wounds. 2000;12:60-67. |
| 18. | Hiskett G. Clinical and economic consequences of discharge from hospital with on-going TNP therapy: a pilot study. J Tissue Viability. 2010;19:16-21. [PubMed] |
| 19. | Philbeck TE, Whittington KT, Millsap MH, Briones RB, Wight DG, Schroeder WJ. The clinical and cost effectiveness of externally applied negative pressure wound therapy in the treatment of wounds in home healthcare Medicare patients. Ostomy Wound Manage. 1999;45:41-50. [PubMed] |
| 20. | Barker DE, Kaufman HJ, Smith LA, Ciraulo DL, Richart CL, Burns RP. Vacuum pack technique of temporary abdominal closure: a 7-year experience with 112 patients. J Trauma. 2000;48:201-206; discussion 201-206;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 273] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Banwell PE, Téot L. Topical negative pressure (TNP): the evolution of a novel wound therapy. J Wound Care. 2003;12:22-28. [PubMed] |
| 22. | Saggi BH, Sugerman HJ, Ivatury RR, Bloomfield GL. Abdominal compartment syndrome. J Trauma. 1998;45:597-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 217] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Kaplan M. Managing the open abdomen. Ostomy Wound Manage. 2004;50:C2, 1-8, quiz 1p following 8. [PubMed] |
| 24. | Bovill E, Banwell PE, Teot L, Eriksson E, Song C, Mahoney J, Gustafsson R, Horch R, Deva A, Whitworth I. Topical negative pressure wound therapy: a review of its role and guidelines for its use in the management of acute wounds. Int Wound J. 2008;5:511-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Gustafsson R, Johnsson P, Algotsson L, Blomquist S, Ingemansson R. Vacuum-assisted closure therapy guided by C-reactive protein level in patients with deep sternal wound infection. J Thorac Cardiovasc Surg. 2002;123:895-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Kamolz LP, Andel H, Haslik W, Winter W, Meissl G, Frey M. Use of subatmospheric pressure therapy to prevent burn wound progression in human: first experiences. Burns. 2004;30:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Teot L, Banwell PE, Ziegler UE. Surgery in Wounds. Berlin Heidelberg: Springer Verlag 2004; . |
| 28. | Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24:742-746. [PubMed] |
| 29. | Zannis J, Angobaldo J, Marks M, DeFranzo A, David L, Molnar J, Argenta L. Comparison of fasciotomy wound closures using traditional dressing changes and the vacuum-assisted closure device. Ann Plast Surg. 2009;62:407-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Yang CC, Chang DS, Webb LX. Vacuum-assisted closure for fasciotomy wounds following compartment syndrome of the leg. J Surg Orthop Adv. 2006;15:19-23. [PubMed] |
| 31. | Banwell PE, Musgrave M. Topical negative pressure therapy: mechanisms and indications. Int Wound J. 2004;1:95-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | DeFranzo AJ, Argenta LC, Marks MW, Molnar JA, David LR, Webb LX, Ward WG, Teasdall RG. The use of vacuum-assisted closure therapy for the treatment of lower-extremity wounds with exposed bone. Plast Reconstr Surg. 2001;108:1184-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 287] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 33. | Meara JG, Guo L, Smith JD, Pribaz JJ, Breuing KH, Orgill DP. Vacuum-assisted closure in the treatment of degloving injuries. Ann Plast Surg. 1999;42:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Josty IC, Ramaswamy R, Laing JH. Vaccum assisted closure: an alternative strategy in the management of degloving injuries of the foot. Br J Plast Surg. 2001;54:363-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | DeFranzo AJ, Marks MW, Argenta LC, Genecov DG. Vacuum-assisted closure for the treatment of degloving injuries. Plast Reconstr Surg. 1999;104:2145-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Banwell P, Téot L. Topical negative pressure (TNP): the evolution of a novel wound therapy. J Tissue Viability. 2006;16:16-24. [PubMed] |
| 37. | Heimbach D, Engrav L, Grube B, Marvin J. Burn depth: a review. World J Surg. 1992;16:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 170] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Morykwas MJ, David LR, Schneider AM, Whang C, Jennings DA, Canty C, Parker D, White WL, Argenta LC. Use of subatmospheric pressure to prevent progression of partial-thickness burns in a swine model. J Burn Care Rehabil. 1999;20:15-21. [PubMed] |
| 39. | Scherer LA, Shiver S, Chang M, Meredith JW, Owings JT. The vacuum assisted closure device: a method of securing skin grafts and improving graft survival. Arch Surg. 2002;137:930-933; discussion 930-933;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 187] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 40. | Schneider AM, Morykwas MJ, Argenta LC. A new and reliable method of securing skin grafts to the difficult recipient bed. Plast Reconstr Surg. 1998;102:1195-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Genecov DG, Schneider AM, Morykwas MJ, Parker D, White WL, Argenta LC. A controlled subatmospheric pressure dressing increases the rate of skin graft donor site reepithelialization. Ann Plast Surg. 1998;40:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 107] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Avery C, Pereira J, Moody A, Whitworth I. Clinical experience with the negative pressure wound dressing. Br J Oral Maxillofac Surg. 2000;38:343-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Avery CM, Pereira J, Brown AE. Suprafascial dissection of the radial forearm flap and donor site morbidity. Int J Oral Maxillofac Surg. 2001;30:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care. 2008;31:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 364] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 45. | Expert Working Group. Vacuum assisted closure: recommendations for use. A consensus document. Int Wound J. 2008;5 Suppl 4:iii-i19. [PubMed] |
| 46. | Jeschke MG, Rose C, Angele P, Füchtmeier B, Nerlich MN, Bolder U. Development of new reconstructive techniques: use of Integra in combination with fibrin glue and negative-pressure therapy for reconstruction of acute and chronic wounds. Plast Reconstr Surg. 2004;113:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Moisidis E, Heath T, Boorer C, Ho K, Deva AK. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg. 2004;114:917-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 48. | Gupta S, Baharestani M, Baranoski S, de Leon J, Engel SJ, Mendez-Eastman S, Niezgoda JA, Pompeo MQ. Guidelines for managing pressure ulcers with negative pressure wound therapy. Adv Skin Wound Care. 2004;17 Suppl 2:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State-of-the-art treatment of chronic leg ulcers: A randomized controlled trial comparing vacuum-assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg. 2006;44:1029-1037; discussion 1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 219] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 50. | Baharestani MM. Use of negative pressure wound therapy in the treatment of neonatal and pediatric wounds: a retrospective examination of clinical outcomes. Ostomy Wound Manage. 2007;53:75-85. [PubMed] |
| 51. | Negosanti L, Aceti A, Bianchi T, Corvaglia L, Negosanti F, Sgarzani R, Morselli PG, Cipriani R, Negosanti M, Patrizi A. Adapting a Vacuum Assisted Closure dressing to challenging wounds: negative pressure treatment for perineal necrotizing fasciitis with rectal prolapse in a newborn affected by acute myeloid leukaemia. Eur J Dermatol. 2010;20:501-503. [PubMed] |
| 52. | European Wound Management Association (EWMA). Position document: Management of Wound Infection. London: MEP Ltd 2006; . |
| 53. | McCord SS, Naik-Mathuria BJ, Murphy KM, McLane KM, Gay AN, Bob Basu C, Downey CR, Hollier LH, Olutoye OO. Negative pressure therapy is effective to manage a variety of wounds in infants and children. Wound Repair Regen. 2007;15:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 1208] [Article Influence: 54.9] [Reference Citation Analysis (2)] |
| 55. | Negosanti L, Sgarzani R, Nejad P, Pinto V, Tavaniello B, Palo S, Oranges CM, Fabbri E, Michelina VV, Zannetti G. VAC therapy for wound management in patients with contraindications to surgical treatment. Dermatol Ther. 2012;25:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 56. | Stannard JP, Volgas DA, McGwin G, Stewart RL, Obremskey W, Moore T, Anglen JO. Incisional negative pressure wound therapy after high-risk lower extremity fractures. J Orthop Trauma. 2012;26:37-42. [PubMed] |
| 57. | Atkins BZ, Wooten MK, Kistler J, Hurley K, Hughes GC, Wolfe WG. Does negative pressure wound therapy have a role in preventing poststernotomy wound complications. Surg Innov. 2009;16:140-146. [PubMed] |
| 58. | Gomoll AH, Lin A, Harris MB. Incisional vacuum-assisted closure therapy. J Orthop Trauma. 2006;20:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 59. | Wilson JA, Clark JJ. Obesity: impediment to postsurgical wound healing. Adv Skin Wound Care. 2004;17:426-435. [PubMed] |
| 60. | Riou JP, Cohen JR, Johnson H. Factors influencing wound dehiscence. Am J Surg. 1992;163:324-330. [PubMed] |
| 61. | Abbas SM, Hill AG. Smoking is a major risk factor for wound dehiscence after midline abdominal incision; case-control study. ANZ J Surg. 2009;79:247-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Stannard JP, Robinson JT, Anderson ER, McGwin G, Volgas DA, Alonso JE. Negative pressure wound therapy to treat hematomas and surgical incisions following high-energy trauma. J Trauma. 2006;60:1301-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 63. | Hollenbeak CS, Murphy DM, Koenig S, Woodward RS, Dunagan WC, Fraser VJ. The clinical and economic impact of deep chest surgical site infections following coronary artery bypass graft surgery. Chest. 2000;118:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 181] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 64. | Karra R, McDermott L, Connelly S, Smith P, Sexton DJ, Kaye KS. Risk factors for 1-year mortality after postoperative mediastinitis. J Thorac Cardiovasc Surg. 2006;132:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 65. | Easterlin B, Bromberg W, Linscott J. A novel technique of vacuum assisted wound closure that functions as a delayed primary closure. Wounds. 2007;19:331-333. |
| 66. | Holm C, Petersen JS, Grønboek F, Gottrup F. Effects of occlusive and conventional gauze dressings on incisional healing after abdominal operations. Eur J Surg. 1998;164:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Gummert JF, Barten MJ, Hans C, Kluge M, Doll N, Walther T, Hentschel B, Schmitt DV, Mohr FW, Diegeler A. Mediastinitis and cardiac surgery--an updated risk factor analysis in 10,373 consecutive adult patients. Thorac Cardiovasc Surg. 2002;50:87-91. [PubMed] |
| 68. | Stannard JP, Atkins BZ, O'Malley D, Singh H, Bernstein B, Fahey M, Masden D, Attinger CE. Use of negative pressure therapy on closed surgical incisions: a case series. Ostomy Wound Manage. 2009;55:58-66. [PubMed] |
| 69. | Wolvos T. Wound instillation--the next step in negative pressure wound therapy. Lessons learned from initial experiences. Ostomy Wound Manage. 2004;50:56-66. [PubMed] |
| 70. | Jerome D. Advances in negative pressure wound therapy: the VAC instill. J Wound Ostomy Continence Nurs. 2007;34:191-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 71. | A symposium: wound infection and occlusion--separating fact from fiction. October 2-5, 1992, London, England. Proceedings. Am J Surg. 1994;167:1S-60S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Falanga V. Growth factors and chronic wounds: the need to understand the microenvironment. J Dermatol. 1992;19:667-672. [PubMed] |
| 73. | D'Hondt M, D'Haeninck A, Dedrye L, Penninckx F, Aerts R. Can vacuum-assisted closure and instillation therapy (VAC-Instill therapy) play a role in the treatment of the infected open abdomen. Tech Coloproctol. 2011;15:75-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Gabriel A, Shores J, Heinrich C, Baqai W, Kalina S, Sogioka N, Gupta S. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J. 2008;5:399-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 75. | Krug E, Berg L, Lee C, Hudson D, Birke-Sorensen H, Depoorter M, Dunn R, Jeffery S, Duteille F, Bruhin A. Evidence-based recommendations for the use of Negative Pressure Wound Therapy in traumatic wounds and reconstructive surgery: steps towards an international consensus. Injury. 2011;42 Suppl 1:S1-12. [PubMed] |
| 76. | SIGN 50: a Guideline developer’s Handbook. Guideline Number 50: ISBN19781905813254, Revised Edition January 2008. Available from: http://www.sign.ac.uk/pdf/sign50.pdf. |