Published online Jul 27, 2014. doi: 10.5313/wja.v3.i2.162
Revised: May 22, 2014
Accepted: June 10, 2014
Published online: July 27, 2014
Processing time: 203 Days and 21.7 Hours
Lumbar radiculopathy, a group of diseases in which the dorsal root ganglia (DRG) or dorsal roots are adversely affected by herniated discs or spinal stenosis, are clinically characterized by spontaneous and evoked types of pain. The pain is underpinned by various distinct pathophysiological mechanisms in the peripheral and central nervous systems. However, the diagnosis of lumbar radiculopathy is still unsatisfactory, because the association of the pain with the neurobiological basis of radiculopathy is largely unknown. Several animal models used to explore the underlying neurobiological basis of lumbar radiculopathy could be classified as mechanical, chemical, or both based on the component of injury. Mechanical injury elevates the intraneural pressure, reduces blood flow, and eventually establishes ischemia in the dorsal root and the DRG. Ischemia may induce ischemic pain and cause nerve damage or death, and the subsequent nerve damage or death may induce neuropathic pain. Chemical injury predominately induces inflammation surrounding the dorsal roots or DRG and consequent inflammatory mediators cause inflammatory pain. Furthermore, DRG neurons sensitized by inflammatory mediators are hypersensitive to innocuous mechanical force (stretch or compression) and responsible for mechanical allodynia in radiculopathy. As well, central sensitization in the spinal cord may play an important role in pain generation in lumbar radiculopathy. Increasing knowledge of pain-generating mechanisms and their translation into clinical symptoms and signs might allow for dissecting the mechanisms that operate in each patient. With precise clinical phenotypic characterization of lumbar radiculopathy and its connection to a specific underlying mechanism, we should be able to design optimal treatments for individuals. This review discusses the present knowledge of lumbar radiculopathy and proposes a novel mechanism-based classification.
Core tip: Lumbar radiculopathy is the most common form of neuropathic pain. However, the diagnosis of lumbar radiculopathy is still not satisfactory because of the largely unknown neurobiological basis of neuropathic pain and paresthesia. Accumulating evidence has shown that lumbar radiculopathy is a multi-factor disease and may involve almost all types of pain, including ischemic, inflammatory, mechanical, and neuropathic pain. Ion channels such as Acid-sensing ion channel 3, Piezo2 and transient receptor potential vanilloid receptor 1 responding to tissue acidosis, mechanical force, and inflammatory mediators may be the pathways transducing the pain.
- Citation: Lin JH, Chiang YH, Chen CC. Lumbar radiculopathy and its neurobiological basis. World J Anesthesiol 2014; 3(2): 162-173
- URL: https://www.wjgnet.com/2218-6182/full/v3/i2/162.htm
- DOI: https://dx.doi.org/10.5313/wja.v3.i2.162
Lumbar radiculopathy or nerve root pain represents one distinct presentation of low back-related leg pain, which is generally characterized by pain radiating to below the knee and into the foot and toes. The annual prevalence of low back pain, varies from 9.9% to 25%. The point prevalence (4.6% to 13.4%) and lifetime prevalence (1.2% to 43%) are high[1], so lumbosacral radicular pain may be the most commonly occurring form of neuropathic pain[2,3].
The terms radicular pain and radiculopathy are sometimes used interchangeably, although they are not synonymous. With radicular pain, only radiating pain is present, whereas with radiculopathy, sensory and/or motor loss can be objectified. Both syndromes frequently occur together and radiculopathy can be a continuum of radicular pain.
Patients with lumbar radiculopathy typically present a chief complaint of pain. The patient may experience the radiating pain as sharp, dull, piercing, throbbing, or burning. Pain caused by a herniated disc classically increases with bending forward, sitting, coughing, or (excessive) stress on the lumbar discs and can be avoided by lying down or sometimes by walking[4]. Conversely, pain due to lumbar spinal-canal stenosis can typically increase during walking and improve immediately with bending forward[5]. In addition to the pain, patients often report paresthesia in affected dermatomes. Although the distribution of pain along a dermatome can determine the affected levels of dorsal roots, the variation in radiation pattern is large. The S1 dermatome seems the most reliable[6]. If present, the dermatomal distribution of paresthesia is more specific[5]. Among the symptoms, pain and paresthesia are often referred to as positive symptoms of radiculopathy, whereas weakness and numbness are considered negative symptoms. Positive symptoms are believed to reflect neuronal hyperactivity, and negative symptoms may stem from diminished neural firing occurring with axonal loss or conduction block[7]. Commonly used physical tests include the straight-leg raise test, Lasègue’s crossed straight-leg raise test, tendon reflexes, and signs of weakness, atrophy or sensory deficits[8-11].
The investigation of the pathway for lumbar radiculopathy in a number of animal models has included mechanical constriction of a nerve root via suture ligation, application of exogenous pro-inflammatory mediators to a nerve root, and application of autologous nucleus pulpous (NP) tissue to a nerve root[12-32]. According to the component of primary injury, these animal models are classified as mechanical or chemical injury or both (Table 1). Mechanical compressors that do not directly produce biochemical effects include silk ligation[26], ameroid constrictors[24], and stainless rods[25,32]; the chemical factors that produce direct biochemical effects include autologous NP application[16,28-30], chromic gut ligatures[27], and Surgiflo[31]. Evidence of mechanical allodynia and thermal hyperalgesia is commonly identified, occurring as early as 2 d post-procedure and persisting for 2 to 6 wk[14,16-23,33,34]. The structural changes in nerve fibers include edema and demyelination, deposition and engulfment of inflammatory cells, and Wallerian degeneration of nerve fibers[29,35]. Mechanical and chemical injury do not differ in pain behaviors or histopathological changes; however, they could have different effects on gene expression in the dorsal root ganglia (DRG) at 7 d after surgery, which suggests that the underlying mechanisms of the 2 types of nerve injury differ[32].
| Injurycomponent | Model | Species | Injury site | Pain behaviors | Motor function |
| Mechanical | Chronic gradual nerve root compression (Cornefjord et al[24]) | Porcine | Preganglion | NA | NA |
| chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis (Hu et al[25]) | Rat (SD) | Dorsal root ganglion | Heat hyperalgesia 5-35 d after surgery | NA | |
| Compression strain of nerve root (Winkelstein et al[26]) | Holtzman rats | Preganglion | Mechanical allodynia | NA | |
| Stainless rod -induced lumbar radiculopathy(Takayama et al[32]) | Rat (SD) | Preganglion | NA | NA | |
| Chemical | Autologus nucleus pulposus-induced lumbar radiculopathy (Olmarker et al[28]) | Porcine | Preganglion | NA | NA |
| Autologus nucleus pulposus-induced lumbar radiculopathy (Yabuki et al[29]) | Rat (SD) | Preganglion | NA | NA | |
| Autologus nucleus pulposus-induced lumbar radiculopathy (Otani et al[30]) | Dog | Preganglion | NA | NA | |
| Autologus nucleus pulposus-induced lumbar radiculopathy (Shamji et al[16]) | Rat (SD) | Dorsal root ganglion | Mechanical allodynia | Marked gait asymmetry, preference to bear weight on the contralateral limb | |
| Mechanical + chemical | Spinal nerve root irritation with chromic gut ligatures (Kawakami et al[27]) | Rat(SD) | Preganglion | Prolonged thermal hyperalgesia 2 to 12 wk | Immeditaely paresis resolved in 2 wk, totally recovered in 6 wk |
| Chronic compression of lumbar dorsal root ganglion with SURGIFLO™(Gu et al[31]) | Rat(SD) | Dorsal root ganglion | Mechanical allodynia and thermal hyperalgesia up to 4 or 5 postoperative week | NA |
The anatomical structure of the nerve root differs from that of DRG. The spinal nerve roots and their nutrient vessels lack a perineurium and feature a poorly developed epineurium. In contrast, DRG, where the soma of sensory neurons reside, feature dense perineurium vascular supply. The blood flow supply is greater in the nerve root proximal than distal to the DRG[36]. Spinal nerve roots are surrounded by cerebrospinal fluid and receive 58% of their nutritional supply from cerebrospinal fluid and 38% from intramural blood vessels, whereas peripheral nerves receive 95% of their nutritional supply from intramural blood vessels[37]. Accordingly, DRG are more sensitive to mechanical compression and consequent ischemia changes than nerve roots and are considered a key player in lumbar radiculopathy. In addition, the direction of information flow from the periphery to DRG to the spinal cord itself is a main factor in the distal lesion inducing strong neuropathic signs. After spinal nerve injury distal to the DRG, the sensory neurons are excited and exhibit ectopic firing. Takiguchi et al[38] observed more severe radiculopathy and more microglia activation in the spinal dorsal horn in rats with injury distal than proximal to the DRG. Another study suggested that spinal-nerve crush injuries produce a greater degree of DRG apoptosis than do corresponding nerve-root crush injuries and that the former injuries are associated with longer-lasting mechanical allodynia[39].
Behavioral changes observed in pre-clinical models of lumbar radicular pain may be similar to painful symptoms observed in human subjects. Patients with low back pain and sciatica report fear of movement and substantial decreases in activity levels[40], and recently, patients with lumbar spinal stenosis reported significantly lower activity levels than both control subjects and patients with knee or hip osteoarthritis[41]. Patients with lumbar radiculopathy have been found with reduced walking velocities, short stride lengths, and increased periods of double limb support[42]. In a rat model of non-compressive disc herniation with autologous NP application, animals exhibited behavioral changes such as heightened behavioral mechanical sensitivity, stance asymmetry, and disturbed gait parameters including symmetry and force analysis[16,43,44]. In animal models of lumbar radiculopathy, motor weakness developed immediately after the injury and pain behaviors developed at the same time. Motor functions recovered gradually within 1 or 2 wk, whereas pain behaviors persisted for at least 6 wk to 3 mo. During the acute or subacute post-injury period, motor weakness is seldom observed in humans but is the predominant symptom in rats. This contradiction may be related to the times of the observations or differences between animal models and humans. Further studies are needed for clarification.
Clinically, in the common lateral type of lumbar disc herniation, radiculopathy is usually ipsilateral, but contralateral radiculopathy exists in some patients[45-48]. Contralateral mechanical allodynia has been shown in some animal models of neuropathic pain[49,50]. In addition, unilateral nerve injuries or inflammation induces molecular changes in the contralateral DRG, which have been demonstrated to contribute to the induction of neuropathic pain[49,51,52]. A previous study suggested that NP application to the unilateral DRG could induce nerve injury, satellite cell activation and upregulation of tumor necrosis factor α (TNF-α) expression in the contralateral DRG[53]. Furthermore, injury of motor neurons might have a significant role in contralateral changes.
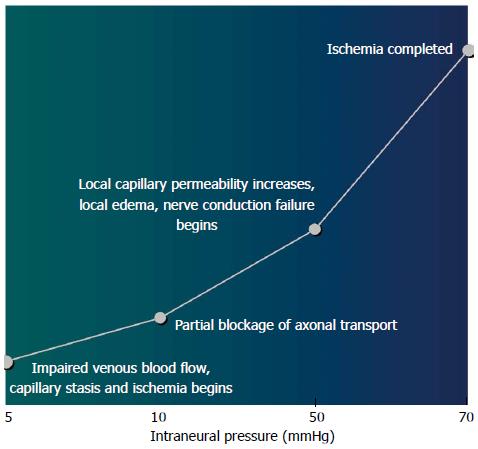
Tissue acidosis and involvement of acid-sensing ion channels: The most pertinent mechanical effect of herniated disc material or degenerative stenosis on neural tissue is likely the result of increased intraneural pressure[7]. With increasing intraneural pressure, mechanical compression of the nerve root induces a decrease in intraradicular blood flow, histological changes such as intramural edema, eletrophysiological changes such as reduced nerve conduction velocity and enhanced excitability of DRG neurons, and reduced mechanical and thermal withdrawal thresholds[54-56] (Figure 1). In addition, compression of the periradicular venous plexus within the foramen and resulting blood stasis can lead to congestion, ischemia, intraneural edema, and increased intraneural pressure[57]. The resulting hypoxia causes tissue acidosis and damage or even death of DRG sensory neurons. Acid-sensing ion channel 3 (ASIC3) is a member of the proton-gated ion channels of the DEG/ENaC/ASIC superfamily, which are two-transmembrane proteins assembling as a trimeric sodium channel that is amiloride-sensitive and voltage-independent[58]. Tissue acidosis activates ASIC3 on DRG sensory neurons and the activation has been reported to be sufficient to cause pain[59]. However, the P2X3 receptor has been demonstrated to mediate nociceptive information of cell damage and inflammation, with activation dependent on peripheral ATP released from the damaged cells. The expression of P2X3 in DRG can be induced by local NP application[60]. Also, ATP may be involved in inducing mechanical and thermal hyperalgesia in experimental animal models. Finally, ATP works together with acid to increase the pH sensitivity of ASIC3 and may enhance the pain caused by acidosis[61].
Neuron damage or death: After damage to the DRG and the dorsal nerve root, primary afferent fibers often show aberrant “ectopic” activity, with an altered pattern of neuronal excitability and conduction causing spontaneous pain and hyperalgesia. The accumulation of sodium channels at or around the site of injury is thought to be responsible for the ectopic activity[62,63] Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels located within the DRG are thought to generate spontaneous rhythmic activity and contribute to neuronal excitability and plasticity[64]. In particular, increased expression of HCN1 channels in large-diameter afferents is responsible for evoking spontaneous pacemaker-driven action potentials in the damaged nerve[65]. In addition, damage to peripheral nerves upregulates vanilloid receptors (TRPV1), which are only marginally expressed under physiological conditions at the membrane of primary afferents[66]. TRPV1 is essential for selective modalities of pain sensation and for tissue injury-induced thermal hyperalgesia[67]. Two studies demonstrated that nerve injury triggers downregulated TRPV1 in damaged afferents but upregulated TRPV1 in uninjured C- and A-fibers[66,68].
Role of mechanosensitive channels: In physiological status, nerve roots and spinal nerves typically demonstrate 2 to 8 mm of glide within their neural foramen depending on the model and measurement technique[69-76]. In compression injury, the presence of periradicular fibrosis will compound the nerve root pain by fixing the nerve in one position, thereby increasing the susceptibility of the nerve root to tension or compression[77-80]. Use of the intraoperative straight-leg raise test in humans has shown that hernia compresses the nerve roots and increases their flatness, thus resulting in a clear disturbance with gliding distance reduced to only a few millimeters, reduced intraradicular blood flow, and significantly deteriorated amplitude of the nerve root action potential after 30 s of the test[79,80]. This transient conduction disturbance probably results from temporary ischemic changes in the nerve root, which suggests that the primary cause of radicular pain is mechanical force of the nerve root induced by periradicular adhesive tissue around the herniated disc[79,80]. However, radicular pain can be produced via stimulation of a swollen or stretched nerve root alone. A normal or uncompressed nerve root could be manipulated, with associated paresthesia but without significant pain[81-83].
Although some intimate contact between the herniated disc material and the nerve root is required for the pain, neither the size of the disc herniation seen on MRI nor the amount of thecal sac deformation is necessarily related to the degree of pain experienced. A common assumption is that some portion of the inflammatory cascade is responsible. Thus, the potentiation or sensitization of the nerve roots or DRG is required for a stretch or tension force on the nerve root to cause radicular pain or ectopic spontaneous activity. The underlying mechanisms of the radicular pain or ectopic spontaneous activity are not clear, and the mechanosensitive channels that allow sensory neurons to transmit noxious mechanical stimuli when the nerve root is under stretch or tension force are still unknown. ASIC3, Piezo2, and channels of transient receptor potential C (TRPC) family have been reported to be essential for a neuron to sense the mechanical force[84-88]. Among these candidate channels, ASIC3 is upregulated in DRG neurons with application of NP[89] or a mixed inflammatory soup[90,91] containing serotonin, bradykinin, interleukins 1 and 6 (IL-1 and IL-6), and TNF-α. Moreover, serotonin potentiates the proton-evoked sustained current of ASIC3[92]. Thereafter, ASIC3, which is upregulated and potentiated in DRG neurons under an inflammatory condition, may be responsible for the neuron to sense the noxious mechanical stimuli.
The application of autologous NP induces electrophysiological changes and similarly enhances DRG neuron excitability, reduces mechanical and thermal withdrawal threshold and nerve blood flow, and causes histological changes such as axonal degeneration, intramural edema, and Schwann cell edema in the nerve root and DRG[28-30,93-99]. Indeed, upon systemic exposure, the NP component of intervertebral disc tissue initiates a specific immune response, likely a consequence of its immune privileged avascular location bounded by the annulus fibrosus[97,100]. In an in vitro canine model, prostaglandin E2, a chemical mediator of inflammation, could provoke an ectopic eruption of impulses from the nerve roots[101]. Leakage of chemical mediators or inflammatory cytokines, which are produced in the painful disk, into the epidural space through anular tears could lead to injury to adjacent nerve roots and the leakage might be the primary pathophysiological mechanism of radiating leg pain without disk herniation[102,103].
Cytokines: TNF-α, IL-1, IL-6: Accumulating evidence shows that sciatica due to disc herniation and low back pain may be related to activation and sensitization of intraspinal nervous structures by disc-derived substances; one key substance for inducing such irritation is TNF-α[12,14,20,35,104-106]. In a rat model of lumbar disc herniation, endoneural macrophages (macrophages infiltrating the DRG), neurons, and activated satellite cells in DRG are the sources of TNF-α[34,51,105,107]. TNF-α can induce neuropathological damage, or neuropathic pain states, which can be prevented by selective TNF-α inhibitors[107,108]. However, initial clinical trials of TNF-α blockers for treating sciatica have shown good[109-112] or inconclusive results[113-116]. Therefore, blockage of other cytokines along with TNF-α may enhance the therapeutic effects because the cytokine network would be inhibited at multiple levels. In fact, cytokines such as IL-1 and IL-6 are strongly linked to radicular pain[21,28,117]. IL-1, IL-6, and TNF-α are activated in the spinal cord, DRG, and Schwann cells in the spinal nerve roots after lumbar spinal stenosis, and their expression is closely related to pain as well as motor nerve dysfunction and degeneration[118].
Glutamate: Discs are avascular and have low rates of cellular metabolism. Because of no reuptake systems for extracellular glutamate in and around cartilage, free glutamate may be cleared quite slowly and much less rapidly in discs than in neural tissue, which contain avid reuptake systems for glutamate[119]. A rat model showed that epidural glutamate infusion at several concentrations created dose-related focal hyperesthesia as measured by von Frey fiber testing[120]. The finding suggests a change in sensory neurotransmission through primary afferents if glutamate cleaved from disc matrix were to diffuse in high enough concentration to the DRG[119], where ionotropic and metabotropic glutamate receptors are found in high densities on cell bodies[121,122].
Protease-activated protein receptor 2: Protease-activated protein receptor 2 (PAR2) is a G-protein–coupled receptor that functions in hemostasis and thrombosis and in the inflammatory and proliferative response triggered by tissue injury[123]. PAR2 is expressed by a subset of sensory neurons and PAR2 agonists to elicit neurogenic inflammation by release of substance P and calcitonin gene-related peptide[124]. PAR2 activation could lower the pain threshold to thermal stimuli via an afferent pathway that involves the activation of spinal neurokinin 1 receptors and prostaglandins[125]. In an animal model of chronic compression of DRG, PAR2 activation was critical for induction of neuronal hyperexcitability induced by nerve injury[126].
Neurotrophic factor and brain-derived trophic factor: Neurotrophic factor (NGF) concentration is increased in response to tissue injury[127,128] and leads to increased brain-derived trophic factor (BDNF) gene expression, mainly in trkA-expressing small- and medium-sized neurons[129-131]. BDNF, a neuromodulator of nociceptive information in the spinal dorsal horn, causes the N-methyl-D-aspartate-mediated depolarization responsible for synaptic plasticity related to central sensitization[132-135]. In a rat model of lumbar disc herniation, Obata et al[34] demonstrated increased NGF-immunoreactive cells and BDNF-immunoreactive neurons within the DRG, which was closely related to pain behaviors, and endoneural injection of NGF led to the same findings in the DRG and in pain behaviors. Thus, increased NGF level in response to tissue or nerve injury upregulates BDNF level in primary sensory afferents, then BDNF causes synaptic plasticity related to central sensitization.
Microgliosis (accumulation of activated microglia) around degenerative neurons is a common pathological feature of various neurological disorders including radiculopathy. Microglia activation in the spinal cord progresses through a hypertrophic morphology, with thickened and retracted processes and an increase in cell number. Peripheral nerve injury leads to marked activation of microglia within the spinal dorsal horn[136] and increases the number of dorsal horn microglia by two- to fourfold[137-141]. Animal models based on compression injury of the DRG demonstrate resultant allodynia and functional deficits associated with increased microglial activation in the spinal cord[105,142-145]. Peripheral nerve injury increases the release of neurotransmitters such as glutamate, substance P, and ATP from primary afferent neurons activating both secondary neurons and surrounding glial cells. These changes appear to be crucial to the ability of glial cells to produce cytokines and other inflammatory agents. The release of inflammatory mediators including TNF-α, IL-1b, IL-6, nitric oxide (NO), and prostaglandins initiates self-propagating enhanced cytokine expression in glial cells. These agents are then capable of sensitizing primary afferent and dorsal horn neurons thereby contributing to neuropathic pain after nerve injury. Therefore, in contrast to behavioral findings, microglia were activated before pain-related behavior and returned to a normal state despite persistent mechanical and thermal hypersensitivity. Increasing evidence shows that microglia cells are involved in the initiation of chronic pain in neuropathic pain models, although no role for microglia in ongoing maintenance of pain has been reported[146].
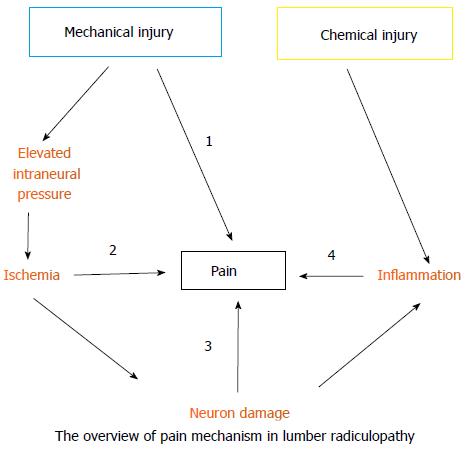
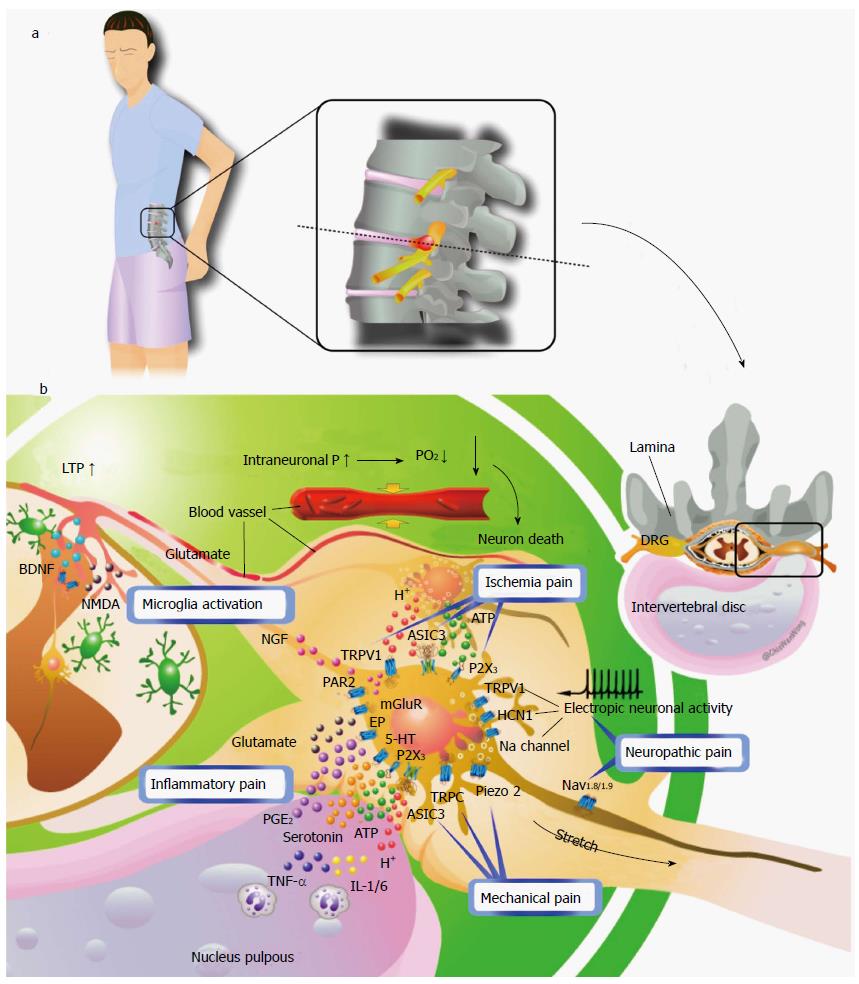
Lumbar radiculopathy is no doubt a multi-factor disease and may involve almost all types of pain, such as ischemic, inflammatory, mechanical, and neuropathic pain (Figure 2). Mechanical injury elevates the intraneural pressure of the dorsal roots and the DRG, reduces blood flow, and eventually establishes ischemia. Ischemia may trigger ischemic pain and cause nerve damage or death. The subsequent nerve damage or death may further induce neuropathic pain. In contrast, chemical injury predominately induces inflammation surrounding the dorsal roots or DRG and the consequent inflammatory mediators cause inflammatory pain. Furthermore, DRG neurons sensitized by inflammatory mediators will produce a nociceptive signal with application of a mechanical force (stretch or compression). As well, central sensitization in the spinal-cord dorsal horn plays an important role in pain generation of lumbar radiculopathy. Here, we propose an overall picture of lumbar radiculopathy and attempt to translate the clinical symptoms and signs based on the present knowledge of the neurobiological basis of pain. (Table 2 and Figure 3)
| Symptoms/signs | Type of pain | Mechanism | Molecules/channels |
| Spontaneous shooting pain | neuropathic | Spontaneous ectopic DRG neuron activity | Na channels |
| Spontaneous ongoing pain | Inflammatory | Inflammation surrounding or within DRG | TNF-α, IL-1/6 |
| Positive straight-leg raise test | Inflammatory, mechanical, ischemic | Induction of ectopic neuron activity or ischemia when a sensitized and constricted nerve root stretches | ASIC3, Piezo2, 5-HTR |
| Sensory deficit | Neuropathic | Apoptosis or phenotype shift of DRG neurons | ? |
| Heat allodynia | Neuropathic | Reduced threshold to heat | TRPV1 |
| Cold allodynia | Neuropathic | Reduced threshold to cold | TRPM8 |
| Static Mechanical allodynia | neuropathic | Reduced threshold to mechanical | ASIC3?, Piezo2? |
| Dynamic Mechanical allodynia | neuropathic | Reduced threshold to mechanical | ASIC3?, Piezo2? |
| Soreness | Inflammatory, ischemic | Increased protons | ASIC3, TNF-α, IL-1, IL-6 |
Lumbar radiculopathy remains an important and largely unresolved medical problem that requires further research into the etiological factors to determine the correct diagnosis, despite pronounced advances in the knowledge of the neurological basis of pain in the past decade. There is clear interest in identifying the cell populations affected by disc herniation-induced radiculopathy, and the role of neurotransmitters and their receptors that mediate the symptomatic and functional deficits of radiculopathy. However, our ability to translate pain complaints and sensory abnormalities into specific pathophysiological mechanisms that have treatment implications is in its infancy. Whether different underlying mechanisms cause different symptoms and signs in patients is unknown. Improvement in the animal models of lumbar radiculopathy and the methods of pain-behaviors is warranted.
P- Reviewer: Hanci V, Kapur S S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Konstantinou K, Dunn KM. Sciatica: review of epidemiological studies and prevalence estimates. Spine (Phila Pa 1976). 2008;33:2464-2472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 367] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 2. | Khoromi S, Patsalides A, Parada S, Salehi V, Meegan JM, Max MB. Topiramate in chronic lumbar radicular pain. J Pain. 2005;6:829-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1450] [Cited by in RCA: 1357] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 4. | Younes M, Béjia I, Aguir Z, Letaief M, Hassen-Zrour S, Touzi M, Bergaoui N. Prevalence and risk factors of disk-related sciatica in an urban population in Tunisia. Joint Bone Spine. 2006;73:538-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Tarulli AW, Raynor EM. Lumbosacral radiculopathy. Neurol Clin. 2007;25:387-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 6. | Murphy DR, Hurwitz EL, Gerrard JK, Clary R. Pain patterns and descriptions in patients with radicular pain: does the pain necessarily follow a specific dermatome? Chiropr Osteopat. 2009;17:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (2)] |
| 7. | Lipetz JS. Pathophysiology of inflammatory, degenerative, and compressive radiculopathies. Phys Med Rehabil Clin N Am. 2002;13:439-449. [PubMed] |
| 8. | Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 1992;268:760-765. [PubMed] |
| 9. | Rebain R, Baxter GD, McDonough S. A systematic review of the passive straight leg raising test as a diagnostic aid for low back pain (1989 to 2000). Spine (Phila Pa 1976). 2002;27:E388-E395. [PubMed] |
| 10. | Rebain R, Baxter GD, McDonough S. The passive straight leg raising test in the diagnosis and treatment of lumbar disc herniation: a survey of United kingdom osteopathic opinion and clinical practice. Spine (Phila Pa 1976). 2003;28:1717-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | van den Hoogen HM, Koes BW, van Eijk JT, Bouter LM. On the accuracy of history, physical examination, and erythrocyte sedimentation rate in diagnosing low back pain in general practice. A criteria-based review of the literature. Spine (Phila Pa 1976). 1995;20:318-327. [PubMed] |
| 12. | Olmarker K, Rydevik B. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity: possible implications for future pharmacologic treatment strategies of sciatica. Spine (Phila Pa 1976). 2001;26:863-869. [PubMed] |
| 13. | Murata Y, Rydevik B, Takahashi K, Larsson K, Olmarker K. Incision of the intervertebral disc induces disintegration and increases permeability of the dorsal root ganglion capsule. Spine (Phila Pa 1976). 2005;30:1712-1716. [PubMed] |
| 14. | Olmarker K, Nutu M, Størkson R. Changes in spontaneous behavior in rats exposed to experimental disc herniation are blocked by selective TNF-alpha inhibition. Spine (Phila Pa 1976). 2003;28:1635-141; discussion 1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Onda A, Murata Y, Rydevik B, Larsson K, Kikuchi S, Olmarker K. Immunoreactivity of brain-derived neurotrophic factor in rat dorsal root ganglion and spinal cord dorsal horn following exposure to herniated nucleus pulposus. Neurosci Lett. 2003;352:49-52. [PubMed] |
| 16. | Shamji MF, Allen KD, So S, Jing L, Adams SB, Schuh R, Huebner J, Kraus VB, Friedman AH, Setton LA. Gait abnormalities and inflammatory cytokines in an autologous nucleus pulposus model of radiculopathy. Spine (Phila Pa 1976). 2009;34:648-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Suzuki M, Inoue G, Gemba T, Watanabe T, Ito T, Koshi T, Yamauchi K, Yamashita M, Orita S, Eguchi Y. Nuclear factor-kappa B decoy suppresses nerve injury and improves mechanical allodynia and thermal hyperalgesia in a rat lumbar disc herniation model. Eur Spine J. 2009;18:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Kato K, Kikuchi S, Konno S, Sekiguchi M. Participation of 5-hydroxytryptamine in pain-related behavior induced by nucleus pulposus applied on the nerve root in rats. Spine (Phila Pa 1976). 2008;33:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Onda A, Murata Y, Rydevik B, Larsson K, Kikuchi S, Olmarker K. Nerve growth factor content in dorsal root ganglion as related to changes in pain behavior in a rat model of experimental lumbar disc herniation. Spine (Phila Pa 1976). 2005;30:188-193. [PubMed] |
| 20. | Cuellar JM, Montesano PX, Carstens E. Role of TNF-alpha in sensitization of nociceptive dorsal horn neurons induced by application of nucleus pulposus to L5 dorsal root ganglion in rats. Pain. 2004;110:578-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Ito T, Ohtori S, Inoue G, Koshi T, Doya H, Ozawa T, Saito T, Moriya H, Takahashi K. Glial phosphorylated p38 MAP kinase mediates pain in a rat model of lumbar disc herniation and induces motor dysfunction in a rat model of lumbar spinal canal stenosis. Spine (Phila Pa 1976). 2007;32:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Igarashi T, Kikuchi S, Shubayev V, Myers RR. 2000 Volvo Award winner in basic science studies: Exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine (Phila Pa 1976). 2000;25:2975-2980. [PubMed] |
| 23. | Sasaki N, Kikuchi S, Konno S, Sekiguchi M, Watanabe K. Anti-TNF-alpha antibody reduces pain-behavioral changes induced by epidural application of nucleus pulposus in a rat model depending on the timing of administration. Spine (Phila Pa 1976). 2007;32:413-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Cornefjord M, Sato K, Olmarker K, Rydevik B, Nordborg C. A model for chronic nerve root compression studies. Presentation of a porcine model for controlled, slow-onset compression with analyses of anatomic aspects, compression onset rate, and morphologic and neurophysiologic effects. Spine (Phila Pa 1976). 1997;22:946-957. [PubMed] |
| 25. | Hu SJ, Xing JL. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain. 1998;77:15-23. [PubMed] |
| 26. | Winkelstein BA, Weinstein JN, DeLeo JA. The role of mechanical deformation in lumbar radiculopathy: an in vivo model. Spine (Phila Pa 1976). 2002;27:27-33. [PubMed] |
| 27. | Kawakami M, Weinstein JN, Chatani K, Spratt KF, Meller ST, Gebhart GF. Experimental lumbar radiculopathy. Behavioral and histologic changes in a model of radicular pain after spinal nerve root irritation with chromic gut ligatures in the rat. Spine (Phila Pa 1976). 1994;19:1795-1802. [PubMed] |
| 28. | Olmarker K, Rydevik B, Nordborg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine (Phila Pa 1976). 1993;18:1425-1432. [PubMed] |
| 29. | Yabuki S, Kikuchi S, Olmarker K, Myers RR. Acute effects of nucleus pulposus on blood flow and endoneurial fluid pressure in rat dorsal root ganglia. Spine (Phila Pa 1976). 1998;23:2517-2523. [PubMed] |
| 30. | Otani K, Arai I, Mao GP, Konno S, Olmarker K, Kikuchi S. Nucleus pulposus-induced nerve root injury: relationship between blood flow and motor nerve conduction velocity. Neurosurgery. 1999;45:614-619; discussion 619-620. [PubMed] |
| 31. | Gu X, Yang L, Wang S, Sung B, Lim G, Mao J, Zeng Q, Yang C, Mao J. A rat model of radicular pain induced by chronic compression of lumbar dorsal root ganglion with SURGIFLO. Anesthesiology. 2008;108:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Takayama B, Sekiguchi M, Yabuki S, Fujita I, Shimada H, Kikuchi S. Gene expression changes in dorsal root ganglion of rat experimental lumber disc herniation models. Spine (Phila Pa 1976). 2008;33:1829-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Kawakami M, Matsumoto T, Tamaki T. Roles of thromboxane A2 and leukotriene B4 in radicular pain induced by herniated nucleus pulposus. J Orthop Res. 2001;19:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Obata K, Tsujino H, Yamanaka H, Yi D, Fukuoka T, Hashimoto N, Yonenobu K, Yoshikawa H, Noguchi K. Expression of neurotrophic factors in the dorsal root ganglion in a rat model of lumbar disc herniation. Pain. 2002;99:121-132. [PubMed] |
| 35. | Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine (Phila Pa 1976). 1998;23:2538-2544. [PubMed] |
| 36. | Naito M, Owen JH, Bridwell KH, Oakley DM. Blood flow direction in the lumbar nerve root. Spine (Phila Pa 1976). 1990;15:966-968. [PubMed] |
| 37. | Rydevik B, Holm S, Brown MD, Lundborg G. Diffusion from the cerebrospinal fluid as a nutritional pathway for spinal nerve roots. Acta Physiol Scand. 1990;138:247-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Takiguchi N, Yoshida M, Taniguchi W, Hashizume H, Yamada H, Miyazaki N, Nishio N, Nakatsuka T. Distinct degree of radiculopathy at different levels of peripheral nerve injury. Mol Pain. 2012;8:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 39. | Sekiguchi M, Sekiguchi Y, Konno S, Kobayashi H, Homma Y, Kikuchi S. Comparison of neuropathic pain and neuronal apoptosis following nerve root or spinal nerve compression. Eur Spine J. 2009;18:1978-1985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | van Wilgen CP, Stewart R, Patrick Stegeman PT, Coppes M, van Wijhe M. Fear of movement in pre-operative patients with a lumbar stenosis and or herniated disc: factor structure of the Tampa scale for kinesiophobia. Man Ther. 2010;15:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Winter CC, Brandes M, Müller C, Schubert T, Ringling M, Hillmann A, Rosenbaum D, Schulte TL. Walking ability during daily life in patients with osteoarthritis of the knee or the hip and lumbar spinal stenosis: a cross sectional study. BMC Musculoskelet Disord. 2010;11:233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 42. | Lee JH, An JH, Lee SH, Seo IS. Three-dimensional gait analysis of patients with weakness of ankle dorsiflexor as a result of unilateral L5 radiculopathy. J Back Musculoskelet Rehabil. 2010;23:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Allen KD, Shamji MF, Mata BA, Gabr MA, Sinclair SM, Schmitt DO, Richardson WJ, Setton LA. Kinematic and dynamic gait compensations in a rat model of lumbar radiculopathy and the effects of tumor necrosis factor-alpha antagonism. Arthritis Res Ther. 2011;13:R137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Hwang PY, Allen KD, Shamji MF, Jing L, Mata BA, Gabr MA, Huebner JL, Kraus VB, Richardson WJ, Setton LA. Changes in midbrain pain receptor expression, gait and behavioral sensitivity in a rat model of radiculopathy. Open Orthop J. 2012;6:383-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Auld AW, DeWall JG. Myelographic defect on the side opposite the leg pain. A case report with an explanation of mechanism of action. Spine (Phila Pa 1976). 1979;4:174-175. [PubMed] |
| 46. | Choudhury AR, Taylor JC, Worthington BS, Whitaker R. Lumbar radiculopathy contralateral to upper lumbar disc herniation: report of 3 cases. Br J Surg. 1978;65:842-844. [PubMed] |
| 47. | Mirovsky Y, Halperin N. Eccentric compression of the spinal canal causing dominantly contralateral-side symptoms. J Spinal Disord. 2000;13:174-177. [PubMed] |
| 48. | Sucu HK, Gelal F. Lumbar disk herniation with contralateral symptoms. Eur Spine J. 2006;15:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Hatashita S, Sekiguchi M, Kobayashi H, Konno S, Kikuchi S. Contralateral neuropathic pain and neuropathology in dorsal root ganglion and spinal cord following hemilateral nerve injury in rats. Spine (Phila Pa 1976). 2008;33:1344-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Schreiber KL, Beitz AJ, Wilcox GL. Activation of spinal microglia in a murine model of peripheral inflammation-induced, long-lasting contralateral allodynia. Neurosci Lett. 2008;440:63-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Dubový P, Tucková L, Jancálek R, Svízenská I, Klusáková I. Increased invasion of ED-1 positive macrophages in both ipsi- and contralateral dorsal root ganglia following unilateral nerve injuries. Neurosci Lett. 2007;427:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Siemionow K, Klimczak A, Brzezicki G, Siemionow M, McLain RF. The effects of inflammation on glial fibrillary acidic protein expression in satellite cells of the dorsal root ganglion. Spine (Phila Pa 1976). 2009;34:1631-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Li Y, Xi C, Niu M, Liu X, Chi Z, Wang X, Yan J. Contralateral neuropathology in dorsal root ganglia in a rat model of noncompressive disc herniation. Neurosci Lett. 2011;493:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 54. | Olmarker K, Rydevik B, Hansson T, Holm S. Compression-induced changes of the nutritional supply to the porcine cauda equina. J Spinal Disord. 1990;3:25-29. [PubMed] |
| 55. | Olmarker K, Rydevik B, Holm S. Edema formation in spinal nerve roots induced by experimental, graded compression. An experimental study on the pig cauda equina with special reference to differences in effects between rapid and slow onset of compression. Spine (Phila Pa 1976). 1989;14:569-573. [PubMed] |
| 56. | Olmarker K, Rydevik B, Holm S, Bagge U. Effects of experimental graded compression on blood flow in spinal nerve roots. A vital microscopic study on the porcine cauda equina. J Orthop Res. 1989;7:817-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 112] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | Hoyland JA, Freemont AJ, Jayson MI. Intervertebral foramen venous obstruction. A cause of periradicular fibrosis? Spine (Phila Pa 1976). 1989;14:558-568. [PubMed] |
| 58. | Wu WL, Cheng CF, Sun WH, Wong CW, Chen CC. Targeting ASIC3 for pain, anxiety, and insulin resistance. Pharmacol Ther. 2012;134:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Su YS, Sun WH, Chen CC. Moleculr mechanism of inflammatory pain. World J Anesthsiol. 2014;27:71-81. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (9)] |
| 60. | Takahashi Sato K, Satoh K, Sekiguchi M, Kikuchi S, Konno S, Murakawa M, Rydevik B, Olmarker K. Local application of nucleus pulposus induces expression OF P2X3 in rat dorsal root ganglion cells. Fukushima J Med Sci. 2012;58:17-21. [PubMed] |
| 61. | Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, Marsh-Haffner J, Adelman JP, Almers W, Elde RP. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron. 2010;68:739-749. [PubMed] |
| 62. | Harvey VL, Dickenson AH. Mechanisms of pain in nonmalignant disease. Curr Opin Support Palliat Care. 2008;2:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | England JD, Happel LT, Kline DG, Gamboni F, Thouron CL, Liu ZP, Levinson SR. Sodium channel accumulation in humans with painful neuromas. Neurology. 1996;47:272-276. [PubMed] |
| 64. | Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 905] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 65. | Chaplan SR, Guo HQ, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM, Dubin AE. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci. 2003;23:1169-1178. [PubMed] |
| 66. | Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur J Neurosci. 2001;13:2105-2114. [PubMed] |
| 67. | Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306-313. [PubMed] |
| 68. | Hong S, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in the expression and function of vanilloid receptor 1. J Biol Chem. 2005;280:618-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 170] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 69. | Goddard MD, Reid JD. Movements induced by straight leg raising in the lumbo-sacral roots, nerves and plexus, and in the intrapelvic section of the sciatic nerve. J Neurol Neurosurg Psychiatry. 1965;28:12-18. [PubMed] |
| 70. | Falconer MA, McGEORGE M, BEGG AC. Observations on the cause and mechanism of symptom-production in sciatica and low-back pain. J Neurol Neurosurg Psychiatry. 1948;11:13-26. [PubMed] |
| 71. | Charnley J. Orthopaedic signs in the diagnosis of disc protrusion. With special reference to the straight-leg-raising test. Lancet. 1951;1:186-192. [PubMed] |
| 72. | Breig A, Troup JD. Biomechanical considerations in the straight-leg-raising test. Cadaveric and clinical studies of the effects of medial hip rotation. Spine (Phila Pa 1976). 1979;4:242-250. [PubMed] |
| 73. | Graham GE. Intraoperative straight-leg raising during laminectomy and disk excision for sciatica. Clin Orthop Relat Res. 1981;343-344. [PubMed] |
| 74. | Smith SA, Massie JB, Chesnut R, Garfin SR. Straight leg raising. Anatomical effects on the spinal nerve root without and with fusion. Spine (Phila Pa 1976). 1993;18:992-999. [PubMed] |
| 75. | Gilbert KK, Brismée JM, Collins DL, James CR, Shah RV, Sawyer SF, Sizer PS. 2006 Young Investigator Award Winner: lumbosacral nerve root displacement and strain: part 1. A novel measurement technique during straight leg raise in unembalmed cadavers. Spine (Phila Pa 1976). 2007;32:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Gilbert KK, Brismée JM, Collins DL, James CR, Shah RV, Sawyer SF, Sizer PS. 2006 Young Investigator Award Winner: lumbosacral nerve root displacement and strain: part 2. A comparison of 2 straight leg raise conditions in unembalmed cadavers. Spine (Phila Pa 1976). 2007;32:1521-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | Spencer DL, Miller JA, Bertolini JE. The effect of intervertebral disc space narrowing on the contact force between the nerve root and a simulated disc protrusion. Spine (Phila Pa 1976). 1984;9:422-426. [PubMed] |
| 78. | Kobayashi S, Baba H, Uchida K, Kokubo Y, Kubota C, Yamada S, Suzuki Y, Yoshizawa H. Effect of mechanical compression on the lumbar nerve root: localization and changes of intraradicular inflammatory cytokines, nitric oxide, and cyclooxygenase. Spine (Phila Pa 1976). 2005;30:1699-1705. [PubMed] |
| 79. | Kobayashi S, Suzuki Y, Asai T, Yoshizawa H. Changes in nerve root motion and intraradicular blood flow during intraoperative femoral nerve stretch test. Report of four cases. J Neurosurg. 2003;99:298-305. [PubMed] |
| 80. | Kobayashi S, Takeno K, Yayama T, Awara K, Miyazaki T, Guerrero A, Baba H. Pathomechanisms of sciatica in lumbar disc herniation: effect of periradicular adhesive tissue on electrophysiological values by an intraoperative straight leg raising test. Spine (Phila Pa 1976). 2010;35:2004-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 81. | Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am. 1991;22:181-187. [PubMed] |
| 82. | Murphy RW. Nerve roots and spinal nerves in degenerative disk disease. Clin Orthop Relat Res. 1977;46-60. [PubMed] |
| 83. | Rydevik B, Brown MD, Lundborg G. Pathoanatomy and pathophysiology of nerve root compression. Spine (Phila Pa 1976). 1984;9:7-15. [PubMed] |
| 84. | Chen CC, Wong CW. Neurosensory mechanotransduction through acid-sensing ion channels. J Cell Mol Med. 2013;17:337-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 85. | Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci USA. 1998;95:10240-10245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 378] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 86. | Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ, Zimmer A. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci USA. 2002;99:8992-8997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 249] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 87. | Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2492] [Cited by in RCA: 2152] [Article Influence: 143.5] [Reference Citation Analysis (0)] |
| 88. | Eijkelkamp N, Linley JE, Torres JM, Bee L, Dickenson AH, Gringhuis M, Minett MS, Hong GS, Lee E, Oh U. A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat Commun. 2013;4:1682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 89. | Ohtori S, Inoue G, Koshi T, Ito T, Doya H, Saito T, Moriya H, Takahashi K. Up-regulation of acid-sensing ion channel 3 in dorsal root ganglion neurons following application of nucleus pulposus on nerve root in rats. Spine (Phila Pa 1976). 2006;31:2048-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 90. | Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22:10662-10670. [PubMed] |
| 91. | Mamet J, Lazdunski M, Voilley N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J Biol Chem. 2003;278:48907-48913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 92. | Wang X, Li WG, Yu Y, Xiao X, Cheng J, Zeng WZ, Peng Z, Xi Zhu M, Xu TL. Serotonin facilitates peripheral pain sensitivity in a manner that depends on the nonproton ligand sensing domain of ASIC3 channel. J Neurosci. 2013;33:4265-4279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 93. | Yabuki S, Kawaguchi Y, Nordborg C, Kikuchi S, Rydevik B, Olmarker K. Effects of lidocaine on nucleus pulposus-induced nerve root injury. A neurophysiologic and histologic study of the pig cauda equina. Spine (Phila Pa 1976). 1998;23:2383-2389; discussion 2389-2390. [PubMed] |
| 94. | Ozawa K, Atsuta Y, Kato T. Chronic effects of the nucleus pulposus applied to nerve roots on ectopic firing and conduction velocity. Spine (Phila Pa 1976). 2001;26:2661-2665. [PubMed] |
| 95. | Takebayashi T, Cavanaugh JM, Cüneyt Ozaktay A, Kallakuri S, Chen C. Effect of nucleus pulposus on the neural activity of dorsal root ganglion. Spine (Phila Pa 1976). 2001;26:940-945. [PubMed] |
| 96. | Anzai H, Hamba M, Onda A, Konno S, Kikuchi S. Epidural application of nucleus pulposus enhances nociresponses of rat dorsal horn neurons. Spine (Phila Pa 1976). 2002;27:E50-E55. [PubMed] |
| 97. | Kawakami M, Tamaki T, Weinstein JN, Hashizume H, Nishi H, Meller ST. Pathomechanism of pain-related behavior produced by allografts of intervertebral disc in the rat. Spine (Phila Pa 1976). 1996;21:2101-2107. [PubMed] |
| 98. | Yabuki S, Igarashi T, Kikuchi S. Application of nucleus pulposus to the nerve root simultaneously reduces blood flow in dorsal root ganglion and corresponding hindpaw in the rat. Spine (Phila Pa 1976). 2000;25:1471-1476. [PubMed] |
| 99. | Olmarker K, Nordborg C, Larsson K, Rydevik B. Ultrastructural changes in spinal nerve roots induced by autologous nucleus pulposus. Spine (Phila Pa 1976). 1996;21:411-414. [PubMed] |
| 100. | Shamji MF, Setton LA, Jarvis W, So S, Chen J, Jing L, Bullock R, Isaacs RE, Brown C, Richardson WJ. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62:1974-1982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 244] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 101. | Muramoto T, Atsuta Y, Iwahara T, Sato M, Takemitsu Y. The action of prostaglandin E2 and triamcinolone acetonide on the firing activity of lumbar nerve roots. Int Orthop. 1997;21:172-175. [PubMed] |
| 102. | Peng B, Wu W, Li Z, Guo J, Wang X. Chemical radiculitis. Pain. 2007;127:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 103. | Bush MS, Allt G. Distribution of anionic sites on the perineurium. J Anat. 1992;181:79-87. [PubMed] |
| 104. | Ozaktay AC, Kallakuri S, Takebayashi T, Cavanaugh JM, Asik I, DeLeo JA, Weinstein JN. Effects of interleukin-1 beta, interleukin-6, and tumor necrosis factor on sensitivity of dorsal root ganglion and peripheral receptive fields in rats. Eur Spine J. 2006;15:1529-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 105. | Otoshi K, Kikuchi S, Konno S, Sekiguchi M. The reactions of glial cells and endoneurial macrophages in the dorsal root ganglion and their contribution to pain-related behavior after application of nucleus pulposus onto the nerve root in rats. Spine (Phila Pa 1976). 2010;35:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 106. | Nakamae T, Ochi M, Olmarker K. Pharmacological inhibition of tumor necrosis factor may reduce pain behavior changes induced by experimental disc puncture in the rat: an experimental study in rats. Spine (Phila Pa 1976). 2011;36:E232-E236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 107. | Wagner R, Myers RR. Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. Neuroreport. 1996;7:2897-2901. [PubMed] |
| 108. | Murata Y, Onda A, Rydevik B, Takahashi K, Olmarker K. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced histologic changes in the dorsal root ganglion. Spine (Phila Pa 1976). 2004;29:2477-2484. [PubMed] |
| 109. | Karppinen J, Korhonen T, Malmivaara A, Paimela L, Kyllönen E, Lindgren KA, Rantanen P, Tervonen O, Niinimäki J, Seitsalo S. Tumor necrosis factor-alpha monoclonal antibody, infliximab, used to manage severe sciatica. Spine (Phila Pa 1976). 2003;28:750-753; discussion 753-754. [PubMed] |
| 110. | Genevay S, Stingelin S, Gabay C. Efficacy of etanercept in the treatment of acute, severe sciatica: a pilot study. Ann Rheum Dis. 2004;63:1120-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 111. | Korhonen T, Karppinen J, Malmivaara A, Autio R, Niinimäki J, Paimela L, Kyllönen E, Lindgren KA, Tervonen O, Seitsalo S. Efficacy of infliximab for disc herniation-induced sciatica: one-year follow-up. Spine (Phila Pa 1976). 2004;29:2115-2119. [PubMed] |
| 112. | Cohen SP, Bogduk N, Dragovich A, Buckenmaier CC, Griffith S, Kurihara C, Raymond J, Richter PJ, Williams N, Yaksh TL. Randomized, double-blind, placebo-controlled, dose-response, and preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica. Anesthesiology. 2009;110:1116-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 113. | Korhonen T, Karppinen J, Paimela L, Malmivaara A, Lindgren KA, Bowman C, Hammond A, Kirkham B, Järvinen S, Niinimäki J. The treatment of disc-herniation-induced sciatica with infliximab: one-year follow-up results of FIRST II, a randomized controlled trial. Spine (Phila Pa 1976). 2006;31:2759-2766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 114. | Korhonen T, Karppinen J, Paimela L, Malmivaara A, Lindgren KA, Järvinen S, Niinimäki J, Veeger N, Seitsalo S, Hurri H. The treatment of disc herniation-induced sciatica with infliximab: results of a randomized, controlled, 3-month follow-up study. Spine (Phila Pa 1976). 2005;30:2724-2728. [PubMed] |
| 115. | Mulleman D, Mammou S, Griffoul I, Watier H, Goupille P. Pathophysiology of disk-related low back pain and sciatica. II. Evidence supporting treatment with TNF-alpha antagonists. Joint Bone Spine. 2006;73:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 116. | Wang H, Schiltenwolf M, Buchner M. The role of TNF-alpha in patients with chronic low back pain-a prospective comparative longitudinal study. Clin J Pain. 2008;24:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 117. | Sekiguchi M, Kikuchi S. Experimental studies of lumbar spinal stenosis. Clin Calcium. 2005;15:51-56. [PubMed] |
| 118. | Sekiguchi M, Kikuchi S, Myers RR. Experimental spinal stenosis: relationship between degree of cauda equina compression, neuropathology, and pain. Spine (Phila Pa 1976). 2004;29:1105-1111. [PubMed] |
| 119. | Osgood DP, Kenney EV, Harrington WF, Harrington JF. Excrescence of neurotransmitter glutamate from disc material has nociceptive qualities: evidence from a rat model. Spine J. 2010;10:999-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 120. | Harrington JF, Messier AA, Hoffman L, Yu E, Dykhuizen M, Barker K. Physiological and behavioral evidence for focal nociception induced by epidural glutamate infusion in rats. Spine (Phila Pa 1976). 2005;30:606-612. [PubMed] |
| 121. | Goudet C, Magnaghi V, Landry M, Nagy F, Gereau RW, Pin JP. Metabotropic receptors for glutamate and GABA in pain. Brain Res Rev. 2009;60:43-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 122. | Larsson M. Ionotropic glutamate receptors in spinal nociceptive processing. Mol Neurobiol. 2009;40:260-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 123. | Traynelis SF, Trejo J. Protease-activated receptor signaling: new roles and regulatory mechanisms. Curr Opin Hematol. 2007;14:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 124. | Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 706] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 125. | Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF, Cirino G, Gerard N, Basbaum AI, Andrade-Gordon P. Proteinase-activated receptor-2 and hyperalgesia: A novel pain pathway. Nat Med. 2001;7:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 364] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 126. | Huang ZJ, Li HC, Cowan AA, Liu S, Zhang YK, Song XJ. Chronic compression or acute dissociation of dorsal root ganglion induces cAMP-dependent neuronal hyperexcitability through activation of PAR2. Pain. 2012;153:1426-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 127. | Woolf CJ, Ma QP, Allchorne A, Poole S. Peripheral cell types contributing to the hyperalgesic action of nerve growth factor in inflammation. J Neurosci. 1996;16:2716-2723. [PubMed] |
| 128. | Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997;121:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 526] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 129. | Donnerer J, Schuligoi R, Stein C. Increased content and transport of substance P and calcitonin gene-related peptide in sensory nerves innervating inflamed tissue: evidence for a regulatory function of nerve growth factor in vivo. Neuroscience. 1992;49:693-698. [PubMed] |
| 130. | Apfel SC, Wright DE, Wiideman AM, Dormia C, Snider WD, Kessler JA. Nerve growth factor regulates the expression of brain-derived neurotrophic factor mRNA in the peripheral nervous system. Mol Cell Neurosci. 1996;7:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 165] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 131. | Cho HJ, Kim JK, Zhou XF, Rush RA. Increased brain-derived neurotrophic factor immunoreactivity in rat dorsal root ganglia and spinal cord following peripheral inflammation. Brain Res. 1997;764:269-272. [PubMed] |
| 132. | Kerr BJ, Bradbury EJ, Bennett DL, Trivedi PM, Dassan P, French J, Shelton DB, McMahon SB, Thompson SW. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J Neurosci. 1999;19:5138-5148. [PubMed] |
| 133. | Kafitz KW, Rose CR, Thoenen H, Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 1999;401:918-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 423] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 134. | Mannion RJ, Costigan M, Decosterd I, Amaya F, Ma QP, Holstege JC, Ji RR, Acheson A, Lindsay RM, Wilkinson GA. Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc Natl Acad Sci USA. 1999;96:9385-9390. [PubMed] |
| 135. | Thompson SW, Bennett DL, Kerr BJ, Bradbury EJ, McMahon SB. Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. Proc Natl Acad Sci USA. 1999;96:7714-7718. [PubMed] |
| 136. | Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol. 1999;157:289-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 350] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 137. | Beggs S, Salter MW. Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain Behav Immun. 2007;21:624-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 138. | Clark AK, Yip PK, Grist J, Gentry C, Staniland AA, Marchand F, Dehvari M, Wotherspoon G, Winter J, Ullah J. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc Natl Acad Sci USA. 2007;104:10655-10660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 369] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 139. | Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain. 2009;13:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 260] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 140. | Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1188] [Cited by in RCA: 1211] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 141. | Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27:12396-12406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 338] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 142. | Hashizume H, DeLeo JA, Colburn RW, Weinstein JN. Spinal glial activation and cytokine expression after lumbar root injury in the rat. Spine (Phila Pa 1976). 2000;25:1206-1217. [PubMed] |
| 143. | Hunt JL, Winkelstein BA, Rutkowski MD, Weinstein JN, DeLeo JA. Repeated injury to the lumbar nerve roots produces enhanced mechanical allodynia and persistent spinal neuroinflammation. Spine (Phila Pa 1976). 2001;26:2073-2079. [PubMed] |
| 144. | Rothman SM, Huang Z, Lee KE, Weisshaar CL, Winkelstein BA. Cytokine mRNA expression in painful radiculopathy. J Pain. 2009;10:90-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 145. | Winkelstein BA, DeLeo JA. Nerve root injury severity differentially modulates spinal glial activation in a rat lumbar radiculopathy model: considerations for persistent pain. Brain Res. 2002;956:294-301. [PubMed] |
| 146. | Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 410] [Article Influence: 19.5] [Reference Citation Analysis (0)] |