Copyright
©2014 Baishideng Publishing Group Inc.
World J Anesthesiol. Jul 27, 2014; 3(2): 181-188
Published online Jul 27, 2014. doi: 10.5313/wja.v3.i2.181
Published online Jul 27, 2014. doi: 10.5313/wja.v3.i2.181
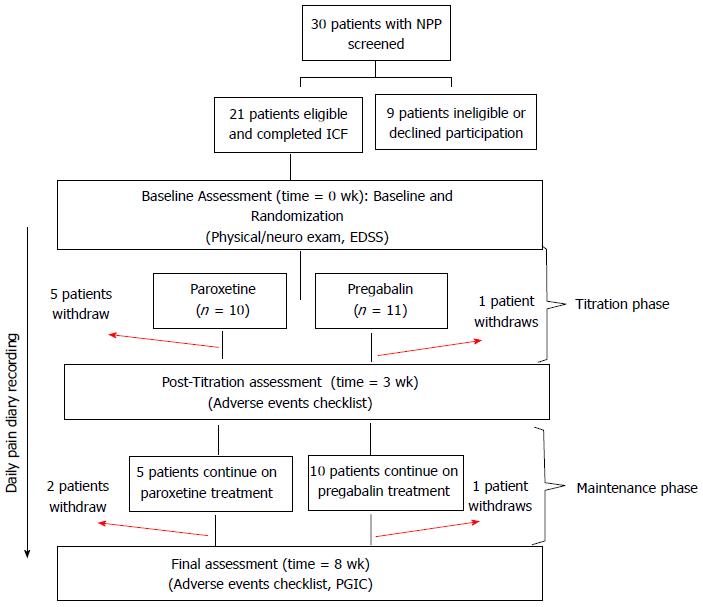
Figure 1 Study schedule summary.
Patient screening outcomes and visit schedule summaries are provided. Bracketed information on specified “Assessment” lines indicates evaluations conducted at each visit. NPP: Neuropathic pain; ICF: Informed consent form; EDSS: Expanded disability status scale; PGIC: Patient rated global Impression of change.
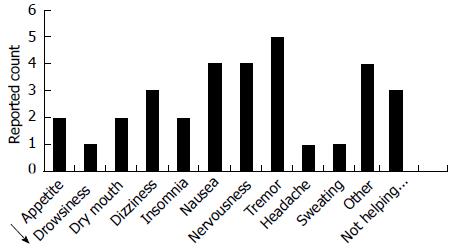
Figure 2 Patient-reported reasons for study attrition: paroxetine arm.
Patient-reported reasons for attrition are presented (n = 7). Patients were permitted to cite multiple reasons for treatment discontinuation. “Other” included complaints of feeling “shaky”, “caffeinated”, “jittery” and “anxious”.
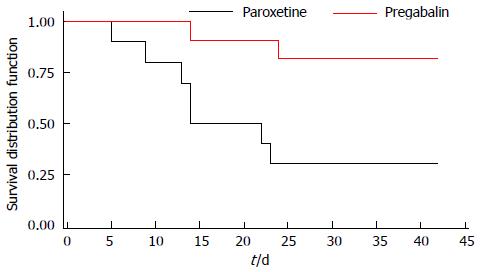
Figure 3 Attrition by study group.
Attrition rates (shown in the format of survival distribution by study day) for each group (paroxetine and pregabalin) are presented. The average study duration (days) for paroxetine = 27.3 and for pregabalin = 49.5.
-
Citation: Turcotte DA, Doupe M, Torabi M, Gomori AJ, Ethans K, Esfahani F, Galloway K, Namaka MP. Paroxetine
vs pregabalin for the management of neuropathic pain in multiple sclerosis. World J Anesthesiol 2014; 3(2): 181-188 - URL: https://www.wjgnet.com/2218-6182/full/v3/i2/181.htm
- DOI: https://dx.doi.org/10.5313/wja.v3.i2.181