Copyright
©The Author(s) 2017.
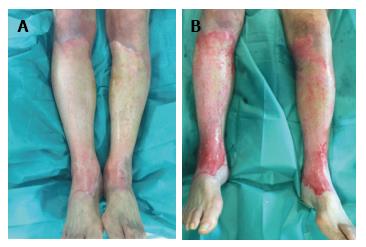
Figure 1 Patient treated with silver sulphadiazine cream for more than 24 h in the pre-Nexobrid® treatment.
A: Patient where silver sulphadiazine cream was applied in the pre-Nexobrid® treatment for more than 24 h; B: After 4 h of exposure to Nexobrid®, the incomplete removal of eschar is shown.
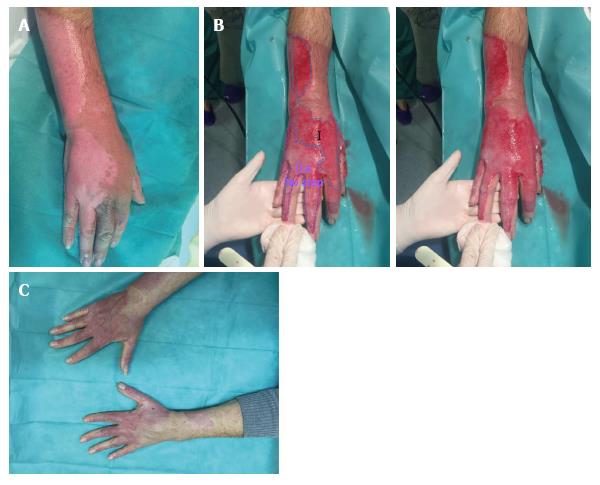
Figure 2 Wound bed Type I and IIa.
A: Burn before Nexobrid® treatment; B: Burn after Nexobrid® treatment with two different wound bed. Type I, with abundance of small diameter pin-point bleeders (uniform shades of red to pink, superficial partial thickness burn). Type IIa, with a sparse pattern of larger diameter bleeders (irregular shades of pink to white colour), not depressed either in relation to the surrounding healthy skin or the own bed surface skin bed (mid/deep partial thickness burn); C: Outcome 38 d post-burn.
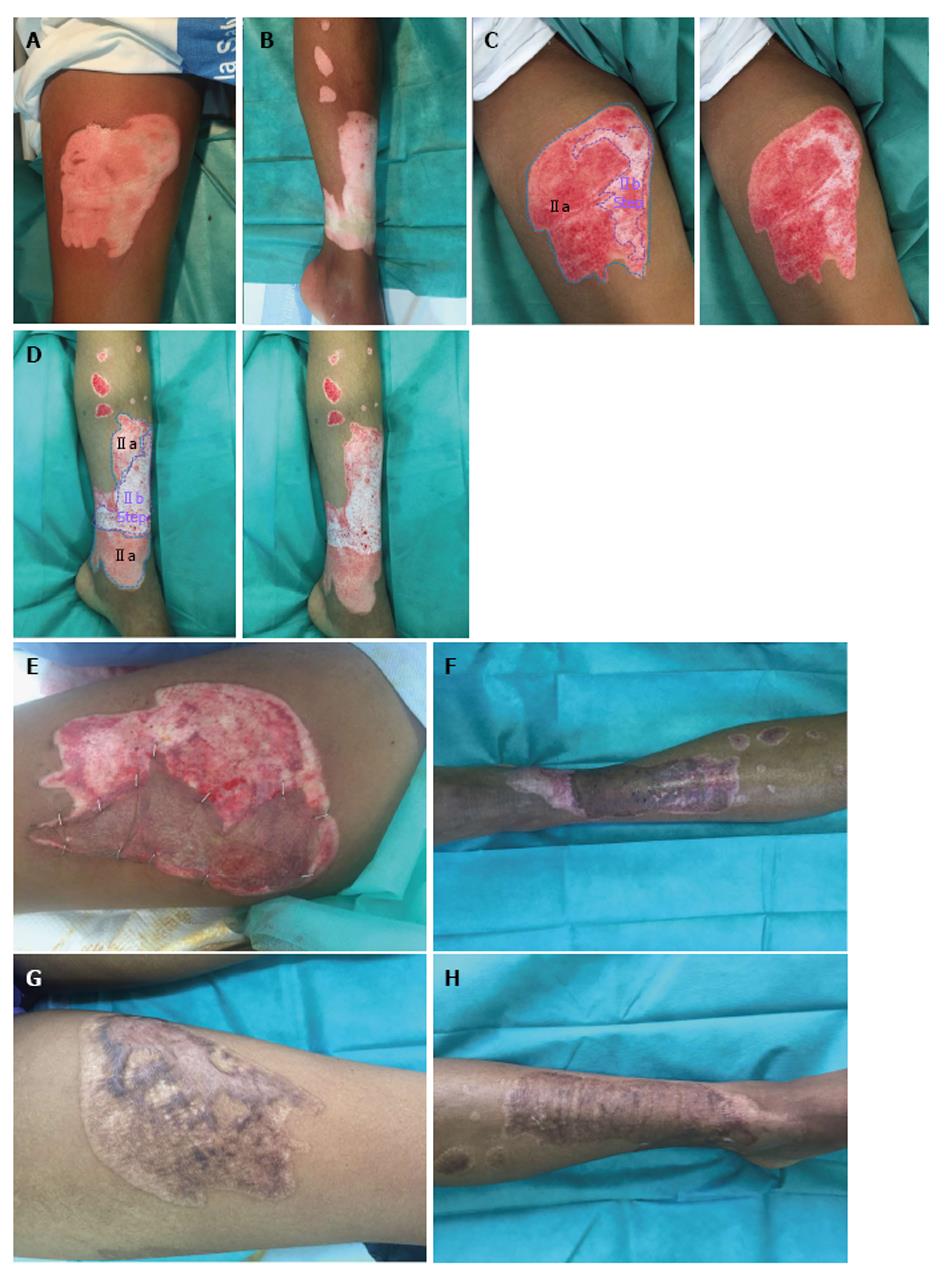
Figure 3 Wound bed IIa and IIb.
A and B: Burn before Nexobrid® treatment; C and D: After Nexobrid® treatment with two different wound bed. Type IIa and Type IIb, sparse pattern of larger diameter bleeders (no shades, white colour) that it is depressed in relation to the surrounding healthy skin, or the own bed surface skin burn (Deep partial thickness burn); E and F: Autografting after two weeks; G and H: Final outcome after 6 mo.
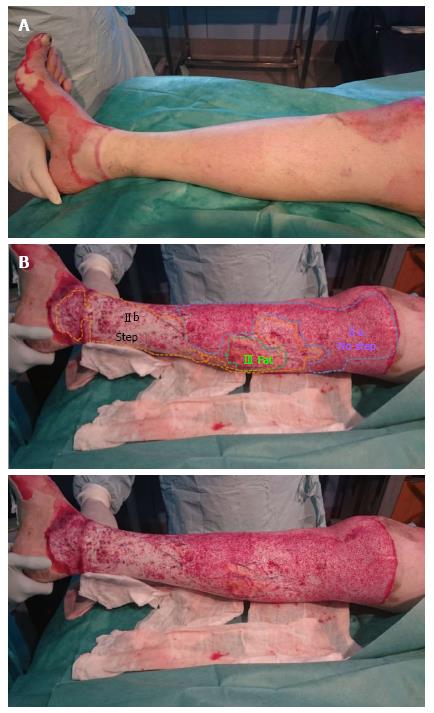
Figure 4 Different wound beds in the same burn.
A: Burn before Nexobrid® treatment; B: After Nexobrid® treatment with three different wound bed. Type IIa, Type IIb and Type III, with a fatty wound bed (full thickness burn)
- Citation: Palao R, Aguilera-Sáez J, Serracanta J, Collado JM, Dos Santos BP, Barret JP. Use of a selective enzymatic debridement agent (Nexobrid®) for wound management: Learning curve. World J Dermatol 2017; 6(2): 32-41
- URL: https://www.wjgnet.com/2218-6190/full/v6/i2/32.htm
- DOI: https://dx.doi.org/10.5314/wjd.v6.i2.32