Published online Mar 27, 2016. doi: 10.5313/wja.v5.i1.15
Peer-review started: June 30, 2015
First decision: September 17, 2015
Revised: November 26, 2015
Accepted: December 13, 2015
Article in press: December 15, 2015
Published online: March 27, 2016
Processing time: 272 Days and 12.9 Hours
Treatment for chronic pain is frequently unsuccessful or characterized by side-effects. The high-frequency repetitive transcranial magnetic stimulation (HF-rTMS) has been suggested in the management of refractory chronic pain. Various studies have shown that HF-rTMS sessions of long-duration applied at primary motor cortex induce pain relief through mechanisms of plastic changes. Efficacy of rTMS mostly depends on stimulation parameters, but this aspect requires better characterization. A rationale to target other cortical areas exists. Current data are promising, but a careful analysis of stimulation settings and maintenance treatment design are need.
Core tip: The high-frequency repetitive transcranial magnetic stimulation (HF-rTMS) is emerging as a possible approach for pain relief. The HF-rTMS delivered to motor cortex modulates brain network implicated in pain processes, facilitating descending pain inhibitory mechainsms. Current data are promising, but a careful analysis of stimulation settings and maintenance treatment design are necessary.
- Citation: Onesti E, Gori MC, Frasca V, Inghilleri M. Transcranial magnetic stimulation as a new tool to control pain perception. World J Anesthesiol 2016; 5(1): 15-27
- URL: https://www.wjgnet.com/2218-6182/full/v5/i1/15.htm
- DOI: https://dx.doi.org/10.5313/wja.v5.i1.15
Chronic pain can be neuropathic, non-neuropathic, mixed, or without demonstrated origin[1]. Whilst acute pain is nociceptive secondary to chemical, mechanical and thermal stimulation of A-delta and C receptors, chronic neuropathic pain (NP) can persist after the initial injury because the nervous system is malfunctioning, becoming the origin of the pain. Examples of NP are trigeminal neuralgia, postherpetic neuralgia, phantom limb pain, monoradiculopathies, complex regional pain syndromes and peripheral neuropathies. The prevalence of NP ranges from 7% to 8%[2-4]. The mechanisms involved in NP are complex and engage both peripheral and central pathophysiologic events. Several NP research studies point to different causal mechanisms including neurogenic inflammation, abnormal ectopic activity in nociceptive nerves, and impaired inhibitory modulation, defining the so-called peripheral and central sensitization[5]. Available treatments provide mainly symptomatic relief, including nonpharmacological, pharmacological, and interventional therapies[6,7]. Unfortunately, the management of NP is not easy because the response to most drugs is not univocal[8,9]. According to recent guidelines, less than 50% of the patients with chronic NP reach symptomatic benefits with drugs[6,10,11].
In this setting, neurostimulation is a promising procedure in the treatment of pain[6,12]. The techniques suggested are: Transcutaneous electrical nerve stimulation, nerve root stimulation, spinal cord stimulation, deep brain stimulation, transcranial direct current stimulation (tDCS), epidural motor cortex stimulation, and repetitive transcranial magnetic stimulation (rTMS)[6].
Specifically, TMS was first introduced in the late 1980s[13]. Initially, rTMS of the motor cortex was used to select patients for chronic stimulation by implanted electrodes[14]. It is a noninvasive method of stimulating cortical motor neurons through the scalp and skull capable of inducing electrical currents and depolarizing neurons in focal brain areas with the use of rapidly changing electromagnetic fields generated by a coil placed over the scalp[15-17]. Since then, several studies used rTMS as an investigational tool and a potential treatment for a variety of neurological and psychiatric disorders. Studies showed that rTMS provided at least partial and transient relief of chronic NP. When applied repetitively, trains of rTMS can modify cortical activity beyond the duration of the stimulation[18]. Three main aspects influence the effect of rTMS: Frequency, intensity, and duration of stimulation. In general, bursts of high-frequency stimulation (≥ 5 Hz) lead to a facilitation of activity in the targeted brain region, whereas continuous low-frequency stimulation (about 1 Hz) provides a suppression in activity of the targeted brain region.
The rTMS produces analgesic effects activating fibres in the motor cortex and projecting to distant areas involved in pain processing[19,20]. In 2007, the EFNS produced the first guidelines on neurostimulation therapy for NP[6]. In recent years, new randomized controlled trials have published in various NP conditions. Therefore, we aimed to review all available evidence for TMS in neuropathic and non-NP, focusing the methods. A narrative synthesis was used to report the results.
A search of literature on the analgesic effect of rTMS in chronic pain published from 1991 to May 2015 was performed using PubMed and the Cochrane Library. Keywords included chronic pain and neurostimulation, chronic pain and transcranial magnetic stimulation, NP and neurostimulation, NP and transcranial magnetic stimulation. The present review included controlled studies with at least 10 subjects enrolled to ensure the quality of the studies. Moreover, we excluded observational studies, and only papers in English were included. To minimize possible bias, the study selection-process was carried out independently by two authors (EO, MI).
We identified 38 controlled studies, including sham stimulations, in patients with NP (spinal cord lesions, central post-stroke pain-CPSP-, trigeminal nerve lesions, peripheral nerve lesions, phantom pain, fibromyalgia and complex regional pain syndrome type II-CRPSII-) or non-NP (migraine, CRPS type I, low back pain, visceral and postoperative pain). Table 1 summarizes these studies. The analysis included 983 patients. Among them, 31 studies showed significant pain reduction with the high-frequency rTMS (HF-rTM) of the motor cortex (Table 1).
| Ref. | Painful syndrome | Study design | Number of patients | Coil | Site stimulation | Frequency, intensity, n sessions | Outcomes | Efficacy |
| NP | ||||||||
| Lefaucheur et al[26] | Intractable neurogenic pain | Double-blind, controlled, crossover | 18 | F8 | Hand M1 | 0.5-10 Hz | Pain intensity | Analgesic effect (only for 10 Hz) |
| (12 central NP; 6 peripheral NP) | 80% RMT | |||||||
| 1 | ||||||||
| Lefaucheur et al[116] | Pain due to thalamic stroke or trigeminal neuropathy | Double-blind, controlled, crossover | 14 | F8 | Hand M1 | 10 Hz | VAS | Decrease in VAS |
| (7 central NP; 7 peripheral NP) | 80% RMT | |||||||
| 1 | ||||||||
| Rollnik et al[41] | Chronic refractory NP | Double-blind, controlled, crossover | 12 | Double coin - Circular coin | M1 | 20 Hz | VAS | No effect |
| (2 central NP; 7 peripheral NP; 2 CRPS; 1 osteomyelitis) | 80% RMT | |||||||
| 1 | ||||||||
| Lefaucheur et al[27] | Pain do to thalamic stroke, brainstem stroke, spinal cord lesion, brachial plexus lesion, or trigeminal nerve lesion | Double-blind, controlled, crossover | 60 | F8 | Hand M1 | 10 Hz | VAS, thermal sensory thresholds | Analgesic effect mainly in trigeminal nerve lesions |
| (36 central NP; 24 peripheral NP) | 80% RMT | |||||||
| 1 | ||||||||
| Khedr et al[25] | Trigeminal neuralgia and post-stroke pain syndrome | Double-blind, controlled | 48 | F8 | Hand M1 | 20 Hz | VAS and the LANSS scale | Analgesic effect |
| (24 central NP; 24 trigeminal NP) | 80% RMT | |||||||
| 5 | ||||||||
| André-Obadia et al[22] | Chronic refractory NP | Double-blind, controlled, crossover | 14 | F8 | Hand M1 | 1-20 Hz | VAS | Analgesic effect (only for 20 Hz) |
| (11 central NP; 3 peripheral NP) | 90% RMT | |||||||
| 1 | ||||||||
| Hirayama et al[104] | Intractable deafferentation pain | Double-blind, controlled, crossover | 20 | F8 | M1 | 5 Hz | VAS and SF-MPQ | Analgesic effect |
| (14 central NP; 6 peripheral NP) | 90% RMT | |||||||
| 1 | ||||||||
| Irlbarcher et al[117] | Chronic NP | Double-blind, controlled | 27 | F8 | M1 | 1-5 Hz | VAS | No effect |
| (13 central NP; 14 phantom p) | 95% RMT | |||||||
| 5 | ||||||||
| Lefaucheur et al[15] | Unilateral hand pain of various neurologic origins | Double-blind, controlled, crossover | 22 | F8 | Hand M1 | 10 Hz | Motor threshold at rest, MEP amplitude, CSP, ICI | ICI increase |
| (14 central NP; 8 peripheral NP) | 90% RMT | |||||||
| 1 | ||||||||
| Lefaucheur et al[118] | Chronic NP | Double-blind, controlled, crossover | 36 | F8 | Face M1 | 10 Hz | VAS | Analgesic effect with the stimulation applied on area adjacent to the cortical representation of the painful zone |
| 80% RMT | ||||||||
| 1 | ||||||||
| Defrin et al[35] | Spinal cord injury | Double-blind, controlled | 12 | F8 | Vertex | 5 Hz | VAS, MPQ, pain threshold | Increased heat pain threshold |
| 115% RMT | ||||||||
| 10 | ||||||||
| Passard et al[29] | Fibromyalgia | Double-blind, controlled | 30 | F8 | M1 | 10 Hz | VAS, MPQ, quality of life (Brief Pain Inventory and the Fibromyalgia Impact Questionnaire) | Decrease in VAS and better quality of life |
| 80% RMT | ||||||||
| 10 | ||||||||
| Saitoh et al[115] | Intractable deafferentation pain | Double-blind, controlled, crossover | 13 | F8 | M1 | 1-5-10 Hz | VAS | Decrease in VAS (only for 5-10 Hz) |
| (9 central NP; 4 peripheral NP) | 90% RMT | |||||||
| 1 | ||||||||
| André-Obadia et al[119] | Chronic NP | Double-blind, randomized, controlled, crossover | 28 | F8 | M1 | 20 Hz | Pain relief, quality of life and rescue drug intake | Analgesic effect |
| 90% RMT | ||||||||
| 1 | ||||||||
| Lefaucheur et al[120] | Chronic refractory NP | Double-blind, controlled, crossover | 46 | F8 | Hand M1 | 10 Hz | Thresholds for thermal and mechanical sensations | Thermal perception improvement |
| (23 central NP; 23 peripheral NP) | 90% RMT | |||||||
| 1 | ||||||||
| Carretero et al[121] | Fibromyalgia | Randomized, single-blinded | 28 | Butterfly coil | DLPFC | 1 Hz | FibroFatigue, Likert pain, HDRS, CBI | No effect |
| 110% RMT | ||||||||
| 20 | ||||||||
| Kang et al[36] | Spinal cord injury | Double-blind, controlled, crossover | 11 | F8 | M1 | 10 Hz | NRS, BPI | No effect |
| 80% RMT | ||||||||
| 5 | ||||||||
| Picarelli et al[33] | CRPS type 1 | Double-blind, controlled | 23 | F8 | M1 | 10 Hz | VAS, MPQ, the SF-36, HDRS | Analgesic effect and improved quality of life |
| 90% RMT | ||||||||
| 10 | ||||||||
| Ahmed et al[108] | Phantom pain | Double-blind, controlled | 27 | F8 | DLPFC | 20 Hz | VAS, LANSS scale | Decrease in VAS and LANSS scale |
| 80% RMT | ||||||||
| 5 | ||||||||
| Mhalla et al[38] | Fibromyalgia | Double-blind, controlled | 40 | F8 | M1 | 10 Hz | Pain intensity over the last 24 h, BPI, quality of life, mood and anxiety, parameters of motor cortical excitability | Analgesic effect |
| 80% RMT | ||||||||
| Short et al[63] | Fibromyalgia | Double-blind, controlled | 20 | F8 | M1 | 14 | BPI, HDRS, Fibromyalgia Impact Questionnaire | Improvement of daily pain, number of tender points, HDRS and FIQ scores |
| 10 Hz | ||||||||
| 120% RMT | ||||||||
| Lefaucheur et al[122] | Chronic refractory NP | Controlled, crossover | 14 | F8 | M1 | 10 | VAS | Analgesic effect |
| (3 localized in the face, 4 upper limb, 3 lower limb, 4 hemibody) | 10 Hz | |||||||
| 90% RMT | ||||||||
| 3 | ||||||||
| Hosomi et al[109] | NP | Double-blind, controlled, crossover | 64 | F8 | M1 | 50 Hz | VAS, SF-MPQ, PGIC, and BDI | Analgesic effect |
| 90% RMT | ||||||||
| 10 | ||||||||
| Onesti et al[28] | Diabetic neuropathy | Double-blind, controlled, crossover | 23 | H-coil | Vertex | 20 Hz | VAS, area and threshold of RIII nociceptive flexion reflex RIII reflex | Decrease in VAS and RIII area |
| 100% RMT | ||||||||
| 5 | ||||||||
| Jetté et al[34] | Spinal cord injury | Randomized, controlled, crossover | 16 | F8 | M1 | 10 Hz | VAS, motor mapping parameters | Decrease in VAS |
| 90%-110% RMT | ||||||||
| 3 | ||||||||
| Boyer et al[30] | Fibromyalgia | Double-blind, randomized, controlled | 38 | F8 | M1 | 10 Hz | FIQ, SF-36, brain metabolism | Improvement of quality of life |
| 90% RMT | ||||||||
| 14 | ||||||||
| Dall’Agnol et al[123] | Myofascial pain syndrome | Double-blind, randomized, controlled | 24 | F8 | M1 | 10 Hz | Pain quantitative sensory testing, conditioned pain modulation, TMS parameters, BDNF | Analgesic effect mediated by mechanisms enhancing the corticospinal inhibitory system and BDNF |
| 80% RMT | ||||||||
| 10 | ||||||||
| Yılmaz et al[40] | Spinal cord injury | Double-blind, randomized, controlled | 17 | F8 | Vertex | 10 Hz | VAS | No effect |
| 110% RMT | ||||||||
| 10 | ||||||||
| Hodaj et al[124] | Chronic refractory facial pain | Open-label study | 55 | F8 | Face M1 | 10 Hz | VAS, CGIC scale | Analgesic effect |
| 80% RMT | ||||||||
| (19 cluster headache; 21 trigeminal neuropathic pain; 15 atypical facial pain) | 12 | |||||||
| Khedr et al[125] | Malignant NP | Randomized, controlled | 34 | F8 | Hand M1 | 20 Hz | VDS, VAS, LANSS, HDRS | Analgesic effect |
| 80% RMT | ||||||||
| 10 | ||||||||
| Lindholm et al[126] | Neuropathic orofacial pain | Randomized, controlled, cross-over | 16 | - | S1/M1, right SII | - | NRS, BPI | Analgesic effect (only for SII) |
| Non-NP | ||||||||
| Brighina et al[46] | Migraine | Double-blind, randomized, controlled | 11 | F8 | DLPFC | 10 Hz | Frequency of attacks, Headache index | Significant reduction of outcome measures |
| 90% RMT | ||||||||
| 12 | ||||||||
| Pleger et al[48] | CRPS | Double-blind, controlled, crossover | 10 | F8 | M1 | 10 Hz | VAS | Analgesic effect |
| 110% RMT | ||||||||
| 1 | ||||||||
| Borckardt et al[127] | Postoperative pain | Double-blind, controlled | 20 | F8 | Left PFC | 10 Hz | VAS for mood, opioid pump use | Reduction in opioid use |
| 100% RMT | ||||||||
| 1 | ||||||||
| Johnson et al[49] | Low back pain | Double-blind, controlled, crossover | 17 | F8 | M1 | 20 Hz | Detection and pain thresholds for cold and heat sensations | Increased heat pain threshold and lowered cold detection |
| 95% RMT | ||||||||
| 1 | ||||||||
| Fregni et al[50] | Pancreatitis | Double-blind, controlled | 17 | F8 | SII | 1 Hz | VAS, BDI | Analgesic effect |
| 70% RMT | ||||||||
| 10 - | ||||||||
| Conforto et al[47] | Migraine | Randomized, double-blind, parallel-group | 18 | - | DLPFC | Number of headache days | No effect | |
| Melchior et al[51] | Irritable bowel syndrome | Double-blind, controlled, crossover | 21 | F8 | M1 | 20 Hz | Pressure pain threshold, changes in maximum tolerated rectal volume, rectal compliance and average pain intensity | Maximun tolerated rectal volume and analgesic effects |
| 80% RMT | ||||||||
| 5 | ||||||||
| Avery et al[52] | Chronic widespread pain | Double-blind, randomized, controlled | 19 | - | DLPFC | - | BIRS | No effect |
| 15 |
Unfortunately, the studies currently available have been performed on groups of patients with different kinds of NP. Evidence at medium follow-up allowing solid conclusions to be drawn is insufficient and conflicting, while evidence at long follow-up is restricted. Future studies on a large number of patients with pain due to specific diseases and the evaluation of maintainance treatment cycles should provide more certain and reproducible data.
Efficacy of rTMS mostly depends on stimulation parameters. When rTMS is applied in the primary motor cortex at low-frequency it is unsuccessful[21-23], while repeated sessions of long-duration (at least 1000 pulses) stimulations at high-frequency (5-20 Hz) applied over repeated sessions induce pain relief[1,24-27]. RTMS seems most effective when stimulation is focal (i.e., figure-of-eight rather than circular coil)[6]. The effect starts a few days later; its duration is less than a week after a single session, 2-3 wk after consecutive sessions of rTMS[28-30]. This last aspect is the keystone for the clinical benefit[31,32]. However, this feature requires better characterization[6]. The TMS parameters vary in the studies, and it is complex to establish the best stimulation parameters to use[12]. The role of coil orientation, time of train of stimulation, inter-train interval, and number of trains, is also to definite[12].
Moreover, 22 of the 32 studies had small sample sizes, with less than 30 enroled patients, and only 16 of 32 studies recruited homogeneous populations of patients (CRPS, spinal cord injury, diabetic polyneuropathy, poststroke pain and fybromialgia), reducing assurance about which states are more responsive to TMS[1,28-30,33-41]. Another unsolved question concerns which site in the motor cortex gives the most effective pain relief. Stimulation is commonly delivered to the contralateral motor cortex to painful area[1,42].
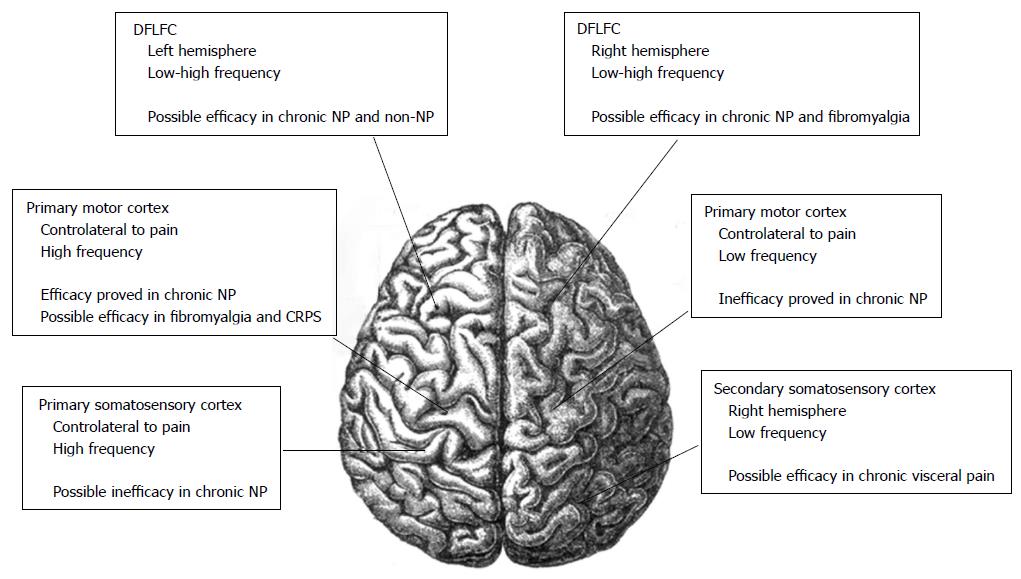
Also the left DLPFC could have a function in nociceptive control, while the left prefrontal cortex has been used in rTMS studies in patients with fibromialgia[39,43] (Figure 1).
Also tDCS, a technique that elicits constant weak electric currents through the scalp throught two electrodes, is able to modulate excitability in cortical tissue. Moreover, it is important to specify that tDCS does not induce action potentials in axons, but it cause polarization of neurons changing their average level of discharge. Several studies examined the tDCS applied to the motor cortex as a possible treatment of chronic pain, but a recent meta-analysis does not suggest a significant analgesic effect of this technique[23].
The mechanisms underlying the effect of rTMS in pain are not clearly identified, but probably involve neuronal plasticity[44,45]. Therefore it is suggested that maintenance therapy for longer intervals should prolong long-lasting effects. Unfortunately, only one study to date evaluated long-term rTMS maintenance therapy[38].
In the past ten years, the rTMS have been also evaluated in different non-NP conditions[1] (Table 1).
Regarding application for migraine, active HF-rTMS delivered over the left DLPFC gave promising results but, in the absence of large controlled studies, no recommendation can be suggested[1,46,47]. Also regarding to the treatment of chronic visceral pain, low back pain and CRPS type I with rTMS, literature is still limited, and no conclusion can be definitely drawn[48-52]. Future research in this field should specifically investigate in a large number of patients the most appropriate cortical target, and the frequency of stimulation[1]. Moreover, a specific analysis regarding to the possible effect of rTMS on other clinical aspects of these syndromes, such as affective-emotional and cognitive components is needed.
In 1985 Barker et al[13] proposed the first magnetic stimulator for the transcranial stimulation of the human brain, giving the prerequisite for subsequent clinical use of TMS. A stimulating coil produces a brief magnetic field when an electrical pulse generator creates a discharge current of several thousand amperes. When the coil is placed on the skull of a subject, it induces an electrical field able to depolarize nerve cells and to stimulate neural networks[1]. The stimulus waveform can be monophasic or biphasic[53]. The rTMS using monophasic pulses activates an homogeneous population of neurons, while biphasic pulses tend to generate a more complex pattern of neural activation, producing local changes but also effects at distance from the stimulus site[1,54].
The first task for pain modulation is to locate primary motor cortex (M1), checking visually the muscle twitch inducing by TMS pulses[12]. Commonly in clinical settings, the intensity of the TMS should be not able to induce a motor response[12]. Specifically, TMS applied in short trains at high frequency and suprathreshold intensity over the M1 elicits a progressive increase in motor evoked potential (MEP) amplitude, demonstrating the phenomenon of MEP amplitude facilitation, through intracortical mechanisms similar to short-term synaptic plasticity[55-59].
However, a rationale for targeting other cortical areas exists. The DLPFC could have a role in nociceptive control[43]. In healthy subjects with pain induced by a capsaicin injection into their hand, the stimulation of the left DLPFC produced a significant pain relief. No improvement was noted when the right DLPFC was stimulated[60]. The effect may be related to the release of endogenous opioids by the left DLPFC[61]. Also rTMS of the cerebellum has been considered for the possible lowering in pain thresholds[62]. Moreover, The left prefrontal cortex has been used in rTMS fibromyalgia studies, but only a small analgesic effect has been noted[63].
The intensity of the stimulation is classically regulated for each patient to obtain the minimal intensity of stimulation applied to M1 that evokes a motor response. It is measured according to the RMT, the lowest stimulation intensity able to generate a MEP small (50 mV) amplitude in 5 of 10 TMS pulses. In the clinical setting, stimulation intensity is frequently subthreshold (80%-90% of RMT)[64]. When the RMT is identified, rTMS is performed in bursts of stimuli (“trains”) with a definite frequency[12].
RTMS can be carried out at low (1 Hz) or high frequencies (5 Hz). When performed at high frequencies, rTMS pulses are delivered in trains divided by specific intertrain intervals. Typically, low-frequency rTMS is considered to have inhibitory properties, whereas HF-rTMS is considered to have excitatory properties[64]. HF-rTMS consists specifically of intermittent bursts of TMS pulses able to induce a long-term potentiation of synaptic activiy, which may clarify why rTMS effects can overcome the period of stimulation[64].
A central question is whether the analgesic effect of rTMS can be prolonged by maintenance sessions performed periodically. To date, 24 of 39 studies have performed repetitive sessions of rTMS to enhance analgesic effects of a single session of stimulation, but maintenance protocol was only tested in one study[38]. Usually, the number of sessions applied range from 5 sessions to 30 sessions. The majority of studies have involved a total of 10 sessions. Based on more recent studies, a general trend indicates a greater number of sessions (> 10) associated with more persisting improvement in pain perception (Table 1).
The total number of pulses in each rTMS session seems related to the analgesic effect, but it is not clear whether a minimum number of pulses is required to obtain the clinical outcome. Usually this value ranges between 1000 to 2000[1]. Moreover an important safety parameter as the intertrain interval (the time in between trains of pulsed energy when no stimulation is occurring) is usually about 10 s[1].
Coil design and orientation are important. The “figure-of-eight” coil is able to induce a focal magnetic field stimulating only superficial cortical regions of the brain[12]. Other novel models are the Tilted double-coil and the Hesed (H)-coil, which drop at a depth of about 6 cm[12]. Specifically, the H-coil lets deep brain stimulation without significantly increasing induced fields in superficial cortical regions, therefore preventing the risk of adverse effects[65,66]. rTMS with the H-coil has already proved effective as an acute treatment for major depressive disorder, bipolar depression, schizophrenia and post-traumatic stress disorder[65,67-69]. Furthermore, there are ongoing studies of its use to treat a very wide range of neurological, psychiatric and medical conditions, including NP[28].
Placebo effects need to be better reported[25]. Theorically, ideal placebo rTMS should be characterized by the same subjective somatic scalp sensation and the acoustic artifacts compared to active coil, and no physiological effect on the targeted cortical region[70]. In the early research, placebo was considered a coil placed in a different area from zone stimulated in the active condition, or a coil oriented with an angle of 45-90 grades on the scalp instead of tangentially[1]. These solutions are not the most reliable, because the stimulation site could be perceived by the subject, or the sham location could cause unexpected effects[1,71]. In the last decades, sham coils have been projected and commercialized in order to block the magnetic field provided, and to produce auditory artifacts and scalp sensation equivalent to that of a real coil[72,73]. Although this stimulation ideally seems a perfect placebo, the cutaneous sensation remains different in about half of the cases, especially when the stimulation intensity is high[72,74].
Although the TMS acts on the superficial cortex, the generated action potentials propagate influencing distant neural networks[12]. The M1 contains pyramidal cells that give rise to numerous excitatory corticospinal projections. Most of These projections are oriented perpendicularly to the brain surface. rTMS applycated on the M1 modulate the cortical excitability producing changes in the following physiological parameters: MT, MEP, silent period, intracortical facilitation, and intracortical inhibition[75]. In chronic pain, the involvement of M1 projections to pain-modulating structures has been demonstrated[23]. Moreover, a rationale for targeting other cortical areas exists. The DLPFC is a cortical target used in studies on major depression, and it is considered to have a function also in nociceptive control[43,61,76]. HF-rTMS on the right DLPFC has shown analgesic effects similar to M1 stimulation[46,77]. Furthermore, left DLPFC stimulation should induce an improvement of pain perception in a model of acute pain[43,78]. The left prefrontal cortex has been used in rTMS studies in patients with fibromyalgia, but it has shown a minor analgesic effect[63].
rTMS seems to modulate cortical plasticity, referred to as the functional reorganization of the inter neuron connections and neuronal properties. Inhibition of the gamma-amminobutyric acid (GABA) pathways produces cortical excitation, rather than a direct enhancement of motor cortex excitability[79-82]. On the other hand, low-frequency rTMS could increase the inhibitory corticospinal control, perhaps through GABA-B transmission, prolonging the CSP duration[1,83-86]. The changes in synaptic plasticity brought by rTMS are explained by long term potentiation (LTP) and long term depression (LTD)[44]. LTP is induced by high frequency stimulation and LTD by low frequency stimulation. The LTP is mediated by the post-synaptic N-methyl-D-aspartate (NMDA) receptors, that lead to calcium flux into the post-synaptic neuron when activated[45]. Calcium activates enzymatic changes in pre- and post-synaptic neurons, increasing the synaptic activity. It also induces the expression of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors on the postsynaptic neuron, increasing the cells sensitivity to glutamate[87]. Furthermore, LTD is characterized by depression of the synaptic transmission, depending on the modulation of NMDA receptors with the reduction of calcium influx, and the internalization of AMPA[87].
The long lasting effect of rTMS (late-LTP) is thought to be exercised by gene induction and protein synthesis[87]. Gene expression has resulted in increased synthesis of c-fos mRNA in the thalamus and parietal cortex, and BDNF mRNA in the hippocampus and parietal cortex[88-90]. Considerable evidence from HF-rTMS studies suggests that short-term synaptic plasticity happens at cortical rather than spinal level[91-94]. When rTMS is delivered in human subjects, the amplitude of the MEP and the duration of the CSP increases during the train[55,75,95-100]. The MEP facilitation also persists after the train ends, and it is probably due to the recruitment of cortical excitatory interneurons[55,75,92,94,99]. It is influenced by the number of stimuli in the train, being greater with longer (20, 40 and 60-stimuli), suggesting mechanisms of short-term synaptic enhancement[59,101].
rTMS has also been found to modulate the activity of brain neurotransmitters, reducing dopamine in the frontal cortex and increasing its levels in the striatum[102]. Moreover, serotonin levels increased in the hippocampus[102]. All these aspects may explain why different rTMS protocols are effective or not, depending on various parameters of stimulation. Further, age, and genetic features could influence the clinical effect of rTMS, with heterogeneous therapeutic responses[1,103].
The results of studies exploring the effects of rTMS on pain are positive but still inconsistent, because of small samples of patients, differences in the TMS methodologies, heterogeneous populations of patients and lack of maintenance protocols. In a Cochrane Review of 2013, a short-term effect on pain of HF-rTMS applied to M1 was confirmed[23]. Moreover, a detailed study to determine which are the best stimulation parameters, is targeted. Studies on image-guided navigation to perform rTMS of M1 in pain patients have provided evidence that the analgesic effect of rTMS links with the integrity of the thalamocortical tract[1,104,105]. Unfortunately, objective indicators of perceived pain, including MEP and RIII, were considered in only two studies neurophysiological[15,28]. New extended studies should improve knowledge in this field of research.
Further rigorously designed studies, particularly of longer courses of stimulation applied on large population of patients, are required to address the issue. Future evidence may significantly confirm the current results. The main question is whether the clinical effect could indeed improve the management of patients with chronic pain in daily clinical practice.
The conclusions of our analysis, related to the actual literature data on rTMS for chronic pain, match with those suggested in previous reviews and meta-analyses[1,6,17,23-25,32,106]. rTMS has become a promising therapeutic tool for a variety of neurological and psychiatric diseases[107]. Different types of NP respond to rTMS, and this is producing a fast growth in researchers interested in rTMS for clinical purposes[6,26,108-110]. Unfortunately at the current time in the lack of large studies, only careful recommendations of rTMS can be suggested[1,6]. The efficacy of a single HF-rTMS session persists for some days, and it could extend with the repetition of sessions[1]. Moreover, the best stimulation settings may be yet to determined.
Studies including neurophysiological evaluation of the effects of the cortex TMS in other brain regions through the use of imaging and electrophysiologic techniques (such as electroencephalography, magnetoencephalography, MRI navigated TMS) could add value at the understanding of the mechanism of action of this technique[111]. New TMS machines have allowed the administration of pulses more focally and at higher frequencies. Moreover, frameless stereotactic systems, have been developed, permitting the identification of specific location in the desired brain target and the precise and comparable placing of the coil during different TMS sessions[112-114].
In future, therapeutic studies need to define the correct utilization of rTMS in the clinical practice for chronic pain, above all if the long-term effect exists. Moreover, studies of rTMS in other diseases associated with chronic pain, such as osteoarthritis, bladder pain syndrome and post-stroke pain, could be of interest. Finally, if rTMS becomes a proven method for the treatment of chronic pain, the development of a home-based rTMS system will be necessary[115].
Active research in pain is still taking place and has the potential to provide useful data (31 open studies on TMS and pain on https://clinicaltrials.gov). Based on this new research, novel therapeutic guidelines may be established in future. Apart from its potential clinical role, rTMS is a valuable probe of brain function that can be used to investigate the neural circuitry. This additional knowledge might help in the development of new treatments. rTMS is non-invasive and can be applied to any patient with drug-resistant NP who could be aspirant for the insertion of a cortical stimulator. In addition, further studies using maintenance sessions of rTMS and evaluating the multiple features of chronic pain are needed to give a more solid basis for its clinical applications.
The authors thank Ms Mali Sion Evans for English language editing.
P- Reviewer: Khashayar P, Saade NE S- Editor: Song XX L- Editor: A E- Editor: Jiao XK
| 1. | Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125:2150-2206. [PubMed] [DOI] [Full Text] |
| 2. | Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630-1635. [PubMed] [DOI] [Full Text] |
| 3. | Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380-387. [PubMed] [DOI] [Full Text] |
| 4. | Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006;7:281-289. [PubMed] [DOI] [Full Text] |
| 5. | Truini A, Cruccu G. Pathophysiological mechanisms of neuropathic pain. Neurol Sci. 2006;27 Suppl 2:S179-S182. [PubMed] [DOI] [Full Text] |
| 6. | Cruccu G, Aziz TZ, Garcia-Larrea L, Hansson P, Jensen TS, Lefaucheur JP, Simpson BA, Taylor RS. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neurol. 2007;14:952-970. [PubMed] [DOI] [Full Text] |
| 7. | Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, Nurmikko T. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113-1e88. [PubMed] [DOI] [Full Text] |
| 8. | Attal N, Lanteri-Minet M, Laurent B, Fermanian J, Bouhassira D. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain. 2011;152:2836-2843. [PubMed] [DOI] [Full Text] |
| 9. | Torrance N, Ferguson JA, Afolabi E, Bennett MI, Serpell MG, Dunn KM, Smith BH. Neuropathic pain in the community: more under-treated than refractory? Pain. 2013;154:690-699. [PubMed] [DOI] [Full Text] |
| 10. | Attal N, Cruccu G, Haanpaa M, Hansson P, Jensen TS, Nurmikko T, Sampaio C, Sindrup S, Wiffen P; EFNS Task Force. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13:1153-1169. [PubMed] [DOI] [Full Text] |
| 11. | Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118:289-305. [PubMed] [DOI] [Full Text] |
| 12. | Treister R, Lang M, Klein MM, Oaklander AL. Non-invasive Transcranial Magnetic Stimulation (TMS) of the Motor Cortex for Neuropathic Pain-At the Tipping Point? Rambam Maimonides Med J. 2013;4:e0023. [PubMed] [DOI] [Full Text] |
| 13. | Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106-1107. [PubMed] [DOI] [Full Text] |
| 14. | Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien). 1991;52:137-139. [PubMed] [DOI] [Full Text] |
| 15. | Lefaucheur JP, Drouot X, Ménard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology. 2006;67:1568-1574. [PubMed] [DOI] [Full Text] |
| 16. | Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, Wagner T, Fecteau S, Rigonatti SP, Riberto M. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115-2122. [PubMed] [DOI] [Full Text] |
| 17. | Leo RJ, Latif T. Repetitive transcranial magnetic stimulation (rTMS) in experimentally induced and chronic neuropathic pain: a review. J Pain. 2007;8:453-459. [PubMed] [DOI] [Full Text] |
| 18. | Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Exp Physiol. 1991;76:159-200. [PubMed] [DOI] [Full Text] |
| 19. | Lefaucheur JP. The use of repetitive transcranial magnetic stimulation (rTMS) in chronic neuropathic pain. Neurophysiol Clin. 2006;36:117-124. [PubMed] [DOI] [Full Text] |
| 20. | Gilhus NE, Barnes MR, Brainin M. European Handbook of Neurological Management. John Wiley & Sons, 2011. . |
| 21. | Lefaucheur JP, Antal A, Ahdab R, Ciampi de Andrade D, Fregni F, Khedr EM, Nitsche M, Paulus W. The use of repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) to relieve pain. Brain Stimul. 2008;1:337-344. [PubMed] [DOI] [Full Text] |
| 22. | André-Obadia N, Peyron R, Mertens P, Mauguière F, Laurent B, Garcia-Larrea L. Transcranial magnetic stimulation for pain control. Double-blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. Clin Neurophysiol. 2006;117:1536-1544. [PubMed] [DOI] [Full Text] |
| 23. | O’Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2014;4:CD008208. [PubMed] [DOI] [Full Text] |
| 24. | Leung A, Donohue M, Xu R, Lee R, Lefaucheur JP, Khedr EM, Saitoh Y, André-Obadia N, Rollnik J, Wallace M. rTMS for suppressing neuropathic pain: a meta-analysis. J Pain. 2009;10:1205-1216. [PubMed] [DOI] [Full Text] |
| 25. | Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry. 2005;76:833-838. [PubMed] [DOI] [Full Text] |
| 26. | Lefaucheur JP, Drouot X, Keravel Y, Nguyen JP. Pain relief induced by repetitive transcranial magnetic stimulation of precentral cortex. Neuroreport. 2001;12:2963-2965. [PubMed] [DOI] [Full Text] |
| 27. | Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Zerah F, Bendib B, Cesaro P, Keravel Y, Nguyen JP. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J Neurol Neurosurg Psychiatry. 2004;75:612-616. [PubMed] [DOI] [Full Text] |
| 28. | Onesti E, Gabriele M, Cambieri C, Ceccanti M, Raccah R, Di Stefano G, Biasiotta A, Truini A, Zangen A, Inghilleri M. H-coil repetitive transcranial magnetic stimulation for pain relief in patients with diabetic neuropathy. Eur J Pain. 2013;17:1347-1356. [PubMed] [DOI] [Full Text] |
| 29. | Passard A, Attal N, Benadhira R, Brasseur L, Saba G, Sichere P, Perrot S, Januel D, Bouhassira D. Effects of unilateral repetitive transcranial magnetic stimulation of the motor cortex on chronic widespread pain in fibromyalgia. Brain. 2007;130:2661-2670. [PubMed] [DOI] [Full Text] |
| 30. | Boyer L, Dousset A, Roussel P, Dossetto N, Cammilleri S, Piano V, Khalfa S, Mundler O, Donnet A, Guedj E. rTMS in fibromyalgia: a randomized trial evaluating QoL and its brain metabolic substrate. Neurology. 2014;82:1231-1238. [PubMed] [DOI] [Full Text] |
| 31. | Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clin Neurophysiol. 2001;112:1367-1377. [PubMed] [DOI] [Full Text] |
| 32. | Galhardoni R, Correia GS, Araujo H, Yeng LT, Fernandes DT, Kaziyama HH, Marcolin MA, Bouhassira D, Teixeira MJ, de Andrade DC. Repetitive transcranial magnetic stimulation in chronic pain: a review of the literature. Arch Phys Med Rehabil. 2015;96:S156-S172. [PubMed] [DOI] [Full Text] |
| 33. | Picarelli H, Teixeira MJ, de Andrade DC, Myczkowski ML, Luvisotto TB, Yeng LT, Fonoff ET, Pridmore S, Marcolin MA. Repetitive transcranial magnetic stimulation is efficacious as an add-on to pharmacological therapy in complex regional pain syndrome (CRPS) type I. J Pain. 2010;11:1203-1210. [PubMed] [DOI] [Full Text] |
| 34. | Jetté F, Côté I, Meziane HB, Mercier C. Effect of single-session repetitive transcranial magnetic stimulation applied over the hand versus leg motor area on pain after spinal cord injury. Neurorehabil Neural Repair. 2013;27:636-643. [PubMed] [DOI] [Full Text] |
| 35. | Defrin R, Grunhaus L, Zamir D, Zeilig G. The effect of a series of repetitive transcranial magnetic stimulations of the motor cortex on central pain after spinal cord injury. Arch Phys Med Rehabil. 2007;88:1574-1580. [PubMed] [DOI] [Full Text] |
| 36. | Kang BS, Shin HI, Bang MS. Effect of repetitive transcranial magnetic stimulation over the hand motor cortical area on central pain after spinal cord injury. Arch Phys Med Rehabil. 2009;90:1766-1771. [PubMed] [DOI] [Full Text] |
| 37. | Matsumura Y, Hirayama T, Yamamoto T. Comparison between pharmacologic evaluation and repetitive transcranial magnetic stimulation-induced analgesia in poststroke pain patients. Neuromodulation. 2013;16:349-354; discussion 354. [PubMed] [DOI] [Full Text] |
| 38. | Mhalla A, Baudic S, Ciampi de Andrade D, Gautron M, Perrot S, Teixeira MJ, Attal N, Bouhassira D. Long-term maintenance of the analgesic effects of transcranial magnetic stimulation in fibromyalgia. Pain. 2011;152:1478-1485. [PubMed] [DOI] [Full Text] |
| 39. | Lee SJ, Kim DY, Chun MH, Kim YG. The effect of repetitive transcranial magnetic stimulation on fibromyalgia: a randomized sham-controlled trial with 1-mo follow-up. Am J Phys Med Rehabil. 2012;91:1077-1085. [PubMed] [DOI] [Full Text] |
| 40. | Yılmaz B, Kesikburun S, Yaşar E, Tan AK. The effect of repetitive transcranial magnetic stimulation on refractory neuropathic pain in spinal cord injury. J Spinal Cord Med. 2014;37:397-400. [PubMed] [DOI] [Full Text] |
| 41. | Rollnik JD, Wüstefeld S, Däuper J, Karst M, Fink M, Kossev A, Dengler R. Repetitive transcranial magnetic stimulation for the treatment of chronic pain - a pilot study. Eur Neurol. 2002;48:6-10. [PubMed] [DOI] [Full Text] |
| 42. | Nizard J, Lefaucheur JP, Helbert M, de Chauvigny E, Nguyen JP. Non-invasive stimulation therapies for the treatment of refractory pain. Discov Med. 2012;14:21-31. [PubMed] |
| 43. | Fierro B, De Tommaso M, Giglia F, Giglia G, Palermo A, Brighina F. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) during capsaicin-induced pain: modulatory effects on motor cortex excitability. Exp Brain Res. 2010;203:31-38. [PubMed] [DOI] [Full Text] |
| 44. | Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187-199. [PubMed] [DOI] [Full Text] |
| 45. | Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129:1659-1673. [PubMed] [DOI] [Full Text] |
| 46. | Brighina F, Piazza A, Vitello G, Aloisio A, Palermo A, Daniele O, Fierro B. rTMS of the prefrontal cortex in the treatment of chronic migraine: a pilot study. J Neurol Sci. 2004;227:67-71. [PubMed] [DOI] [Full Text] |
| 47. | Conforto AB, Amaro E, Gonçalves AL, Mercante JP, Guendler VZ, Ferreira JR, Kirschner CC, Peres MF. Randomized, proof-of-principle clinical trial of active transcranial magnetic stimulation in chronic migraine. Cephalalgia. 2014;34:464-472. [PubMed] [DOI] [Full Text] |
| 48. | Pleger B, Janssen F, Schwenkreis P, Völker B, Maier C, Tegenthoff M. Repetitive transcranial magnetic stimulation of the motor cortex attenuates pain perception in complex regional pain syndrome type I. Neurosci Lett. 2004;356:87-90. [PubMed] [DOI] [Full Text] |
| 49. | Johnson S, Summers J, Pridmore S. Changes to somatosensory detection and pain thresholds following high frequency repetitive TMS of the motor cortex in individuals suffering from chronic pain. Pain. 2006;123:187-192. [PubMed] [DOI] [Full Text] |
| 50. | Fregni F, Potvin K, Dasilva D, Wang X, Lenkinski RE, Freedman SD, Pascual-Leone A. Clinical effects and brain metabolic correlates in non-invasive cortical neuromodulation for visceral pain. Eur J Pain. 2011;15:53-60. [PubMed] [DOI] [Full Text] |
| 51. | Melchior C, Gourcerol G, Chastan N, Verin E, Menard JF, Ducrotte P, Leroi AM. Effect of transcranial magnetic stimulation on rectal sensitivity in irritable bowel syndrome: a randomized, placebo-controlled pilot study. Colorectal Dis. 2014;16:O104-O111. [PubMed] [DOI] [Full Text] |
| 52. | Avery DH, Zarkowski P, Krashin D, Rho WK, Wajdik C, Joesch JM, Haynor DR, Buchwald D, Roy-Byrne P. Transcranial magnetic stimulation in the treatment of chronic widespread pain: a randomized controlled study. J ECT. 2015;31:57-66. [PubMed] [DOI] [Full Text] |
| 53. | Sommer M, Alfaro A, Rummel M, Speck S, Lang N, Tings T, Paulus W. Half sine, monophasic and biphasic transcranial magnetic stimulation of the human motor cortex. Clin Neurophysiol. 2006;117:838-844. [PubMed] [DOI] [Full Text] |
| 54. | Arai N, Okabe S, Furubayashi T, Terao Y, Yuasa K, Ugawa Y. Comparison between short train, monophasic and biphasic repetitive transcranial magnetic stimulation (rTMS) of the human motor cortex. Clin Neurophysiol. 2005;116:605-613. [PubMed] [DOI] [Full Text] |
| 55. | Di Lazzaro V, Oliviero A, Berardelli A, Mazzone P, Insola A, Pilato F, Saturno E, Dileone M, Tonali PA, Rothwell JC. Direct demonstration of the effects of repetitive transcranial magnetic stimulation on the excitability of the human motor cortex. Exp Brain Res. 2002;144:549-553. [PubMed] [DOI] [Full Text] |
| 56. | Berardelli A, Inghilleri M, Cruccu G, Manfredi M. Descending volley after electrical and magnetic transcranial stimulation in man. Neurosci Lett. 1990;112:54-58. [PubMed] [DOI] [Full Text] |
| 57. | Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255-266. [PubMed] [DOI] [Full Text] |
| 58. | Inghilleri M, Berardelli A, Cruccu G, Priori A, Manfredi M. Corticospinal potentials after transcranial stimulation in humans. J Neurol Neurosurg Psychiatry. 1989;52:970-974. [PubMed] [DOI] [Full Text] |
| 59. | Gilio F, Conte A, Vanacore N, Frasca V, Inghilleri M, Berardelli A. Excitatory and inhibitory after-effects after repetitive magnetic transcranial stimulation (rTMS) in normal subjects. Exp Brain Res. 2007;176:588-593. [PubMed] [DOI] [Full Text] |
| 60. | Brighina F, De Tommaso M, Giglia F, Scalia S, Cosentino G, Puma A, Panetta M, Giglia G, Fierro B. Modulation of pain perception by transcranial magnetic stimulation of left prefrontal cortex. J Headache Pain. 2011;12:185-191. [PubMed] [DOI] [Full Text] |
| 61. | Taylor JJ, Borckardt JJ, George MS. Endogenous opioids mediate left dorsolateral prefrontal cortex rTMS-induced analgesia. Pain. 2012;153:1219-1225. [PubMed] [DOI] [Full Text] |
| 62. | Zunhammer M, Busch V, Griesbach F, Landgrebe M, Hajak G, Langguth B. rTMS over the cerebellum modulates temperature detection and pain thresholds through peripheral mechanisms. Brain Stimul. 2011;4:210-217.e1. [PubMed] [DOI] [Full Text] |
| 63. | Short EB, Borckardt JJ, Anderson BS, Frohman H, Beam W, Reeves ST, George MS. Ten sessions of adjunctive left prefrontal rTMS significantly reduces fibromyalgia pain: a randomized, controlled pilot study. Pain. 2011;152:2477-2484. [PubMed] [DOI] [Full Text] |
| 64. | Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Cañete C, Catalá MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:333-343. [PubMed] [DOI] [Full Text] |
| 65. | Zangen A, Roth Y, Voller B, Hallett M. Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin Neurophysiol. 2005;116:775-779. [PubMed] [DOI] [Full Text] |
| 66. | Roth Y, Amir A, Levkovitz Y, Zangen A. Three-dimensional distribution of the electric field induced in the brain by transcranial magnetic stimulation using figure-8 and deep H-coils. J Clin Neurophysiol. 2007;24:31-38. [PubMed] [DOI] [Full Text] |
| 67. | Kranz G, Shamim EA, Lin PT, Kranz GS, Hallett M. Transcranial magnetic brain stimulation modulates blepharospasm: a randomized controlled study. Neurology. 2010;75:1465-1471. [PubMed] [DOI] [Full Text] |
| 68. | Harel EV, Zangen A, Roth Y, Reti I, Braw Y, Levkovitz Y. H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World J Biol Psychiatry. 2011;12:119-126. [PubMed] [DOI] [Full Text] |
| 69. | Harel EV, Rabany L, Deutsch L, Bloch Y, Zangen A, Levkovitz Y. H-coil repetitive transcranial magnetic stimulation for treatment resistant major depressive disorder: An 18-week continuation safety and feasibility study. World J Biol Psychiatry. 2014;15:298-306. [PubMed] [DOI] [Full Text] |
| 70. | Loo CK, Taylor JL, Gandevia SC, McDarmont BN, Mitchell PB, Sachdev PS. Transcranial magnetic stimulation (TMS) in controlled treatment studies: are some “sham” forms active? Biol Psychiatry. 2000;47:325-331. [PubMed] [DOI] [Full Text] |
| 71. | Lisanby SH, Gutman D, Luber B, Schroeder C, Sackeim HA. Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biol Psychiatry. 2001;49:460-463. [PubMed] [DOI] [Full Text] |
| 72. | Rossi S, Ferro M, Cincotta M, Ulivelli M, Bartalini S, Miniussi C, Giovannelli F, Passero S. A real electro-magnetic placebo (REMP) device for sham transcranial magnetic stimulation (TMS). Clin Neurophysiol. 2007;118:709-716. [PubMed] [DOI] [Full Text] |
| 73. | Mennemeier M, Triggs W, Chelette K, Woods A, Kimbrell T, Dornhoffer J. Sham Transcranial Magnetic Stimulation Using Electrical Stimulation of the Scalp. Brain Stimul. 2009;2:168-173. [PubMed] [DOI] [Full Text] |
| 74. | Arana AB, Borckardt JJ, Ricci R, Anderson B, Li X, Linder KJ, Long J, Sackeim HA, George MS. Focal electrical stimulation as a sham control for repetitive transcranial magnetic stimulation: Does it truly mimic the cutaneous sensation and pain of active prefrontal repetitive transcranial magnetic stimulation? Brain Stimul. 2008;1:44-51. [PubMed] [DOI] [Full Text] |
| 75. | Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847-858. [PubMed] [DOI] [Full Text] |
| 76. | Rumi DO, Gattaz WF, Rigonatti SP, Rosa MA, Fregni F, Rosa MO, Mansur C, Myczkowski ML, Moreno RA, Marcolin MA. Transcranial magnetic stimulation accelerates the antidepressant effect of amitriptyline in severe depression: a double-blind placebo-controlled study. Biol Psychiatry. 2005;57:162-166. [PubMed] [DOI] [Full Text] |
| 77. | de Andrade DC, Mhalla A, Adam F, Texeira MJ, Bouhassira D. Neuropharmacological basis of rTMS-induced analgesia: the role of endogenous opioids. Pain. 2011;152:320-326. [PubMed] [DOI] [Full Text] |
| 78. | Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079-1091. [PubMed] [DOI] [Full Text] |
| 79. | Ziemann U, Tergau F, Wischer S, Hildebrandt J, Paulus W. Pharmacological control of facilitatory I-wave interaction in the human motor cortex. A paired transcranial magnetic stimulation study. Electroencephalogr Clin Neurophysiol. 1998;109:321-330. [PubMed] [DOI] [Full Text] |
| 80. | Wu T, Sommer M, Tergau F, Paulus W. Lasting influence of repetitive transcranial magnetic stimulation on intracortical excitability in human subjects. Neurosci Lett. 2000;287:37-40. [PubMed] [DOI] [Full Text] |
| 81. | Di Lazzaro V, Oliviero A, Saturno E, Pilato F, Insola A, Mazzone P, Profice P, Tonali P, Rothwell JC. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp Brain Res. 2001;138:268-273. [PubMed] [DOI] [Full Text] |
| 82. | Ziemann U. TMS induced plasticity in human cortex. Rev Neurosci. 2004;15:253-266. [PubMed] [DOI] [Full Text] |
| 83. | Cincotta M, Borgheresi A, Gambetti C, Balestrieri F, Rossi L, Zaccara G, Ulivelli M, Rossi S, Civardi C, Cantello R. Suprathreshold 0.3 Hz repetitive TMS prolongs the cortical silent period: potential implications for therapeutic trials in epilepsy. Clin Neurophysiol. 2003;114:1827-1833. [PubMed] [DOI] [Full Text] |
| 84. | Daskalakis ZJ, Möller B, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res. 2006;174:403-412. [PubMed] [DOI] [Full Text] |
| 85. | Murase N, Rothwell JC, Kaji R, Urushihara R, Nakamura K, Murayama N, Igasaki T, Sakata-Igasaki M, Mima T, Ikeda A. Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer’s cramp. Brain. 2005;128:104-115. [PubMed] [DOI] [Full Text] |
| 86. | Filipović SR, Rothwell JC, Bhatia K. Slow (1 Hz) repetitive transcranial magnetic stimulation (rTMS) induces a sustained change in cortical excitability in patients with Parkinson’s disease. Clin Neurophysiol. 2010;121:1129-1137. [PubMed] [DOI] [Full Text] |
| 87. | Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5-21. [PubMed] [DOI] [Full Text] |
| 88. | Ji RR, Schlaepfer TE, Aizenman CD, Epstein CM, Qiu D, Huang JC, Rupp F. Repetitive transcranial magnetic stimulation activates specific regions in rat brain. Proc Natl Acad Sci USA. 1998;95:15635-15640. [PubMed] [DOI] [Full Text] |
| 89. | Hausmann A, Weis C, Marksteiner J, Hinterhuber H, Humpel C. Chronic repetitive transcranial magnetic stimulation enhances c-fos in the parietal cortex and hippocampus. Brain Res Mol Brain Res. 2000;76:355-362. [PubMed] [DOI] [Full Text] |
| 90. | Müller MB, Toschi N, Kresse AE, Post A, Keck ME. Long-term repetitive transcranial magnetic stimulation increases the expression of brain-derived neurotrophic factor and cholecystokinin mRNA, but not neuropeptide tyrosine mRNA in specific areas of rat brain. Neuropsychopharmacology. 2000;23:205-215. [PubMed] [DOI] [Full Text] |
| 91. | Rothwell J, Burke D, Hicks R, Stephen J, Woodforth I, Crawford M. Transcranial electrical stimulation of the motor cortex in man: further evidence for the site of activation. J Physiol. 1994;481:243-250. [PubMed] [DOI] [Full Text] |
| 92. | Berardelli A, Inghilleri M, Rothwell JC, Romeo S, Currà A, Gilio F, Modugno N, Manfredi M. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res. 1998;122:79-84. [PubMed] [DOI] [Full Text] |
| 93. | Di Lazzaro V, Oliviero A, Tonali PA, Marra C, Daniele A, Profice P, Saturno E, Pilato F, Masullo C, Rothwell JC. Noninvasive in vivo assessment of cholinergic cortical circuits in AD using transcranial magnetic stimulation. Neurology. 2002;59:392-397. [PubMed] [DOI] [Full Text] |
| 94. | Inghilleri M, Conte A, Frasca V, Gilio F, Lorenzano C, Berardelli A. Synaptic potentiation induced by rTMS: effect of lidocaine infusion. Exp Brain Res. 2005;163:114-117. [PubMed] [DOI] [Full Text] |
| 95. | Jennum P, Winkel H, Fuglsang-Frederiksen A. Repetitive magnetic stimulation and motor evoked potentials. Electroencephalogr Clin Neurophysiol. 1995;97:96-101. [PubMed] [DOI] [Full Text] |
| 96. | Berardelli A, Inghilleri M, Gilio F, Romeo S, Pedace F, Currà A, Manfredi M. Effects of repetitive cortical stimulation on the silent period evoked by magnetic stimulation. Exp Brain Res. 1999;125:82-86. [PubMed] [DOI] [Full Text] |
| 97. | Chen R. Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve Suppl. 2000;9:S26-S32. [PubMed] [DOI] [Full Text] |
| 98. | Romeo S, Gilio F, Pedace F, Ozkaynak S, Inghilleri M, Manfredi M, Berardelli A. Changes in the cortical silent period after repetitive magnetic stimulation of cortical motor areas. Exp Brain Res. 2000;135:504-510. [PubMed] [DOI] [Full Text] |
| 99. | Modugno N, Nakamura Y, MacKinnon CD, Filipovic SR, Bestmann S, Berardelli A, Rothwell JC. Motor cortex excitability following short trains of repetitive magnetic stimuli. Exp Brain Res. 2001;140:453-459. [PubMed] [DOI] [Full Text] |
| 100. | Lorenzano C, Gilio F, Inghilleri M, Conte A, Fofi L, Manfredi M, Berardelli A. Spread of electrical activity at cortical level after repetitive magnetic stimulation in normal subjects. Exp Brain Res. 2002;147:186-192. [PubMed] [DOI] [Full Text] |
| 101. | Inghilleri M, Conte A, Frasca V, Curra’ A, Gilio F, Manfredi M, Berardelli A. Antiepileptic drugs and cortical excitability: a study with repetitive transcranial stimulation. Exp Brain Res. 2004;154:488-493. [PubMed] [DOI] [Full Text] |
| 102. | Ben-Shachar D, Belmaker RH, Grisaru N, Klein E. Transcranial magnetic stimulation induces alterations in brain monoamines. J Neural Transm (Vienna). 1997;104:191-197. [PubMed] [DOI] [Full Text] |
| 103. | Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3:95-118. [PubMed] [DOI] [Full Text] |
| 104. | Hirayama A, Saitoh Y, Kishima H, Shimokawa T, Oshino S, Hirata M, Kato A, Yoshimine T. Reduction of intractable deafferentation pain by navigation-guided repetitive transcranial magnetic stimulation of the primary motor cortex. Pain. 2006;122:22-27. [PubMed] [DOI] [Full Text] |
| 105. | Goto T, Saitoh Y, Hashimoto N, Hirata M, Kishima H, Oshino S, Tani N, Hosomi K, Kakigi R, Yoshimine T. Diffusion tensor fiber tracking in patients with central post-stroke pain; correlation with efficacy of repetitive transcranial magnetic stimulation. Pain. 2008;140:509-518. [PubMed] [DOI] [Full Text] |
| 106. | Lefaucheur JP. Use of repetitive transcranial magnetic stimulation in pain relief. Expert Rev Neurother. 2008;8:799-808. [PubMed] [DOI] [Full Text] |
| 107. | Wasserman EM, Epstein CM, Ziemann U, Walsh V, Paus T, Lisanby SH. The Oxford Handbook of Transcranial Stimulation. Oxford: Oxford University Press 2008; . |
| 108. | Ahmed MA, Mohamed SA, Sayed D. Long-term antalgic effects of repetitive transcranial magnetic stimulation of motor cortex and serum beta-endorphin in patients with phantom pain. Neurol Res. 2011;33:953-958. [PubMed] [DOI] [Full Text] |
| 109. | Hosomi K, Shimokawa T, Ikoma K, Nakamura Y, Sugiyama K, Ugawa Y, Uozumi T, Yamamoto T, Saitoh Y. Daily repetitive transcranial magnetic stimulation of primary motor cortex for neuropathic pain: a randomized, multicenter, double-blind, crossover, sham-controlled trial. Pain. 2013;154:1065-1072. [PubMed] [DOI] [Full Text] |
| 110. | Ceccanti M, Inghilleri M, Attilia ML, Raccah R, Fiore M, Zangen A, Ceccanti M. Deep TMS on alcoholics: effects on cortisolemia and dopamine pathway modulation. A pilot study. Can J Physiol Pharmacol. 2015;93:283-290. [PubMed] [DOI] [Full Text] |
| 111. | Kakigi R, Inui K, Tamura Y. Electrophysiological studies on human pain perception. Clin Neurophysiol. 2005;116:743-763. [PubMed] [DOI] [Full Text] |
| 112. | Cincotta M, Giovannelli F, Borgheresi A, Balestrieri F, Toscani L, Zaccara G, Carducci F, Viggiano MP, Rossi S. Optically tracked neuronavigation increases the stability of hand-held focal coil positioning: evidence from “transcranial” magnetic stimulation-induced electrical field measurements. Brain Stimul. 2010;3:119-123. [PubMed] [DOI] [Full Text] |
| 113. | Ahdab R, Ayache SS, Brugières P, Goujon C, Lefaucheur JP. Comparison of “standard” and “navigated” procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Neurophysiol Clin. 2010;40:27-36. [PubMed] [DOI] [Full Text] |
| 114. | Bashir S, Edwards D, Pascual-Leone A. Neuronavigation increases the physiologic and behavioral effects of low-frequency rTMS of primary motor cortex in healthy subjects. Brain Topogr. 2011;24:54-64. [PubMed] [DOI] [Full Text] |
| 115. | Saitoh Y. Validation and the future of stimulation therapy of the primary motor cortex. Neurol Med Chir (Tokyo). 2012;52:451-456. [PubMed] [DOI] [Full Text] |
| 116. | Lefaucheur JP, Drouot X, Nguyen JP. Interventional neurophysiology for pain control: duration of pain relief following repetitive transcranial magnetic stimulation of the motor cortex. Neurophysiol Clin. 2001;31:247-252. [PubMed] [DOI] [Full Text] |
| 117. | Irlbacher K, Kuhnert J, Röricht S, Meyer BU, Brandt SA. [Central and peripheral deafferent pain: therapy with repetitive transcranial magnetic stimulation]. Nervenarzt. 2006;77:1196, 1198-1203. [PubMed] [DOI] [Full Text] |
| 118. | Lefaucheur JP, Hatem S, Nineb A, Ménard-Lefaucheur I, Wendling S, Keravel Y, Nguyen JP. Somatotopic organization of the analgesic effects of motor cortex rTMS in neuropathic pain. Neurology. 2006;67:1998-2004. [PubMed] [DOI] [Full Text] |
| 119. | André-Obadia N, Mertens P, Gueguen A, Peyron R, Garcia-Larrea L. Pain relief by rTMS: differential effect of current flow but no specific action on pain subtypes. Neurology. 2008;71:833-840. [PubMed] [DOI] [Full Text] |
| 120. | Lefaucheur JP, Drouot X, Ménard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS in chronic neuropathic pain: pain relief is associated with thermal sensory perception improvement. J Neurol Neurosurg Psychiatry. 2008;79:1044-1049. [PubMed] [DOI] [Full Text] |
| 121. | Carretero B, Martín MJ, Juan A, Pradana ML, Martín B, Carral M, Jimeno T, Pareja A, Montoya P, Aguirre I. Low-frequency transcranial magnetic stimulation in patients with fibromyalgia and major depression. Pain Med. 2009;10:748-753. [PubMed] [DOI] [Full Text] |
| 122. | Lefaucheur JP, Ayache SS, Sorel M, Farhat WH, Zouari HG, Ciampi de Andrade D, Ahdab R, Ménard-Lefaucheur I, Brugières P, Goujon C. Analgesic effects of repetitive transcranial magnetic stimulation of the motor cortex in neuropathic pain: influence of theta burst stimulation priming. Eur J Pain. 2012;16:1403-1413. [PubMed] [DOI] [Full Text] |
| 123. | Dall’Agnol L, Medeiros LF, Torres IL, Deitos A, Brietzke A, Laste G, de Souza A, Vieira JL, Fregni F, Caumo W. Repetitive transcranial magnetic stimulation increases the corticospinal inhibition and the brain-derived neurotrophic factor in chronic myofascial pain syndrome: an explanatory double-blinded, randomized, sham-controlled trial. J Pain. 2014;15:845-855. [PubMed] [DOI] [Full Text] |
| 124. | Hodaj H, Alibeu JP, Payen JF, Lefaucheur JP. Treatment of Chronic Facial Pain Including Cluster Headache by Repetitive Transcranial Magnetic Stimulation of the Motor Cortex With Maintenance Sessions: A Naturalistic Study. Brain Stimul. 2015;8:801-807. [PubMed] [DOI] [Full Text] |
| 125. | Khedr EM, Kotb HI, Mostafa MG, Mohamad MF, Amr SA, Ahmed MA, Karim AA, Kamal SM. Repetitive transcranial magnetic stimulation in neuropathic pain secondary to malignancy: a randomized clinical trial. Eur J Pain. 2015;19:519-527. [PubMed] [DOI] [Full Text] |
| 126. | Lindholm P, Lamusuo S, Taiminen T, Pesonen U, Lahti A, Virtanen A, Forssell H, Hietala J, Hagelberg N, Pertovaara A. Right secondary somatosensory cortex-a promising novel target for the treatment of drug-resistant neuropathic orofacial pain with repetitive transcranial magnetic stimulation. Pain. 2015;156:1276-1283. [PubMed] [DOI] [Full Text] |
| 127. | Borckardt JJ, Weinstein M, Reeves ST, Kozel FA, Nahas Z, Smith AR, Byrne TK, Morgan K, George MS. Postoperative left prefrontal repetitive transcranial magnetic stimulation reduces patient-controlled analgesia use. Anesthesiology. 2006;105:557-562. [PubMed] [DOI] [Full Text] |