Published online Oct 13, 2021. doi: 10.5313/wja.v10.i1.1
Peer-review started: June 7, 2021
First decision: July 27, 2021
Revised: August 10, 2021
Accepted: September 26, 2021
Article in press: September 26, 2021
Published online: October 13, 2021
Processing time: 127 Days and 13.3 Hours
The intraoperative management of patients undergoing orthotopic liver transplantation (OLT) frequently encounters hemodynamic instability after reperfusion of the new liver graft. The resulting post-reperfusion syndrome is characterized by an increase in pulmonary vascular resistance and decrease in systemic vascular resistance. In the presence of a left to right intracardiac shunt, this hemodynamic perturbance can lead to shunt reversal followed by hypoxemia and embolization of air and debris into the systemic circulatory system.
A 43 years-old male with end-stage liver disease due to primary sclerosing cholangitis complicated by portal hypertension and hepatocellular carcinoma presented for an OLT. A bedside transthoracic echocardiography (TTE) was performed immediately before the procedure and unexpectedly identified a ventricular septal defect (VSD). The patient and the surgical team agreed to proceed with the surgery as it was a time critical donation after circulatory organ death. We developed an intraoperative plan to optimize pulmonary and systemic pressures using vasoactive support, optimized mechanical ventilation, and used transesophageal echocardiography (TEE) for intraoperative monitoring. During reperfusion, considerable turbulent flows with air were noted in the right ventricle, but no air was visualized in the left ventricle. Color flow Doppler showed no reversal flow in the VSD. At the end of the procedure, the patient was extubated in the operating room without complication and was transferred to the transplant unit for recovery.
Our case highlights the importance of echocardiography in the perioperative assessment of patients undergoing liver transplantation. The TTE findings obtained immediately before the procedure and the real-time use of intraoperative TEE to modify our management during the critical phases of the transplant resulted in continuity of care and a good surgical outcome for this patient.
Core Tip: A liver transplant is a challenging case which can involve significant hemodynamic instability. It is also a situation where organ waitlists can prolong time to surgery leading to significant deterioration of the recipient’s condition. This can be compounded by any unexpected cardiac findings diagnosed in the immediate preoperative period by echocardiography. Our findings of a ventricular septal defect on transthoracic echocardiography (TTE) led to a clinical dilemma of proceeding with surgery knowing there was a risk of paradoxical embolism or hypoxemia. On the other hand, rejecting a matched donation after circulatory death liver graft would have been a waste of precious resources. By using intraoperative transesophageal echocardiography (TEE) we carefully titrated intraoperative hemodynamics and prevented intracardiac shunting. Our case highlights the importance of bedside TTE as well as intraoperative TEE in patients undergoing orthotopic liver transplants.
- Citation: Desai TV, Dhir A, Quan D, Zamper R. Intraoperative management of liver transplant in a patient with an undiagnosed ventricular septal defect: A case report. World J Anesthesiol 2021; 10(1): 1-6
- URL: https://www.wjgnet.com/2218-6182/full/v10/i1/1.htm
- DOI: https://dx.doi.org/10.5313/wja.v10.i1.1
The intraoperative hemodynamic management of patients undergoing orthotopic liver transplantation (OLT) is challenging and often involves significant instability. After reperfusion of the graft, post-reperfusion syndrome can occur which is characterized by an increase in pulmonary vascular resistance (PVR), central venous pressure (CVP) and pulmonary artery pressure, as opposed to a decrease in systemic vascular resistance (SVR)[1].
Amongst the cardiovascular diseases, intracardiac shunts are less common in OLT candidates[2]. During reperfusion there may be catastrophic consequences in the presence of an intracardiac shunt, as hypoxemia can occur due to the combination of increased PVR and decreased SVR leading to a right-to-left shunt. With the shunt reversal, air and debris can cross over to systemic circulation causing paradoxical embolization.
We report the case of a patient who presented for an urgent donation after cardiac death (DCD) OLT in whom preoperative transthoracic echocardiography (TTE) discovered the presence of a ventricular septal defect (VSD).
The chief complaints are not applicable.
A 43-year-old male with end-stage liver disease secondary to primary sclerosing cholangitis with a sodium–MELD of 19 presented for an urgent OLT. He had features of portal hypertension and had exception points due to the development of hepatocellular carcinoma. Routine preoperative investigations included a TTE performed eleven months before the surgery which was reported as normal.
The history of past illness is not applicable.
The personal and family history is not applicable.
The physical examination is not applicable.
The laboratory examinations are not applicable.
A bedside TTE performed immediately before the procedure identified the presence of abnormal flow in the right ventricular (RV) outflow tract on the parasternal long-axis view and RV parasternal inflow-outflow view. The patient then mentioned that he had a VSD in childhood which had closed spontaneously. This information was missed in all prior interactions between the patient and the multidisciplinary transplant team.
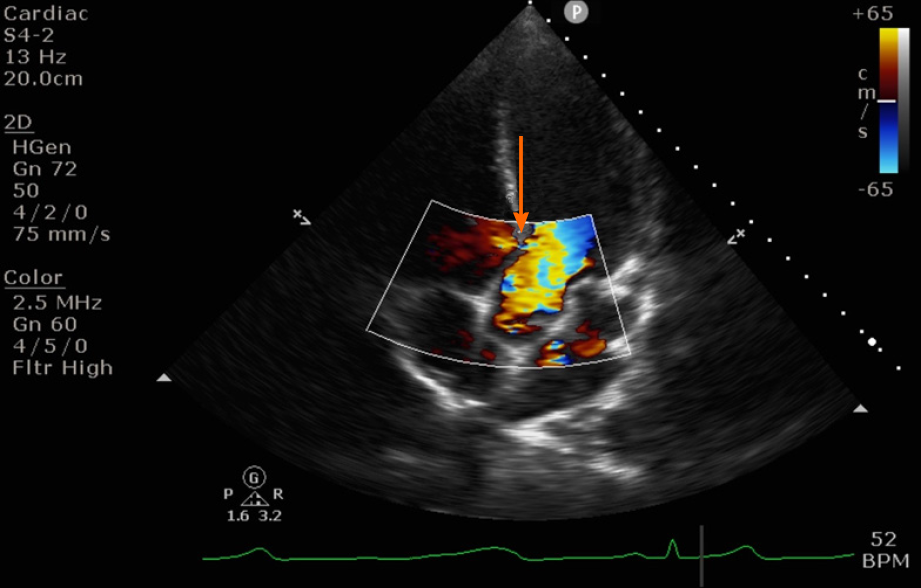
Further examination demonstrated a single jet with left-to-right shunt across the interventricular septum on the apical 4 chamber view, suggestive of a perimembranous VSD (Figure 1). Significant pulmonary hypertension was ruled out with an estimated RV systolic pressure (RVSP) of 25-30 mmHg by the tricuspid regurgitation jet, considering a CVP of 10 mmHg.
Our findings were discussed with the patient and the surgical team with the consensus to proceed with the surgery due to the time sensitive nature of the DCD graft. We then devised an intraoperative plan of optimizing the pulmonary and systemic pressures using vasoactive support and the most appropriate mode of mechanical ventilation for this case. We monitored changes in the magnitude of the shunt with intraoperative transesophageal echocardiography (TEE).
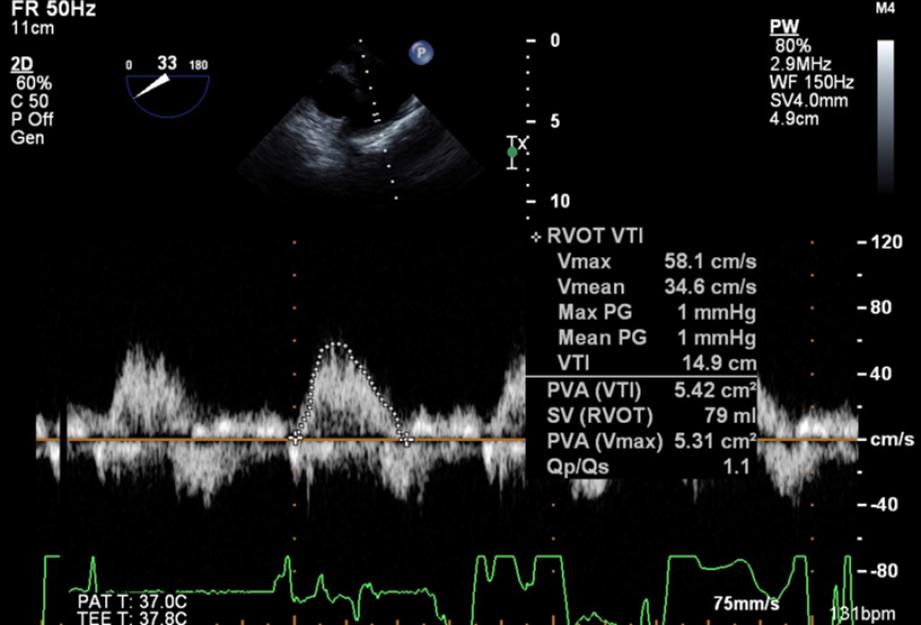
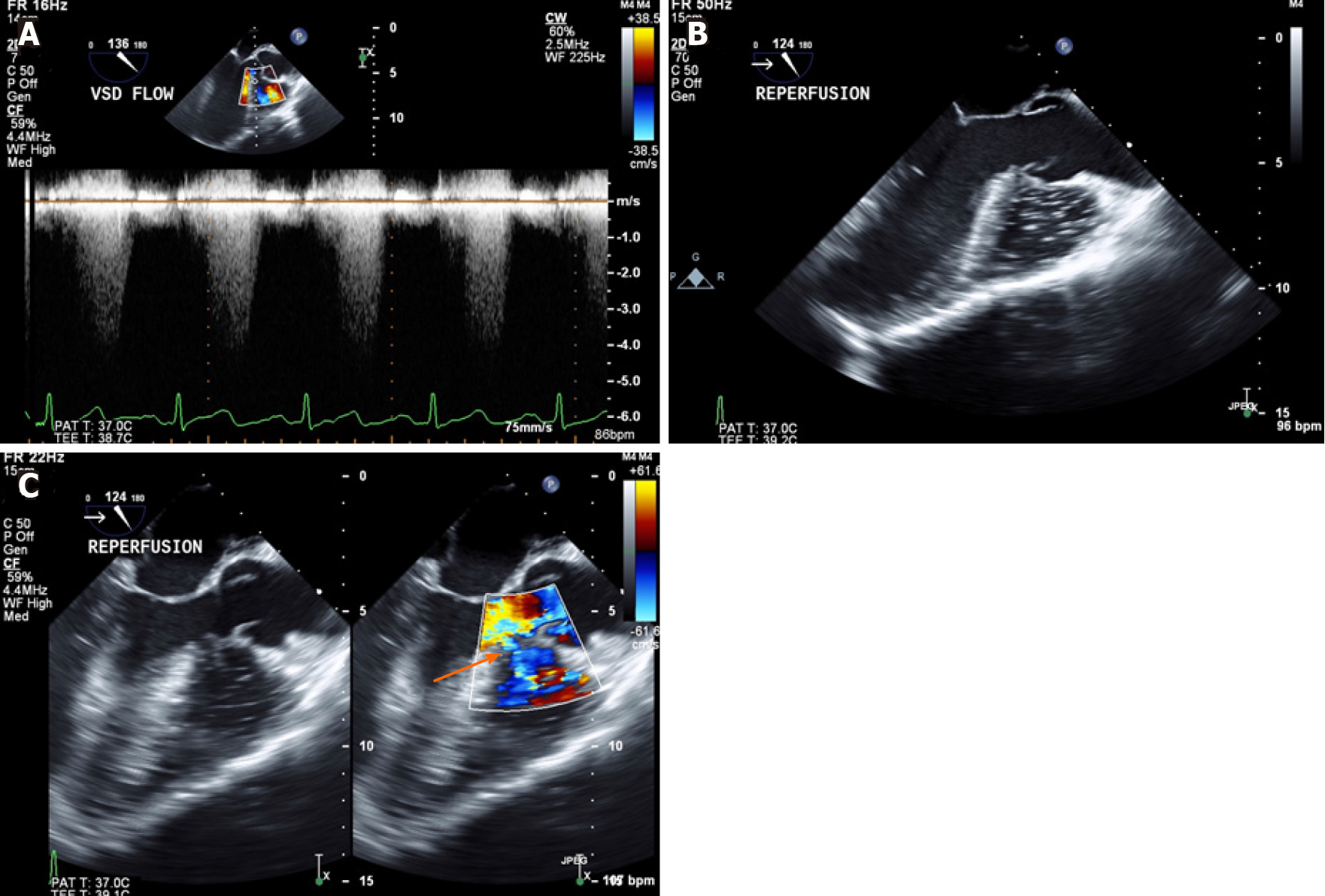
After induction of general anesthesia, TEE showed a shunt fraction (Qp:Qs) of 1.1 and flow velocity across the VSD of 4.5-5 m/s (Figures 2 and 3A) with no indirect signs of volume or pressure overload to the RV. During the procedure, the pressure controlled ventilation mode was used with an inspiratory to expiratory (I:E) ratio of 1:3 to reduce the peak and mean airway pressures. We aimed for hyperventilation (ETCO2 < 25-30 mmHg) and hyperoxia (FiO2 > 70%) in an attempt to minimize the pulmonary vascular resistance, whilst continuously monitoring the flows through the VSD for shunt reversal.
After release of the inferior vena cava clamp, considerable turbulent flows with air were noted in the RV, but no air was visualized in the left ventricle (LV) (Figure 3B). Systemic hypotension occurred, and velocity across the VSD reduced to 2.5 m/s, but the shunt did not reverse (Figure 3C). Using the LV systolic blood pressure (LVSP) of 80 mmHg at that moment, we estimated a RVSP of 55 mmHg using the hemodynamic calculation of LVSP-RVSP = 4 (VSD velocity)2. Vasopressin was the vasopressor of choice to treat the hemodynamic instability as it has a differential effect on the pulmonary and systemic circulation, increasing the SVR more than the PVR. At the end of the procedure, the patient was extubated in the operating room, and transferred to the intensive care unit.
The final diagnosis of the case presented is a perimembranous VSD in an OLT recipient.
The patient’s VSD was small and the shunt fraction did not warrant closure of the VSD pretransplant. Intraoperatively we managed the hemodynamics of the patient to minimize the fluctuation in the shunt direction.
The patient’s postoperative clinical course was complicated by non-cardiac musculoskeletal chest and arm pain which were unrelated to the ventricular septal defect. It resolved with appropriate management and the patient was discharged home 10 d following surgery. We had advised the patient to follow up with a cardiologist due to the persistent finding of congenital VSD.
To our knowledge, this is the first report in the literature of a patient with an undiagnosed VSD undergoing an OLT. An increased risk of embolic events in patients with a patent foramen ovale during OLT has already been described[3]. In addition, a retrospective study of patients undergoing liver transplants showed that intracardiac shunts were not associated with an increased risk of perioperative stroke[4].
Since our patient’s VSD was diagnosed immediately prior to surgery, a time-dependent, critical decision had to be made of whether to proceed with the transplant or postpone the surgery and refer the patient to a cardiologist. AHA/ACC guidelines show that small restrictive VSDs are managed clinically, and surgical interventions are indicated in VSDs with a Qp:Qs ratio greater than 1.5 or if there is prolapse of an aortic valve cusp into the VSD causing progressive aortic regurgitation[5]. None of these features were present in our patient and after a discussion involving the anesthesia team, surgical team, and the patient, we decided to proceed. It was suggested to use intraoperative TEE to guide hemodynamic management and to monitor the shunt direction for risk of paradoxical embolism.
Our case highlights the importance of performing a bedside focused TTE exam immediately before the OLT procedure, and this has become standard practice at our center. Patients might be on the transplant list for a substantial length of time before a suitable deceased donor organ is available, and cardiac diseases may have progressed or developed in the interim. Furthermore, as anesthesiologists, we understand intraoperative nuances and the impact of significant findings on hemodynamic management.
Another important aspect is the use of intraoperative TEE in OLT, which has become common practice amongst transplant centers. Whether its use in our case, along with additional intraoperative management techniques (goal-directed management of PVR and SVR to avoid reversal of the shunt by optimization of ventilatory parameters and vasoactive drugs during reperfusion), have led us to a positive outcome or not can be questionable. However, having a tool to modify our surgical management using real time images during the critical phases of liver transplant allows us to provide optimal patient care.
Manuscript source: Unsolicited manuscript
Specialty type: Anesthesiology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Samant H S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Jeong SM. Postreperfusion syndrome during liver transplantation. Korean J Anesthesiol. 2015;68:527-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Garg A, Armstrong WF. Echocardiography in liver transplant candidates. JACC Cardiovasc Imaging. 2013;6:105-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Ellis JE, Lichtor JL, Feinstein SB, Chung MR, Polk SL, Broelsch C, Emond J, Thistlethwaite JR, Roizen MF. Right heart dysfunction, pulmonary embolism, and paradoxical embolization during liver transplantation. A transesophageal two-dimensional echocardiographic study. Anesth Analg. 1989;68:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 82] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 4. | Harinstein ME, Iyer S, Mathier MA, Flaherty JD, Fontes P, Planinsic RM, Edelman K, Katz WE, Lopez-Candales A. Role of baseline echocardiography in the preoperative management of liver transplant candidates. Am J Cardiol. 2012;110:1852-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:1494-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 406] [Article Influence: 58.0] [Reference Citation Analysis (0)] |