Published online Mar 27, 2014. doi: 10.5313/wja.v3.i1.12
Revised: December 9, 2013
Accepted: February 16, 2014
Published online: March 27, 2014
Processing time: 132 Days and 23.1 Hours
Ultrasound (US) is being extensively used an imaging tool in regional anesthesia (RA) and pain practice. Although it was first used in a regional block in 1978, it was only in 1994 that the first direct use of US in RA was reported. Like any other medical tool, its utility is only realized when the performing physician is able to understand the principles behind its application. Efficient use of US also requires an understanding of physical variables which can be suitable modified to produce a clear image of the structure of importance. This brief narrative review summarises the advantages of US in RA and pain practice over the conventionally used localising or imaging tools. The second section deals with the physics behind US. It highlights the necessary physical concepts such as wavelength, frequency and generation of US waves. It also informs the reader about the possible US and tissue interactions, use of US transducers and their differences. The third section deals with understanding the control variables in a typical US machine and how they could be modified to improve the image quality. The final section highlights the various artifacts that could be associated.
Core tip: This review summarises the essential concepts of ultrasound (US) physics and equipment operation. To make the best use of US in regional anesthesia and pain practice, it is important to obtain a quality image. Since anesthesiologists do not depend on any image technician, it is necessary for them to understand the operating principles of the equipment. The physician will also have to choose an appropriate transducer, and make suitable adjustments in gain and focus. All these concepts are detailed in simple terms along with necessary figures for easy understanding.
- Citation: Shanthanna H. Review of essential understanding of ultrasound physics and equipment operation. World J Anesthesiol 2014; 3(1): 12-17
- URL: https://www.wjgnet.com/2218-6182/full/v3/i1/12.htm
- DOI: https://dx.doi.org/10.5313/wja.v3.i1.12
Ultrasound (US) is being increasingly employed in the field of anesthesiology and pain medicine. Medical use of US, for diagnostic brain imaging was first done by Dr Karl Theo Dussik[1]. The published report of Doppler technology for regional block came in 1978[2]. Subsequently it was not popularly used in the field of anesthesia until the last couple of centuries. The first direct use of US for regional block was performed in 1994[3]. The number of physicians who use it in their daily practice has increased significantly. Although tremendously useful, it is still a machine and it is up to the physician using it to make the most of it. As Dr. Marhofer et al[4] has said, informed scepticism should accompany an increasing enthusiasm. The appropriate use of US technology in regional anesthesia (RA) requires a thorough knowledge of anatomy, an essential understanding of its working, and adequate training in the use of needling techniques. Accordingly essential categories of training in US guided interventional procedures are supposed to involve: (1) understanding device operations; (2) image optimization; (3) image interpretation; and (4) visualisation of needle insertion and injection[5]. This review summarises the physical principles involved in US operation and also an understanding of the equipment involved and its appropriate use.
US has several advantages over the conventional imaging or nerve localisation modalities. In the field of RA, US clearly has several potential advantages (Table 1) over the nerve stimulation method. Although improvement in patient safety during RA procedures could be debatable, there continues to be a surge of publications reporting better technical performance, lesser complications and increased safety with the use of US[6].
| Advantages of ultrasound nerve localisation in regional anesthesia[4] |
| Real time imaging-helps to modify the technique based on individual differences in anatomy |
| Actual visualisation of neural structures |
| Guides approximate needle positioning (not too near or too far) |
| Lesser patient discomfort-motor confirmation/muscle twitches may not be necessary |
| Can visualise vascular structures-helps to avoid intravascular injections |
| Visualisation of local anesthetic spread-less volume block-decreases the chances of toxicity |
| Can visualise important organs and avoid injury-lung shadows in supraclavicular block, paravertebral block |
| Other advantages of ultrasound as compared to fluoroscopy or computed tomography scan |
| Easy to use-less bulky |
| Does not need a separate environment-such as radiation shielded walls |
| No radiation exposure-safe |
| Cheaper |
| Produces tomographic images |
| Does not need a technician to operate |
In the field of interventional pain medicine (IPM), fluoroscopy to a major extent and computed tomography scan to some extent have been used as imaging modalities. There are important challenges for the use of US guidance in IPM. Most involve precision placement of needles around the neuraxis. However US is still very useful for injection of superficial structures such as stellate ganglion, inguinal nerves, intercostals nerves; deep intramuscular injections such as piriformis, trigger points, BOTOX injections. Narouze et al[7] detail the pain interventions that can be done with the use of US. They also list the level of difficulty associated.
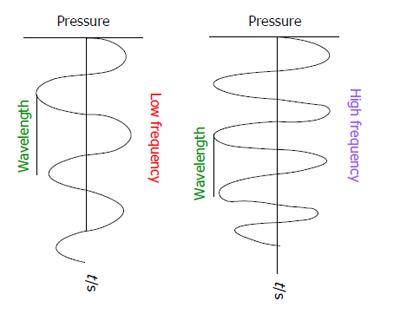
US waves are a form of sound waves. US wave frequencies exceed the upper limit of audible human hearing. Medical US frequencies are in the range of 1-20 MHz. Each US wave is characterised by a specific frequency and wavelength, which are inversely related (Figure 1). The higher the frequency, lower the wavelength. Frequency is the number of cycles per second and is measured in Hertz.
Wavelength is the distance between two consecutive, similar positions in the pressure wave. It is determined by the frequency of the wave, and the speed of propagation in the medium it is passing through. Actually the speed of sound is different based on the tissues through which it propagates: air-330 m/s, water-1525 m/s, bone 3000 m/s, fat-1450 m/s, muscle-1600 m/s and blood-1560 m/s. However it is averaged as approximately 1540 m/s for the entire body and is referred to as propagation velocity or acoustic velocity[8,9].
The US waves are produced by piezoelectric effect, which was discovered by Curie brothers in 1880[10]. It involves the generation of an electrical charge, by a piezoelectric material, when subjected to mechanical stress and the reverse piezoelectric effect involves such an electrical charge being converted to mechanical vibration. In the available US machines the transducer holding the piezoelectric material acts both as a generator and receiver of such signals. US used in medical imaging is referred to as B-mode (2D), meaning brightness mode display. This means the brightness of the pixel on the image is a representation of the strength of reflection.
A source of alternating current makes the piezoelectric crystals to vibrate at high frequency producing US waves. This is then transmitted to the patient through a conductive gel. These sound waves go through structures underneath the transducer and information is relayed back as reflected sound waves. A transducer acts as a generator of US waves for only 1% of the time and acts as a microphone to listen to the incoming waves for the rest 99% of the time. Frame rate refers to number of such pulses of US waves[11]. Higher frequency produces more waves of compression and rarefaction in a given time and increases the resolution (ability to discriminate between 2 separate structures along the axial plane).
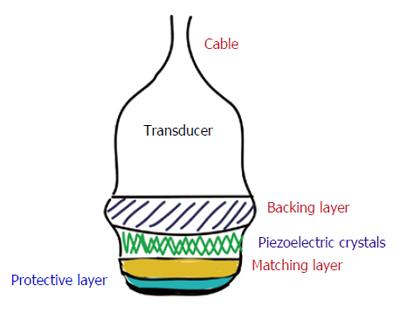
Medical US utilises a transducer attached to a display monitor which also holds the operating console. A transducer (also known as probe) contains a damping material, piezoelectric crystals, a matching layer and a protective layer (Figure 2). Each crystal is isolated and hence transmits its own US wave. The damping layer, present just behind the crystals acts to reduce their resonance so that they are sensitive to the returning signal. The matching layer in front acts to reduce the impedance mismatch and is covered by a protective layer[10].
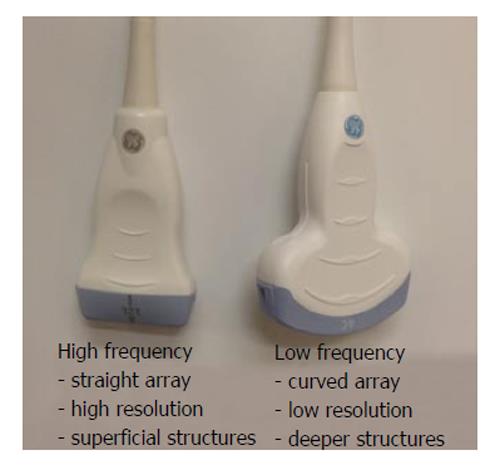
There are several types of transducers and it is necessary to choose the right one for the procedure. Based on the frequency range, commonly 2 types of transducers are used for RA procedures[10] (Figure 3). Some others give 3 varieties, based on the range: high- (8-12 MHz), medium- (6-10 MHz), and low- (2-5 MHz)[12]. The smaller one with a straight contact surface is called a linear array transducer due to the linear arrangement of crystals. It also produces high frequency waves in the range of 8-12 MHz. Its penetration, and hence resolution is usually good for structures within 3-4 cm. The larger one with a curved contact surface is called a curved array or curvilinear transducer because of the curved arrangement of crystals[8,9]. It creates a wedge shaped US beam and produces a much broader view with the image of deeper structures being wider than the footprint of the probe. It is used to visualise deeper structures, beyond 4 cm. It is important to know that the width of the image is equal to the probe footprint size only at the uppermost part of the image and hence any determination of depth is tricky[13].
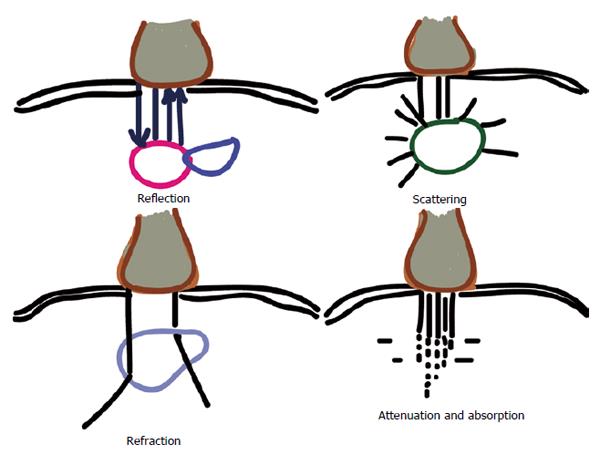
Once generated the wave passes through various tissue structures and thereby interacts with them. This interaction results in various possibilities as shown in Figure 4. US waves are primarily influenced by physical changes involving reflection, refraction and attenuation. The reflected wave, which gives rise to the resulting signal, is dependent upon the underlying structures the waves encounter. This property is called acoustic impedance[14]. It is unique to each tissue type and is defined as the density of the medium times the velocity of US wave transmitted through it. Less dense organs such as lungs have a lower impedance in contrast to bones which would have a higher impedance. The greater the differences in acoustic impedance between 2 adjacent tissues, more waves are reflected back. So it is not individual acoustic impedances but the relative difference of it among adjacent tissues that control the amount of energy reflected back[15].
Specular reflection involves a large smooth surface, such as a needle. Depending upon the incident angle, a large amount of US waves are reflected back to the transducer. Scattered reflection involves an irregular surface giving rise to scattering and hence loss of signal. However due to the wide range of angles, regardless of the incident angle, some US energy is always reflected back. Most biological tissues fall into this category and give rise to the speckled appearance observed of most soft tissues[16]. Refraction involves changes in the direction of US waves due to an interface of tissues with different speeds of sound transmission. This is similar to seeing a bent spoon when observed in a glass of water. This gives rise to loss of energy when not captured from the transducer[16]. Even when captured, acts as a source of several artifacts (duplication error) commonly encountered[15]. Attenuation refers to the loss of energy as the sound waves travel through increasing depth. It is related to the depth of beam penetration, type of tissue being imaged and the frequency of the wave. Due to friction, a vast amount of energy is lost as heat. More dense structures have higher attenuation coefficients as the oscillatory tissue motion produced by the sound wave creates more friction and heat[15]. Higher frequency signals are attenuated more than the lower frequency signals. Hence a high frequency probe cannot be of much help in visualising deeper structures such as the sciatic nerve. Posterior acoustic enhancement, a commonly observed artefact, is largely due to an intrinsic compensating mechanism provided to counter the attenuation and loss of signal when imaging highly hyperechoic structures such as blood vessels[15]. Transmission refers to loss of signal due to unopposed transmission away from the probe[16].
A good use of US guidance can only be made when one understands how to operate the equipment and also how to modify the variables to get the best possible image. The following section gives an understanding of these elements (Figure 5).
Resolution: It describes the ability to separately identify to individual structures[8,12]. Axial resolution refers to the possible differentiation between the 2 objects in the plane of US beam. Higher frequencies and superficial structures give better axial resolution[10].
Temporal resolution refers to the rate at which consecutive images are visualised. It depends on the frame rate or pulses. A transducer emits the next pulse only after it has received the previous pulse. Increasing the depth of US beam affects the temporal resolution. Similarly, using Doppler has the same effect as it requires more time to process the incoming signals and hence lower temporal resolution. Lateral resolution refers to separation of structures lying side by side. Inappropriate use of focus zone-as explained below can decrease the lateral resolution. Contrast resolution is referred to the optimal visualisation achieved in terms of hyper and hypo-echogenic structures displayed on the screen. To enhance visualisation and to improve resolution there are 3 important settings which can be altered.
GAIN: This simply refers to the strength of the signal. The brightness of the image is proportional to the strength of the signal received by the transducer. A highly reflective structure sends back proportionately more sound signals causing whiter shadow-hyperechogenic, where as less denser and less reflective structures send back less signals to the transducer causing blacker shadow-hypoechogenic. Increasing the gain increases the signal strength and brightness in general. This may not be optimal as even the background structures (noise) are also increased[12]. The optimal gain necessary for visualisation might be different from what is set as auto-gain and might need individual adjustments. Such well adjusted image is referred to as contrast resolution. Increasing the gain can also affect lateral resolution.
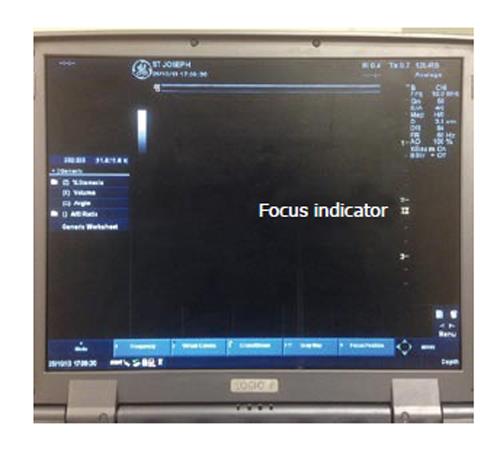
FOCUS: The sound waves converge to a point called focal zone and then diverge[10]. The divergence of these waves beyond the focal zone can allow for missed information in a horizontal plane. To minimise this loss, it is important to set the focal zone at the same level as the target of interest. It is achieved usually by a dial setting and is observed on the monitor as a small arrow on either side of the screen (Figure 6).
Time gain compensation: As the name suggests there is an increase in gain (signal strength) which is restricted to a set field of depth. Attenuation increases with increasing depth. To compensate for this time gain compensation (TGC) allows for stepwise increase in gain which can be adjusted for a particular depth. It is suggested that TGA adjustments are made less frequently than gain adjustments, which is not usually optimal[16].
Frequency: Waves of higher frequency are more attenuated. One should choose a higher frequency probe for superficial structures, and low frequency probe for deeper structures.
Color doppler: This function helps to detect structures with movement, like blood flow. It is based on the doppler principle. Structures moving away from the probe appear blue and those towards the probe appear red. One important thing to remember is that the angle of incidence should be as less as possible. With an angle of incidence of 90°, no flow is detected and might provide a false negative implication. To help visualise even smaller vessels and also to be independent of the incident angle, newer machines have power Doppler[9,13]. This function provides only a single color pattern.
The image produced on the monitor is a 2 dimensional image obtained from converting mechanical energy into electrical signals. The actual conversion of signals into images involves several assumptions on the part of equipment’s software[8,9]. These give rise to artifacts: could be a distortion in the image brightness, duplication, absence of echoes, etc.
It is difficult to avoid them altogether and hence must be able to distinguish them[17]. Commonly understood artifacts are described below.
Acoustic shadowing: This happens when a superficial structure has greater attenuation coefficient than the structures deep to it. Due to this the underlying structure appears less echogenic than normal. This is typically seen under a bone as a black shadow[8,18].
Posterior acoustic enhancement: This is almost the opposite of shadowing. Due to the presence of a less attenuating structure superficially, the region behind that structure produces stronger echoes than the surrounding structures. This is typically seen underneath or posterior to a blood vessel and can be mistaken as a nerve due to its hyperechoic quality[8,17].
Reverberation: It is the multiple representation of the same structure at different depths of display. It is usually caused by a specular reflector such as a needle. It reflects a strong signal back to the tranducer, some of which is again reflected back to cause a repeat of the shadow at a different depth, because of the time delay involved. The lumen and the walls of a hollow needle can also give rise to reverberation artifacts due to differences in the time of reflected wave and appear as multiple but similar shadows. They also give rise to comet tail shadows[19].
Mirror image: It is a type of reverberation artifact, commonly produced due to a significant mismatch in the acoustic impedance between 2 adjacent structures such as air-bone, soft tissue-lung etc. Interestingly this artifact appears in all modes including doppler.
Refraction: This is also called as bayonet effect[20]. This appears as a subtle bend in the length of the needle due to refraction.
Dealing with artifacts[8,17]: (1) Have a high degree of suspicion; (2) Confirm in 2 views, longitudinal and cross-sectional; (3) Change the position of transducer-move proximal or distal; (4) Reduce gain; and (5) Move the patient.
Appropriate and effective use of US requires a thorough knowledge of its operating principles. This helps one to utilise the controls to get an optimal image which is clear, with more signal transmission than background noise.
Key points include: selection of the right transducer, using focus adjustment, using TGC to allow for compensation at the required depth, using appropriate doppler imaging. All of these help to minimise the artifacts.
P- Reviewers: Cheung, CW, Gomez RS, Huang J S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Edler I, Lindström K. The history of echocardiography. Ultrasound Med Biol. 2004;30:1565-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | la Grange P, Foster PA, Pretorius LK. Application of the Doppler ultrasound bloodflow detector in supraclavicular brachial plexus block. Br J Anaesth. 1978;50:965-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 84] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Kapral S, Krafft P, Eibenberger K, Fitzgerald R, Gosch M, Weinstabl C. Ultrasound-guided supraclavicular approach for regional anesthesia of the brachial plexus. Anesth Analg. 1994;78:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 234] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Marhofer P, Harrop-Griffiths W, Kettner SC, Kirchmair L. Fifteen years of ultrasound guidance in regional anaesthesia: part 1. Br J Anaesth. 2010;104:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Sites BD, Chan VW, Neal JM, Weller R, Grau T, Koscielniak-Nielsen ZJ, Ivani G. The American Society of Regional Anesthesia and Pain Medicine and the European Society Of Regional Anaesthesia and Pain Therapy Joint Committee recommendations for education and training in ultrasound-guided regional anesthesia. Reg Anesth Pain Med. 2009;34:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Griffin J, Nicholls B. Ultrasound in regional anaesthesia. Anaesthesia. 2010;65 Suppl 1:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Narouze SN, Provenzano D, Peng P, Eichenberger U, Lee SC, Nicholls B, Moriggl B. The American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, and the Asian Australasian Federation of Pain Societies Joint Committee recommendations for education and training in ultrasound-guided interventional pain procedures. Reg Anesth Pain Med. 2012;37:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Sanders RC, Winter TC. Clinical Sonography A Practical Guide. Lippincott, Williams and Wilking. 2006;. |
| 10. | Lawrence JP. Physics and instrumentation of ultrasound. Crit Care Med. 2007;35:S314-S322. [PubMed] |
| 11. | Arbona FL, Khabiri B, Norton JA. Ultrasound basics for the busy novice practitioner. Int Anesthesiol Clin. 2011;49:34-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Brull R, Macfarlane AJ, Tse CC. Practical knobology for ultrasound-guided regional anesthesia. Reg Anesth Pain Med. 2010;35:S68-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Ihnatsenka B, Boezaart AP. Ultrasound: Basic understanding and learning the language. Int J Shoulder Surg. 2010;4:55-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 14. | Kossoff G. Basic physics and imaging characteristics of ultrasound. World J Surg. 2000;24:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Narouze SN. Atlas of ultrasound guided procedures in interventional pain management. 1st ed. New York, NY: Springer 2011; . [DOI] [Full Text] |
| 16. | Sites BD, Brull R, Chan VW, Spence BC, Gallagher J, Beach ML, Sites VR, Hartman GS. Artifacts and pitfall errors associated with ultrasound-guided regional anesthesia. Part I: understanding the basic principles of ultrasound physics and machine operations. Reg Anesth Pain Med. 2007;32:412-418. [PubMed] |
| 17. | Sites BD, Brull R, Chan VW, Spence BC, Gallagher J, Beach ML, Sites VR, Abbas S, Hartman GS. Artifacts and pitfall errors associated with ultrasound-guided regional anesthesia. Part II: a pictorial approach to understanding and avoidance. Reg Anesth Pain Med. 2007;32:419-433. [PubMed] |
| 18. | Kremkau FW, Taylor KJ. Artifacts in ultrasound imaging. J Ultrasound Med. 1986;5:227-237. [PubMed] |
| 19. | Thickman DI, Ziskin MC, Goldenberg NJ, Linder BE. Clinical manifestations of the comet tail artifact. J Ultrasound Med. 1983;2:225-230. [PubMed] |
| 20. | Gray AT, Schafhalter-Zoppoth I. “Bayonet artifact” during ultrasound-guided transarterial axillary block. Anesthesiology. 2005;102:1291-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |