Published online Jul 27, 2015. doi: 10.5313/wja.v4.i2.30
Peer-review started: April 8, 2015
First decision: April 27, 2015
Revised: May 12, 2015
Accepted: June 1, 2015
Article in press: June 2, 2015
Published online: July 27, 2015
Processing time: 111 Days and 13.7 Hours
AIM: To investigate the utility of transthoracic echocardiography in confirming appropriate pulmonary artery catheter (PAC) placement.
METHODS: Three commonly used transthoracic echocardiography (TTE) views were used to confirm PAC position in 103 patients undergoing elective cardiac surgery - the parasternal short axis right ventricular inflow-outflow view; the subcostal short axis right ventricular inflow-outflow view; and the parasternal short axis ascending aortic view. All PACs were inserted by the managing anesthesiologist under pressure waveform guidance alone, who was blinded to all sonographic information. A sonographer blinded to all pressure waveform information confirmed visualisation of an “empty” PA before PAC insertion, and visualisation of the PAC balloon in the main PA (MPA) or right PA (RPA) after attempts at placement were complete. Agreement, sensitivity and specificity of TTE in confirming appropriate PAC placement was compared against pressure waveform guidance as the “gold standard”. The successful view used was compared against patients’ anthropomorphic indices, presence of lung hyperinflation, and insertion of PAC during positive pressure ventilation. Agreement between TTE and pressure waveform guidance was analysed using Cohen’s Kappa statistic. The relative proportion of total RPA seen by subcostal vs parasternal TTE views was also compared with a further 20 patients’ computed tomography (CT) pulmonary angiograms (CTPA), to determine efficacy in detection of distal RPA PAC placement.
RESULTS: Appropriate positioning of the PAC balloon, and its to-and-fro movement consistent with a non-wedged state, within the MPA or RPA was confirmed by TTE in 98 of the 103 patients [sensitivity 95% (95%CI: 89%-98%)], and absence of the PAC balloon before insertion correctly established in 100 patients [specificity 97% (92%-99%)]. This was in very good agreement with pressure waveform guidance [Cohen’s Kappa 0.92, (0.87-0.98)]. The subcostal view was the best view to visualise the PAC tip when it was placed in the right pulmonary artery (OR 70, P < 0.0001), was more successful in patients with COAD (OR 9.5, P = 0.001), and visualized 61% (vs 44% by parasternal views, P < 0.001) of mean RPA lengths compared with CTPA; however the parasternal views were more successful in patients with higher body mass indexs (OR 0.78 for success with subcostal views, P < 0.001). There was a trend towards insertion during intermittent positive pressure ventilation favoring visualisation by subcostal views (OR 3.9, P = 0.08). The subcostal view visualized a greater length of the RPA than parasternal views (3.9 cm vs 2.9 cm, P < 0.0001). PACs were more often placed in the MPA than RPA (80 vs 18 patients). Three patient’s pulmonary arteries were not visible by any TTE view; in a further 2 patients, despite pre-insertion visualisation of their pulmonary arteries, the PAC balloon was not visible by any view with TTE where correct placement by pressure waveform was unequivocal.
CONCLUSION: TTE can assist appropriate PAC placement by visualization of an unwedged PAC balloon in the PA.
Core tip: Transthoracic echocardiography (TTE) is an efficacious adjunct to pressure waveform guidance for guiding appropriate pulmonary artery catheter (PAC) placement. With the required equipment and expertise, TTE is a rapid and safe tool for confirming whether the PAC is placed too far (the PAC balloon seen beyond the proximal RPA) or not far enough (the body of the PAC seen in the right ventricle but the PAC balloon not seen in the main PA or right PA). This application may assist in reducing complications related to PA rupture or PAC induced arrhythmias.
- Citation: Tan CO, Weinberg L, Story DA, McNicol L. Transthoracic echocardiography assists appropriate pulmonary artery catheter placement: An observational study. World J Anesthesiol 2015; 4(2): 30-38
- URL: https://www.wjgnet.com/2218-6182/full/v4/i2/30.htm
- DOI: https://dx.doi.org/10.5313/wja.v4.i2.30
Pulmonary artery catheters (PACs) are variably used in critical care[1] and cardiac anesthesiology[2]. In proficient hands, complication rates are low[3] but include a 1:650-1:4300 risk of death secondary to pulmonary arterial rupture[4], with inappropriate balloon inflation or catheter advancement beyond the main pulmonary artery (MPA) as presumed contributory factors. Conversely, failure to advance the PAC beyond the right ventricle (RV) may result in endocardial irritation and arrhythmias; these complications may occur not only during insertion but also subsequently due to retrograde catheter migration. Pressure waveform analysis, X-ray guidance, and transesophageal echocardiography (TEE)[5] have all been used to confirm placement of the tip of the catheter in the PA. Pressure waveform analysis is the most commonly used technique[6] because of its accuracy and accessibility. Pressure waveform guided placement is usually straightforward, however fast or irregular heart rates, low pulmonary pressures, or gross waveform distortion by widely varying intrathoracic pressures may make pressure waveforms difficult to interpret. This situation occurs more frequently in the ICU setting where patients requiring PACs often display severely abnormal haemodynamic states, at times also with tachyarrhythmias.
TEE is an ultrasound modality that offers advantages over transthoracic echocardiography (TTE) but is semiinvasive, and its use is difficult to justify for PAC placement as a primary indication. Portable X-ray offers ideal information on PAC position but may not be immediately available during PAC insertion and also utilises ionising radiation. TTE however is non-invasive, utilises no ionising radiation, and is usually immediately available in most critical care settings. It has also been used to successfully visualise PACs in a small sample in a non-surgical scenario[7]. We aimed to explore how TTE could serve as an adjunct to pressure waveform guidance for confirmation of PAC placement in the MPA or RPA, as well as how TTE could assist in ideal final positioning of the PAC tip in the MPA to assist prevention of hazardous distal or proximal PAC migration.
We hypothesized that: (1) use of three basic TTE views could reliably confirm PAC placement in the MPA or RPA when pressure waveform analysis is used as the primary technique; (2) particular TTE views could visualise a greater proportion the distal RPA as determined by CTPA, assisting confirmation of ideal PAC placement proximal to the RPA 1st division; and (3) that other factors including: chronic obstructive airways disease (COAD), body mass index (BMI), concomitant intermittent positive pressure ventilation (IPPV) or final PAC position influenced which echocardiographic view used to successfully visualise the MPA, RPA and PAC.
This study was approved by the Austin Health Human Research and Ethics Committee (H2012/04776) and was carried out in compliance with the Helsinki Declaration on research involving human participants. Written informed consent was obtained from all participants. We recruited 103 cardiac surgery patients who were planned for PAC insertion as part of their anaesthesia care. The PACs inserted were the Swan-Ganz VIP+ (Edwards Lifesciences, CA, United States). Information on patients BMI and previous diagnosis of COAD or asthma were obtained preoperatively. The study protocol was as follows.
All PACs were inserted preoperatively by observation of the pressure waveform during advancement of the catheter.
A single anesthesiologist experienced in TTE, who was not the patient’s treating anesthesiologist at the time, performed all ultrasound examinations and was blinded to all pressure waveform information obtained during PAC insertion.
Three commonly used TTE views were used in the following sequential order to visualize the MPA and right pulmonary artery (RPA) prior to PAC placement.
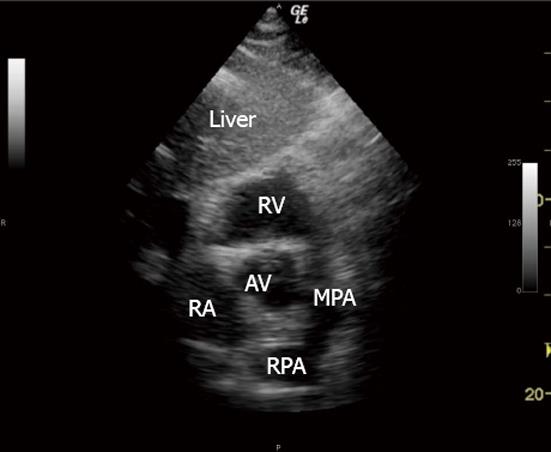
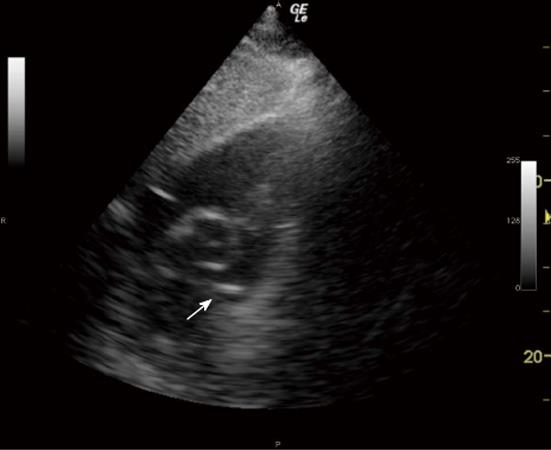
The MPA and RPA were visualised and confirmed as “empty” of the catheter before PAC insertion to establish the specificity of the technique (Figure 1).
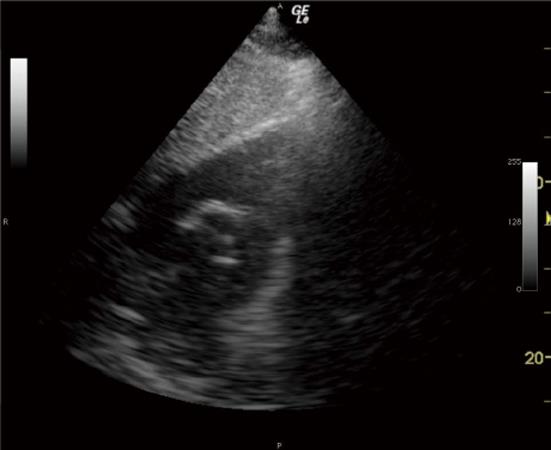
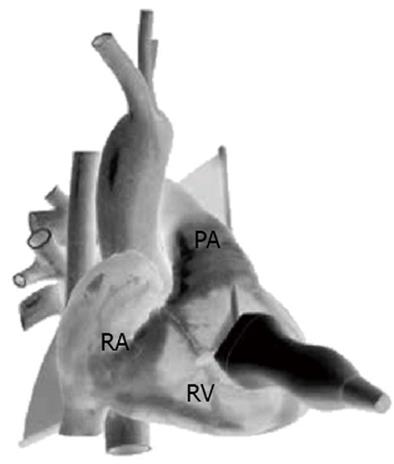
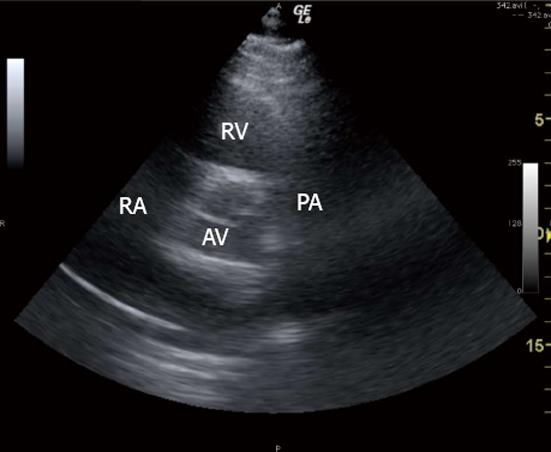
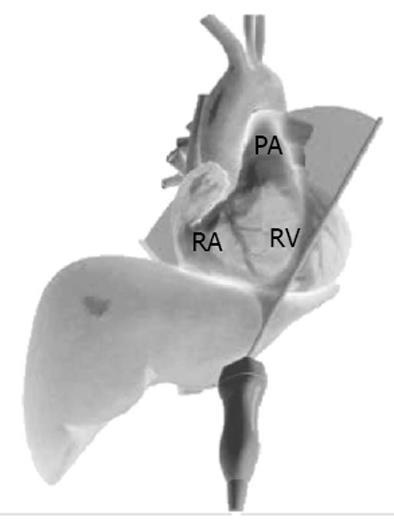
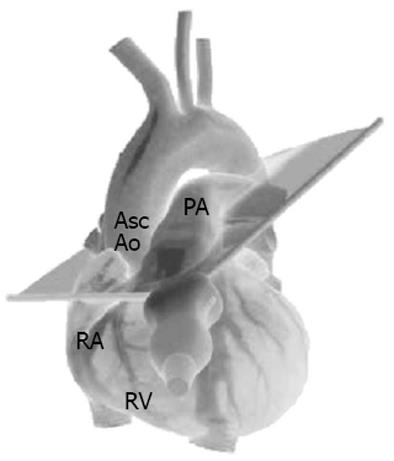
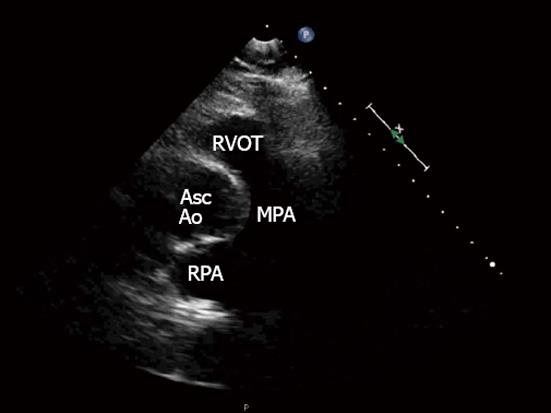
The 3 TTE views used were: (1) the parasternal short axis right ventricular inflow-outflow (PSRVIO) view (Figures 2 and 3); (2) the subcostal short axis right ventricular inflow-outflow (SCRVIO) view (Figures 4 and 5); and (3) the parasternal short axis ascending aortic (PSAscAo) view (Figures 6 and 7).
These particular views were selected as they offer optimal visualisation of the MPA and RPA, with the structures in question positioned in the near field.
Patients in whom none of the three TTE views of the MPA and RPA were attainable were noted and included in the results of the study.
The sonographer was then notified by the proceduralist inserting the PAC that attempts at insertion had begun.
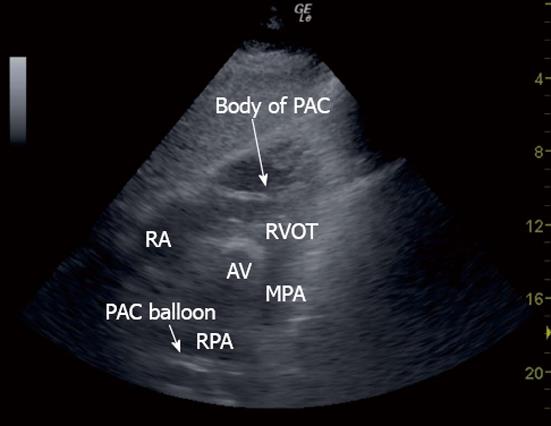
The sonographer then used the same TTE view that was successfully used to visualise the MPA and RPA to examine for the appearance of a PAC balloon or tip, and the right ventricular outflow tract (RVOT) visualised to examine for the presence of the body of the PAC line (Figure 8).
The sonographer was notified by the proceduralist inserting the PAC that attempts at correct placement were complete. The sonographer was not given any information about presumed success or failure of appropriate PAC placement according to pressure waveform information.
If the PAC balloon or tip were not already seen in the PA, or PAC body in the RVOT, then any of the 3 remaining views not used were henceforth utilised in the above described sequence to examine for those structures.
If either PAC line in the RVOT or PAC balloon in the PA were seen by TTE, the case was recorded as a “test positive” for the first view used that, in the above sequential order, was able to identify those structures. We did not attempt to visualise PAC structures further by different views once positively identified. If not seen, the case was recorded as a “test negative”.
If the PAC balloon had not been seen in the MPA or RPA by any of the 3 designated TTE views, but examination of the RVOT positively identified the body of the PAC, the PAC tip was presumed to be placed in the distal RPA beyond the view of TTE.
All sonograms were recorded and de-identified, then reviewed independently at a later date by a second anesthesiologist skilled in perioperative TTE to confirm absence or presence of the PAC balloon or tip in the PA, and PAC body in the RVOT. The second assessor was blinded to the diagnosis of “test positive” or “test negative” by the initial sonographer. Disagreement between observers was recorded as interobserver variability.
A single portable ultrasound machine (LogiqE, GE, New York) and 2-5 MHz phased array ultrasound probe (3S, GE, New York) was used to perform each sonogram. The probe was positioned on the praecordium and subcostal region carefully under the patient’s sterile drapes. PAC balloon position was identified by the hyperechoic appearance of its air-filled balloon and motion away from the transducer in concert with ventricular systole (Figure 9, Movie Clip 1). The body of the PAC was identified as a hyperechoic line positioned between and distinct from the RVOT free wall and anterior border of the aortic root (Figure 8). Insertion was performed by a cardiac anesthesiologist or a supervised cardiac anaesthesiology registrar, both of whom were blinded to all sonographic information. In keeping with the majority practice in our department of PAC insertion until PA pressure waveforms were observed, in all cases bar those inserted by a single anesthesiologist, PACs were advanced until this point, the balloon then deflated, and the catheter position secured. The remaining practitioner deliberately obtained a pulmonary capillary wedge pressure waveform before balloon deflation, 1-2 cm withdrawal, and fixation of catheter position. Patients were awake and spontaneously ventilating or anaesthetised and positive pressure ventilated (IPPV) as per the cardiac anesthesiologist’s preference. Difficulties in placing the PAC under pressure waveform guidance were recorded by the inserting proceduralist but not communicated to the sonographer at any time.
To establish how TTE could assist in confirming a PAC placed potentially too distally or proximally, the distance from MPA bifurcation to the furthest point of RPA visualisation in each sonogram was also measured and compared between views. This was then compared with the dimensions of the RPA at its narrowest point, and its 1st divisions, measured from the CT pulmonary angiography scans (CTPA) of 20 adult patients. With this information the approximate expected wedge position was estimated by comparison of the known width of the inflated PAC balloon[8]. TTE measurements were performed offline (Pixmeo, Geneva, Switzerland). CTPA RPA measurements were taken at the narrowest points of the vessels with the midpoints of bifurcation (MPA to RPA, RPA to 1st divisions) used as measurement endpoints. CTPA measurements were performed via Impax Web1000 (Agfa, Mortsel, Belgium).
All calculations were performed using SPSS V21 (IBM, New York, United States) statistical software. As described by Gwet[9], based on a mimimum limit of agreement of 70% between TTE and pressure waveform guidance by Cohen’s Kappa statistic, 51 subjects were required. We chose a convenient sample of 100 patients anticipating difficulty in obtaining appropriate MPA and RPA views. Continuous data was assessed for normality by histogram frequency distribution analysis and the Kolgomorov-Smirnoff normality test, and was considered suitable for parametric testing using unpaired t-tests, or one-way ANOVA for multiple groups. Single independent variable, binary outcome data was analysed with Fisher’s Exact test. Multivariate logistic regression was used to identify the effect of simultaneous patient factors and PAC position in the MPA or RPA on likelihood of successful TTE views used. We reported this study using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines[10].
One hundred and three patients were recruited (Table 1). Three patients’ pulmonary arteries were not visible in any of the 3 TTE views [2% (95%CI: 1%-8%)]. Appropriate placement of the PAC balloon within the MPA or RPA was confirmed by TTE in 98 patients [sensitivity 95% (95%CI: 89%-98%)], measured against pressure waveform guidance as the “gold standard”. Absence of the PAC balloon in the MPA or RPA before PAC insertion was correctly established by TTE in 100 patients [specificity 97% (95%CI: 92%-99%)]. Confirmation of correct PAC placement by TTE was in very good agreement with pressure waveform guidance [Cohen’s Kappa 0.92, (95%CI: 0.87-0.98)]. There was no interobserver disagreement found regarding presence or absence of the PAC balloon. Visualisation of the body of the PAC in the RVOT was confirmed in 76 patients [74% (95%CI: 63%-82%)] more often by parasternal than subcostal views (69% vs 21%, P < 0.0001). PACs were more often placed in the MPA than the RPA and the PAC balloon most often seen in the PSRVIO view (Table 1).
| Parameter | Number (proportion or 95%CI) or mean (SD or 95%CI) | |
| Age (yr) | 67 (10) | |
| BMI (kg/m2) | 29.6 (5.6) | |
| IPPV during PAC insertion | 24 (24%) | |
| Diagnosis of COAD or Asthma | 28 (28%) | |
| Final PAC position in MPA | 80 (72%-88%) | P < 0.0001 |
| Final PAC position in RPA | 18 (13%-29%) | P < 0.0001 |
| TTE view in which PAC was seen | ||
| PSRVIO | 52 (43%-63%) | P < 0.0001 |
| SCRVIO | 33 (26%-45%) | P < 0.0001 |
| PSAscAo | 13 (6%-24%) | P < 0.0001 |
All PACs were successfully floated into the MPA or RPA. When the PAC was visualised during insertion, the PAC balloon was easily visible in its transition from RV to PA. Three patients suffered from rapid atrial fibrillation preoperatively, making the pressure waveform transition from RV to PA difficult to interpret. In these instances the balloon was clearly seen on TTE in the MPA. In 2 patients, despite detection of the body of the PAC in the RVOT and clear confirmation of correct placement in the PA by pressure waveform analysis, the inflated balloon of the PAC was not visible by TTE; in one of these patients, despite good visualisation of the PA in all 3 views, and in the other where only the PSRVIO view was able to be obtained. Neither of these 2 patients had a preoperative diagnosis of COAD, were positively pressure ventilated, or had a BMI over 30.
Successful PAC visualisation by subcostal views compared to parasternal views was more likely in patients with preoperative diagnoses of COAD, PAC insertion during IPPV and PAC positioning in the RPA (Table 2), but less likely in patients with higher BMI. All factors except insertion during IPPV reached statistical significance in the multivariate logistic regression model.
| Odds ratio | P-value | 95%CI | |
| Diagnosis of COAD or Asthma | 9.5 | 0.001 | 2.5-36 |
| Insertion during IPPV | 3.9 | 0.08 | 0.8-17.8 |
| BMI (kg/m2) | 0.78 | < 0.0001 | 0.67-0.89 |
| RPA PAC position | 70.0 | < 0.0001 | 9.6-502 |
Length of RPA visualised by TTE and CTPA measured dimensions of the RPA and its 1st divisions at their narrowest points are presented in Tables 3 and 4. The SCRVIO view consistently visualised a greater length of the RPA compared with parasternal views, and hence a greater proportion of the complete RPA as established by CTPA (61% vs 45% of the complete RPA length respectively, P < 0.0001).
| TTE | Length (cm) | P-value | |
| Mean (SD) | Range | ||
| Parasternal views: RPA | 2.9 (0.8) | 1.2-4.8 | < 0.0001 |
| SCRVIO view: RPA | 3.9 (0.8) | 2.8-5.6 | |
| CTPA | Length (cm) | Width (cm) | ||
| Mean (SD) | Range | Mean (SD) | Range | |
| RPA | 6.4 (1.0) | 4.5-8.1 | 2.0 (0.4) | 1.1-2.6 |
| RPA 1st division (anterior) | 0.8 (0.02) | 0.4-1.4 | ||
| RPA 1st division (posterior) | 0.7 (0.02) | 0.4-1.3 | ||
The use of TTE in acute medicine continues to enjoy rapidly increasing uptake and application in many areas of acute care[11]. Point-of-care ultrasound machines now offer high quality imaging and are smaller and more affordable, making their use in the critical care setting ideal. Although now superseded, the point-of-care machine used in our study was fully enabled to perform quantitative spectral and colour Doppler analyses. Focused TTE training for critical care physicians outside of conventional cardiology and sonography training has demonstrated utility in assisting clinical decision making[12,13].
We conducted a prospective observational study of focused transthoracic echocardiography used to confirm appropriate pulmonary artery catheter placement in the main or right pulmonary artery in patients undergoing elective cardiac surgery. Consistent with our hypotheses we found that: (1) Use of three basic TTE views could reliably confirm appropriate PAC placement in most patients, by direct visualisation of a mobile “to-and-fro” PAC balloon in MPA or RPA, and by the presence of the body of the PAC catheter in the RVOT confirming adequate PAC advancement; (2) Subcostal TTE views visualised a greater proportion of the RPA as defined by CTPA than parasternal TTE views, allowing more reliable estimation of ideal (“not too far”) PAC positioning; and (3) that patient factors and final PAC position affect the type of echocardiographic view successfully used for PAC visualisation.
Tempe et al[14] demonstrated a mean distance from PA to wedge pressure trace of 6.8 cm. The MPA is known to be approximately 5 cm in length; as our data suggests that as the mean length of the RPA is 6.4 cm, in the majority of cases a PAC would wedge in proximal-to-mid RPA. Such a wedge position is consistent with our CT pulmonary angiogram data suggesting that small RPAs at their distal narrowest point may not accommodate an inflated PAC balloon (Table 3), as the balloon is purported to be 1 cm in diameter when inflated[8]. Hence a PAC placed approximately halfway between the origin of the MPA and 2 cm beyond the MPA bifurcation should have the least likelihood of migrating too distally to spontaneously wedge in the RPA, as well as too proximally causing RV irritation. Even the TTE parasternal views, which visualized less of the RPA than the SCRVIO view, consistently visualized the proximal RPA (Table 3). Hence successful use of any view that captures the PAC balloon, together with visualization of its to-and-fro motion over the cardiac cycle, should confirm placement of a PAC balloon within this “safe” depth of proximal to mid RPA.
Use of parasternal views also offered good visualization of the body of the PAC in the RVOT more frequently; this was most likely because the lie of the PAC in the RVOT is perpendicularly aligned to the incoming ultrasound beams, and is in the near field, in these views. In practice, if the body of the PAC is seen in the RVOT, but to-and-fro movement of the PAC balloon is not seen in the MPA or RPA, it is highly likely that the balloon is wedged; additionally, if the catheter is not seen in both the RVOT and the PA/MPA in this instance, then the PAC must be inserted “not far enough” (i.e., placed proximal to the RVOT). Hence, TTE should be used to: (1) confirm that a PAC is not unintentionally wedged; (2) confirm that a PAC is inserted distal to the RVOT; (3) assist “ideal” PAC balloon final positioning approximately 3-4 cm beyond the origin of the MPA; and (4) confirm subsequently by repeat examination that proximal or distal PAC migration has not occurred if the monitored PAC pressure waveforms become equivocal.
Where the PACs were placed in the RPA, the SCRVIO was significantly better at visualizing the PAC balloon than parasternal views. This may be because the caudo-cranial direction of the ultrasound beams from the SCRVIO are well aligned with the final path taken by the PAC from RVOT to RPA (Figures 5 and 6).
In the 2 patients where the balloon of the PAC was not seen when an obvious PA waveform was displayed, one case was most likely because the SCRVIO was not obtained and the PAC positioned in the RPA. In the other patient, despite good visualization of the MPA and RPA in both parasternal and subcostal views, the PAC balloon was not seen. It is possible that further non-standard views not utilised in this study, such as the parasternal long axis RV outflow view[15], may have assisted in PAC visualisation.
Although serious complications are rare with the use of PACs, should pulmonary artery rupture occur, the associated mortality is up to 70%[16]. The important pressure waveform transitions to recognize during insertion are the changes from RV to PA, and if this is unrecognized, the transition from PA to wedge. Even in experienced hands these changes can occasionally be obscure. Patients who have received a sedating premedication can partially obstruct their airway and create large baseline swings in their intrathoracic, and hence cardiac, pressures. Right ventricular systolic failure and hypovolemia may also reduce the pulse pressures in RV and PA whilst raising the central venous pressure a, c and v waves. Absolute changes between systolic and diastolic pressures from RA, RV, PA and then to wedge are difficult to appreciate in these circumstances. The dicrotic notch seen in a PA pressure trace may be subtle and hidden within an underdamped pressure trace, and the falling diastolic phase seen when in the PA can be hard to distinguish from the flat or rising diastolic phase of the RV during rapid and/or irregular heart rates; for example in rapid atrial fibrillation or other supraventricular tachycardias. All of these confounders may mislead even experienced practitioners in identifying appropriate PAC placement; in our study 3% of patients had rapid atrial fibrillation making pressure waveform interpretation difficult. These situations will be more frequent in the emergency cardiac surgery or intensive care setting. Scenarios in which TTE would provide utility in confirming appropriate PAC placement are summarized in Table 5.
| Timing | Utility |
| Pre-insertion | Identify RV dilation, suggesting a longer than standard PAC insertion distance until the MPA/RPA is reached by the PAC balloon |
| Identify small calibre MPA/RPA dimensions, usually associated with hypovolemia, and possibly predisposing to shorter depths of insertion from RV to “wedge” | |
| Quantify RA, TV and PV abnormalities and/or degree of regurgitation prior to PAC insertion | |
| Insertion | Establish absence of the body of the PAC within the RVOT, suggesting PAC coiling or failure of passage past the TV |
| Establish presence of the body of the PAC within the RVOT, confirming that the PAC balloon (1) is not coiled in the RV and (2) must be either in or distal to the MPA/RPA | |
| Visualisation of an “un-wedged” PAC balloon by the appearance of “to-and-fro” movement of the echogenic air-filled PAC balloon in the MPA or RPA | |
| Imply a wedge position and/or “too distal” placement of the PAC balloon if (1) the body of the PAC is seen within the RVOT and (2) the PAC balloon is not seen in the MPA or RPA | |
| Optimise final PAC balloon position to distal MPA/proximal RPA | |
| Post-insertion | Repetition of the above TTE signs to identify proximal or distal migration of the PAC from the initial insertion point |
| When in doubt, confirmation of the PAC balloon inflation status by visualisation of the “to-and-fro” movement of the echogenic air-filled PAC balloon | |
| Quantify possible contribution of decline in RV/TV/PV function with presence of the PAC |
Our study is limited by its single centre sample and observational design. Selection bias cannot be excluded as only elective patients in whom a PAC was deemed necessary for the operative procedure were included in the study. The moderate sample size used also precludes any conclusions when lack of a statistical difference between compared samples was found. The designation of TTE in the study as an adjunct to pressure waveform guidance, rather than as a sole alternative was purposeful; in most cases, insertion of a PAC is straightforward with the latter technique. Whilst we have not investigated a direct comparison between the two insertion techniques, we believe this would not have practical significance as use of pressure waveform guidance should remain the proceduralist’s insertion technique of choice. Catheter migration due to unfolding PAC loops, changes in patient position, and/or unintentional catheter manipulation is known to occur after insertion. To take full advantage of the potential for TTE to assist in the prevention of PAC related complications, repeated examinations would be required to exclude further PAC migration into the RPA or regression back to the RVOT. The sequential, rather than systematic use of TTE views from PSRVIO to PSAscAo then SCRVIO may have also skewed the proportion of “true positive” views in favor of the parasternal approach. This was again a deliberate aspect of the study design as the author’s experience in teaching TTE is that parasternal views are more intuitive and easier to apply in a beginner’s hands. Finally, the fact that in 3 patients no TTE views were obtained is a reminder that despite the excellent sensitivity and specificity of the technique when the MPA and RPA are visualized, there remains a very small proportion of patients in whom the technique will not provide useful information.
We conclude that TTE is a highly sensitive and specific second line technique when confirmation of appropriate PAC placement is required. Visualization of the air-filled PAC balloon in the MPA or RPA, together with its free “to-and-fro” movement, assists in confirming that a PAC is not wedged, whereas visualization of the body of the PAC in the RVOT confirms PAC placement beyond the RV. TTE used to guide final PAC positioning approximately 3-4 cm beyond the MPA origin, and used again as needed in the postoperative period, could assist in reducing the incidence of complications related to proximal or distal migration. Parasternal views are useful in patients with higher BMI, for PACs placed in the MPA and for visualisation of the PAC body, whereas subcostal views are more successful for PACs placed in the RPA. With the appropriate equipment and an available pracitioner with experience in perioperative TTE relevant to obtaining views of the pulmonary artery, use of focussed TTE as an adjunct to pressure waveform guidance may assist in reducing complications related to PAC malposition.
With the advent of point-of-care ultrasound machines with high quality imaging, more and more applications of ultrasound in critical care are finding their place. Transthoracic echocardiography (TTE) in particular was previously the sole domain of cardiologists and cardiac sonographers; however with the use of a focussed TTE examination critical care physicians can obtain reliable and useful information. Appropriate placement of pulmonary artery catheters (PAC) is usually a routine procedure with the use of pressure waveform guidance, but can be challenging in certain circumstances. The authors explored how focussed TTE could assist in confirming appropriate PAC placement.
Other examples of new applications of point-of-care ultrasound (U/S) in critical care include U/S diagnosis of pneumothorax, U/S localisation of the cricothyroid membrane for cricothyrotomy, and U/S estimation of stomach residual volumes to assist decision making on pre anaesthesia aspiration risk.
Point-of-care TTE utilised by critical care physicians has added great value to cardiovascular aspects of clinical decision making (Potter A. Echocardiography in acute medicine: a clinical review. Br J Hosp Med 2010; 71: 626-630). Guidance on degree of volume replacement, requirement for administration of inotropes or vasocstrictors, and postoperative level of care planning decisions have all been assisted by information by TTE (Cowie B. Three years’ experience of focused cardiovascular ultrasound in the peri-operative period. Anaesthesia 2011; 66: 268-273). However, use of echocardiography to guide invasive procedures outside of U/S guided regional anaesthesia has been restricted to case series of transoesophageal echocardiographic confirmation of PAC placement (Tempe DK, Datt V, Banerjee A, et al (2004). Case 5: Transesophageal echocardiography-guided insertion of a pulmonary artery catheter. J Cardiothorac Vasc Anesth 2004; 18: 657-662). This study is the first of its kind to be used in a large sample in the perioperative context demonstrating the utility of the less invasive transthoracic modality. The authors have successfully shown how TTE can safely and efficaciously confirm appropriate TTE placement.
With appropriate training and available equipment, PAC positioning in the main or right pulmonary artery can be confirmed reliably when PAC insertion by pressure waveform methods are equivocal. This can be performed rapidly and safely in time critical situations. Other applications of the technique may include pre-insertion quantification of right heart conditions that may be affected by PAC insertion, as well as post-insertion assessment of placement when catheter migration may have occurred.
TTE: Transthoracic echocardiogropahy; TEE: Transesophageal echocardiography; RV: Right ventricle; RVOT: Right ventricular outflow tract; RA: Right atrium; PAC: Pulmonary artery catheter; PA: Pulmonary artery; MPA: Main pulmonary artery; RPA: Right pulmonary artery; PSRVIO: Parasternal right ventricular inflow-outflow view; SCRVIO: Subcostal right ventricular inflow-outflow view; PSAscAo: Parasternal ascending aorta short axis view; CTPA: Computerised tomographic pulmonary angiogram; COAD: Chronic obstructive airways disease; IPPV: Intermittent positive pressure ventilation; ICU: Intensive care unit; BMI: Body mass index; ANOVA: Analysis of variance statistical test.
This is an interesting and well conducted study reinforcing the usefulness of TTE during invasive procedure.
P- Reviewer: Schoenhagen P, Sicari R S- Editor: Ji FF L- Editor: A E- Editor: Yan JL
| 1. | Gore JM, Goldberg RJ, Spodick DH, Alpert JS, Dalen JE. A community-wide assessment of the use of pulmonary artery catheters in patients with acute myocardial infarction. Chest. 1987;92:721-727. [PubMed] |
| 2. | Ranucci M. Which cardiac surgical patients can benefit from placement of a pulmonary artery catheter? Crit Care. 2006;10 Suppl 3:S6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Cowie BS. Does the pulmonary artery catheter still have a role in the perioperative period? Anaesth Intensive Care. 2011;39:345-355. [PubMed] |
| 4. | Damen J, Bolton D. A prospective analysis of 1,400 pulmonary artery catheterizations in patients undergoing cardiac surgery. Acta Anaesthesiol Scand. 1986;30:386-392. [PubMed] |
| 5. | Orihashi K, Nakashima Y, Sueda T, Yamanoue T, Yuge O, Matsuura Y. Usefulness of transesophageal echocardiography for guiding pulmonary artery catheter placement in the operating room. Heart Vessels. 1994;9:315-321. [PubMed] |
| 6. | Kaplan JA. Essentials of Cardiac Anesthesia: A Volume in Essentials of Anesthesia and Critical Care. : Elsevier Health Sciences 2008; . |
| 7. | Wang XF, Wang JE, Cao LS, Huang YZ, Huang HQ, Deng YB, Wu Y. Application of two-dimensional echocardiography in location of balloon of the Swan-Ganz catheter. Chin Med J (Engl). 1990;103:117-124. [PubMed] |
| 8. | Edwards Lifesciences LLC, Irvine, CA 92614-5686 USA. Swan-Ganz® Balloon-Tipped Catheter Specifications [Internet]. [accessed 2014 Aug 10]. Available from: http: //ht.edwards.com/scin/edwards/sitecollectionimages/products/transcatheter/f75rbh002.pdf. |
| 9. | Gwet KL. Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol. 2008;61:29-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 895] [Cited by in RCA: 914] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 10. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495-1499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2924] [Cited by in RCA: 3448] [Article Influence: 191.6] [Reference Citation Analysis (0)] |
| 11. | Potter A. Echocardiography in acute medicine: a clinical review. Br J Hosp Med (Lond). 2010;71:626-630. [PubMed] |
| 12. | Hall A, Walker J, Welters I. Echocardiography in the ICU: an audit of 3 years practice. Crit Care. 2012;16:P192. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Cowie B. Three years’ experience of focused cardiovascular ultrasound in the peri-operative period. Anaesthesia. 2011;66:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Tempe DK, Gandhi A, Datt V, Gupta M, Tomar AS, Rajesh V, Virmani S, Banerjee A. Length of insertion for pulmonary artery catheters to locate different cardiac chambers in patients undergoing cardiac surgery. Br J Anaesth. 2006;97:147-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Royse C, Donnan GA, Royse A. McGraw-Hill’s pocket guide to perioperative and critical care echocardiography. Sydney: McGraw-Hill 2006; . |