Published online Sep 18, 2018. doi: 10.5312/wjo.v9.i9.165
Peer-review started: April 7, 2018
First decision: June 14, 2018
Revised: July 1, 2018
Accepted: August 2, 2018
Article in press: August 3, 2018
Published online: September 18, 2018
Processing time: 162 Days and 20.2 Hours
To investigate the additional value of physiotherapy after a corticosteroid injection in stage one or two idiopathic frozen shoulders (FSs).
A two center, randomized controlled trial was done. Patients with a painful early stage idiopathic FS were eligible for inclusion. After written consent, patients were randomly allocated into two groups. All patients received an ultrasound-guided intra-articular corticosteroid injection. One group underwent additional physiotherapy treatment (PT) and the other group did not (non-PT). The primary outcome measure was the Shoulder Pain and Disability Index (SPADI). Secondary outcomes were pain (numeric pain rating scale), range of motion (ROM), quality of life (RAND-36 score), and patient satisfaction. Follow-up was scheduled after 6, 12 and 26 wk.
Twenty-one patients were included, 11 patients in the non-PT and ten in the PT group, with a mean age of 52 years. Both treatment groups showed a significant improvement at 26 wk for SPADI score (non-PT: P = 0.05, PT: P = 0.03). At the 6 wk follow-up, median SPADI score was significant decreased in the PT group (14 IQR: 6-38) vs the non-PT group (63 IQR: 45-76) (P = 0.01). Pain decreased significantly in both groups but no differences were observed between both treatment groups at any time point, except for night pain at 6 wk in favor of the PT group (P = 0.02). Significant differences in all three ROM directions were observed after 6 wk in favor of the PT group (P ≤ 0.02 for all directions). A significantly greater improvement in abduction (P = 0.03) and external rotation (P = 0.04) was also present in favor of the PT group after 12 wk. RAND-36 scores showed no significant differences in health-related quality of life at all follow-up moments. At 26 wk, both groups did not differ significantly with respect to any of the outcome parameters. No complications were reported in both groups.
Additional physiotherapy after corticosteroid injection improves ROM and functional limitations in early-stage FSs up to the first three months.
Core tip: Corticosteroids and physiotherapy are the most widely used treatment modalities in frozen shoulders (FSs). However, the role of physiotherapy, especially in early FSs, is controversial. Corticosteroid injection with additional physiotherapy leads to better Shoulder Pain and Disability Index scores and range of motion up to three months compared to corticosteroid injection alone. Although a trend was recognized in favor of the physiotherapy group, both groups did not differ significantly with respect to any of the outcome parameters at the final follow-up after 26 wk.
- Citation: Kraal T, Sierevelt I, van Deurzen D, van den Bekerom MP, Beimers L. Corticosteroid injection alone vs additional physiotherapy treatment in early stage frozen shoulders. World J Orthop 2018; 9(9): 165-172
- URL: https://www.wjgnet.com/2218-5836/full/v9/i9/165.htm
- DOI: https://dx.doi.org/10.5312/wjo.v9.i9.165
Frozen shoulder (FS), a common cause of shoulder pain and disability, affects approximately 2% to 4% of the general population[1-3]. The peak incidence of FS is between the fifth and sixth decade of life, occurring slightly more frequently in women than in men. The pathophysiology of FS is poorly understood[4]. The generally accepted theory comprises an inflammatory cascade causing contracture of the anterosuperior capsule, the rotator interval and the coracohumeral ligaments of the shoulder joint. These events lead to the typical loss of the passive external rotation seen in FS[2]. Although there are histopathological similarities with Dupuytren’s disease, FS follows a different natural course[5]. Historically, FS is considered to be self-limiting with three different stages; the freezing, frozen, and thawing stages[6,7]. However, clear distinction between separate stages is difficult without clear cut-off criteria, and a continuing spectrum is more appropriate. Functional recovery mainly takes place within one to three years[8,9]. However, the remaining pain and restriction in range of motion (ROM) of the shoulder joint can even persist long-term[10-12].
There is no widely agreed consensus about the most optimal treatment regimen for FS. Systematic reviews point to a large gap in evidence for treatment strategies for FS[13-15]. Currently, there seems to be a trend towards more invasive treatments, like manipulation under anesthesia and particularly arthroscopic capsular release[16]. However, there is insufficient evidence to recommend these treatment modalities[13]. Less invasive treatment options are intra-articular corticosteroid injections and physiotherapy. These are the most widely used treatment modalities in FS in both primary and secondary healthcare settings[2,17,18]. Corticosteroid injections demonstrated a positive effect on shoulder pain and ROM, at least in the short-term[19,20]. However, the role of physiotherapy in the treatment of FS is more uncertain[14,21,22]. Supervised neglect, consisting of supportive therapy and exercises within pain limits, has been advocated as an appropriate treatment for FS[23]. In a systematic review, Blanchard et al[24] hypothesized a potential beneficial effect of combining corticosteroid injections with physiotherapy. Conclusive evidence to support this is lacking, which warrants further trials. The objective of this randomized controlled trial was therefore to investigate the additional value of physiotherapy treatment (PT) after an intra-articular corticosteroid injection in the management of early-stage idiopathic FSs. It is hypothesized that, with respect to ROM and shoulder function, additional physiotherapy is superior to corticosteroid injection alone.
Approval for a prospective randomized clinical trial (D-FROST; Dutch frozen shoulder study) was obtained by the MC Slotervaart Hospital Medical Ethics Committee (NL47325.048.13). The trial was registered in the Dutch Trial Register (NTR4587). The study was undertaken in accordance with the declaration of Helsinki. Patients were recruited between February 2014 and December 2015 in two participating hospitals in Amsterdam. Patients were eligible for participation if they exhibited clinical signs of FS, including pain and stiffness of the involved shoulder without preliminary trauma persisting for more than three months. The required level of pain was a minimum score of six out of ten on a numeric pain scale. Restriction of the passive ROM of the shoulder joint of more than 30° in external rotation and a second direction (i.e., abduction and/or forward flexion) when compared to the unaffected contralateral side was required for inclusion. Conventional radiographs of the shoulder joint and ultrasound studies were used to rule out osteoarthritis and rotator cuff ruptures. Exclusion criteria were: Corticosteroid injection in the shoulder joint region in the previous 6 wk, previous surgery to the shoulder, systemic inflammatory disease, neurological disorder with impairment of the upper limb, and the use of anti-coagulation therapy using a therapeutic dosage. These selection criteria are intended to select a clearly defined population of patients with early-stage (stage one or two) idiopathic FSs. Patients were informed both in word and with an information leaflet. Informed consent was obtained from all included patients.
Patients were randomly assigned into two groups. The intervention group undergoing a PT program (PT-group), or the control group without physiotherapy (non-PT). Patients were allocated to one of the study groups using an online website. Randomization was stratified by the participating hospital and performed in variable blocks using computer-generated randomization software. Participating orthopaedic surgeons who assessed patient eligibility had no access to the randomization software, hereby securing allocation concealment. Within two weeks after inclusion, patients in both study groups received an ultrasound-guided glenohumeral joint injection of 1 mL kenacort 40 mg in 4 mL lidocaine 1%, administered by an experienced radiologist. Both groups were informed about the possible self-limiting nature of FS, and received counseling about optional analgesics like acetaminophen, nonsteroidal anti-inflammatory drugs or tramadol, if needed. The non-PT group did not receive PT. Advice was given to try to use the affected arm in daily life activities within their pain limits. Patients in the PT group were referred to a participating physiotherapy clinic. All participating physiotherapists treated the referred study patients according to a standardized protocol, twice a week with a maximum duration of three months. This physiotherapy protocol was composed after a thorough literature review by the participating shoulder surgeons in accordance with two experienced shoulder-treating physiotherapists. The aim of the PT was to increase ROM of the shoulder, decrease pain, and restore the function of the shoulder for daily activities. Tissue irritability of the shoulder joint was taken into account to guide the intensity of the treatment[25]. Passive mobilization techniques were used, except for Maitland grade five mobilizations[26]. Attention was paid to scapulothoracic movement, with the purpose to improve the scapulohumeral kinematics. Also, active and auto-assisted stretching techniques were part of the physiotherapy program. If there was an increase in pain lasting for more than four hours after the PT session, the next session had to be less intense. Hot packs, icing, and massage techniques were allowed to reduce pain. Transcutaneous electrical nerve stimulation, pulsed electromagnetic field, infrared, dry needling and medical taping were not allowed due to the lack of evidence of these treatment modalities in the treatment of FS[27].
The main outcome parameter of this study was the Shoulder Pain and Disability Index (SPADI) at the 26 wk follow-up, consisting of 13 questions divided into two domains (pain and disability). Item responses were rated on a eleven-point scale (0-10) leading to a score between 0 (best) and 100 (worst)[28]. The SPADI has been translated and validated in Dutch[29,30]. Pain on average last week, and pain at night were scored on a ten-point numeric pain-rating scale (NPRS). Health-related quality of life was assessed using the RAND-36[31,32]. Passive ROM was measured in the standing position with the use of a goniometer. External rotation was measured in the horizontal plane, with the elbow at the side. Abduction was measured in the frontal plane and anteflexion in the sagittal plane. Patient satisfaction about their change in pain and function was assessed on a five-point Likert scale (“worse’’, ‘‘unchanged’’, ‘‘unsatisfactory improved’’, ‘‘satisfactory improved’’ and ‘‘good to very good improved”)[33]. Repeated corticosteroid injections were allowed after 6 wk if the level of pain had not dropped by at least 50%. Follow-up was scheduled after 6, 12 and 26 wk.
Statistical analysis was performed by use of the SPSS statistical package software (version 22.0; Armonk, NY: IBM Corp) according to the intention to treat principle. Statistical review was performed by a clinical epidemiologist. Due to the small sample sizes and skewed distributions, analyses were performed non-parametrically. Patient demographics and baseline characteristics were described and compared between groups according to their distributions. Continuous and ordinal data are presented as medians with interquartile ranges (IQR) and differences between the treatment groups were assessed by use of Mann Whitney U tests. Wilcoxon Signed Ranks tests were performed to assess changes from baseline at 26 wk. χ2 tests were performed in case of categorical variables. A P-value < 0.05 was considered statistically significant.
A total of 21 patients were included, with 11 patients in the non-PT and ten in the PT group (Table 1). All patients had conventional radiographs of the shoulder without abnormalities. At baseline, external rotation was limited in both patient groups with a median external rotation measuring five degrees for all patients (IQR: 0-20). Median NPRS on average last week was eight (IQR: 7-8.5). In both groups, two patients were too disabled to work due to their FS symptoms. Two patients in both groups had received a previous corticosteroid injection more than three months prior to inclusion. After 26 wk, ROM measurements were available for 81% of the patients. Questionnaires were completed by 15 out of 21 patients (71%). An intra-articular corticosteroid injection was repeated after 12 wk in two patients in both groups. No complications or adverse events were reported in both groups.
| Characteristic | Total | Non-PT | PT | P-value |
| No. of patients | 21 | 11 | 10 | |
| Age (yr) | 51.9 (SD 5.1) | 50.4 (SD 6.1) | 53.3 (SD 3.8) | 0.17 |
| Gender | ||||
| Male | 9 (43) | 4 (36) | 5 (50) | |
| Female | 12 (57) | 7 (64) | 5 (50) | 0.67 |
| Stage of frozen shoulder | ||||
| Freezing (stage I) | 8 (38) | 6 (55) | 2 (20) | |
| Frozen (stage II) | 13 (62) | 5 (45) | 8 (80) | 0.18 |
| Duration of symptoms prior to intervention | ||||
| < 6 mo | 13 (62) | 9 (82) | 4 (40) | |
| > 6 mo | 8 (38) | 2 (18) | 6 (60) | 0.08 |
| Previous injection around the shoulder | 11 (52) | 5 (45) | 6 (60) | 0.67 |
| Previous PT | 15 (71) | 7 (64) | 8 (80) | 0.64 |
| Disabled to work related to shoulder | 4 (19) | 2 (18) | 2 (20) | 1.00 |
| Diabetes mellitus | 2 (10) | 2 (18) | 0 (0) |
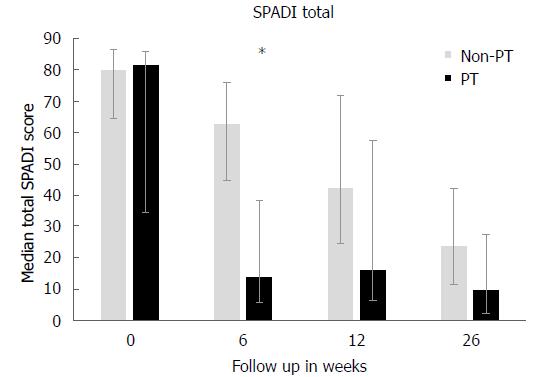
The median total SPADI scores for all patients at baseline was 81 (IQR: 58-87), which confirmed the severe pain and disabilities of FS in the early stages. Both treatment groups showed a significant improvement at the primary endpoint of 26 wk for SPADI scores (non-PT: P = 0.05, PT: P = 0.03). At the 6 wk follow-up, median SPADI scores had decreased to 63 (IQR: 45-76) in the non-PT group and 14 (IQR: 6-38) in the PT group. This difference was significant (P = 0.01) and exceeded the minimal clinical important difference (range 8-13) of the SPADI[34], but this difference had disappeared after 26 wk (P = 0.23). At the final follow-up, median SPADI scores were 24 (IQR: 12-19) in the non-PT and ten (IQR: 2-28) in the PT group (Figure 1 and Table 2). Passive ROM increased significantly compared to baseline in both groups (P < 0.03 for all comparisons). Significant differences in all three ROM directions were observed after 6 wk in favor of the PT group (P ≤ 0.02 for all comparisons). At the final follow-up, all ROM measurements were still in favor of the PT group, but were not significant (Table 3).
| Non-PT | PT | P-value | |
| SPADI pain | |||
| Baseline (wk) | 82 (70-90) | 86 (46-92) | 0.68 |
| 6 | 71 (24-79) | 18 (9-43) | 0.09 |
| 12 | 48 (22-68) | 20 (9-57) | 0.17 |
| 26 | 14 (8-30) | 13 (4-32) | 0.94 |
| SPADI limitations | |||
| Baseline (wk) | 81 (58-88) | 74 (28-84) | 0.42 |
| 6 | 69 (47-76) | 11 (4-36) | 0.01 |
| 12 | 38 (25-72) | 14 (5-58) | 0.15 |
| 26 | 10 (9-50) | 8 (1-25) | 0.35 |
| SPADI total | |||
| Baseline (wk) | 80 (65-87) | 82 (35-86) | 0.54 |
| 6 | 63 (45-76) | 14 (6-38) | 0.01 |
| 12 | 42 (25-72) | 16 (7-58) | 0.17 |
| 26 | 14 (11-39) | 10 (2-28) | 0.44 |
| Non-PT | PT | P-value | |
| Abduction | |||
| Baseline (wk) | 50 (40-60) | 50 (41-102) | 0.39 |
| 6 | 70 (43-90) | 100 (80-140) | 0.01 |
| 12 | 80 (65-98) | 100 (90-165) | 0.03 |
| 26 | 85 (80-149) | 130 (85-170) | 0.33 |
| Anteflexion | |||
| Baseline (wk) | 70 (70-80) | 95 (48-120) | 0.25 |
| 6 | 90 (75-111) | 140 (105-165) | 0.02 |
| 12 | 90 (80-146) | 130 (115-155) | 0.06 |
| 26 | 100 (90-160) | 155 (110-170) | 0.17 |
| External rotation | |||
| Baseline (wk) | 0 (0-5) | 8 (0-24) | 0.14 |
| 6 | 13 (5-26) | 40 (30-43) | 0.01 |
| 12 | 18 (8-29) | 40 (25-65) | 0.04 |
| 26 | 30 (13-44) | 50 (35-60) | 0.07 |
Both of the NPRS items “night pain” and “average pain last week” showed significant decreases at the 26 wk follow-up for both groups (P < 0.03 for all comparisons). However, significant differences between both treatment groups were not observed at any time point, except for night pain at 6 wk in favor of the PT group (P = 0.02, Table 4). The results of the RAND-36 showed no significant differences between both groups regarding health-related quality of life at all follow-up moments. A slightly higher satisfaction score was reported by the PT group compared to the non-PT group at the 6 wk follow-up (P = 0.02). At all other follow-up moments, the degree of satisfaction was comparable between the two treatment groups (Table 4).
| Non-PT | PT | P-value | |
| NPRS average last week | |||
| Baseline (wk) | 8 (7-9) | 8 (5-8) | 0.37 |
| 6 | 4 (2-8) | 2 (1-4) | 0.19 |
| 12 | 4 (2-7) | 1 (0.5-5) | 0.17 |
| 26 | 3 (1-4) | 2 (0-3) | 0.41 |
| NPRS night | |||
| Baseline (wk) | 8 (8-9) | 9 (7-9) | 0.94 |
| 6 | 4 (3-7) | 2 (0-3) | 0.02 |
| 12 | 5 (2-7) | 1 (0-6) | 0.11 |
| 26 | 2 (1-3) | 2 (0-3) | 0.48 |
| RAND-36 PCS | |||
| Baseline (wk) | 33 (31-40) | 39 (34-46) | 0.11 |
| 6 | 43 (35-46) | 47 (44-52) | 0.10 |
| 12 | 45 (43-50) | 47 (43-55) | 0.63 |
| 26 | 43 (35-56) | 40 (46-56) | 0.56 |
| RAND-36 MCS | |||
| Baseline (wk) | 47 (36-54) | 44 (35-54) | 0.94 |
| 6 | 49 (35-52) | 50 (42-56) | 0.33 |
| 12 | 43 (29-51) | 52 (40-55) | 0.20 |
| 26 | 52 (50-57) | 52 (35-57) | 0.56 |
| Satisfaction (wk) | |||
| 6 | 3 (2-3) | 4 (3-4) | 0.02 |
| 12 | 2 (0-4) | 3 (2-4) | 0.22 |
| 26 | 3 (3-4) | 3.5 (3-4) | 1.00 |
The aim of this trial was to investigate whether physiotherapy is of additional value after an intra-articular corticosteroid injection into the shoulder joint in the treatment of patients with FS in stage one or two. At the final follow-up after 26 wk, no clinical or functional differences were observed between both groups, with or without additional PT. However, total SPADI scores, ROM measurements and NPRS for pain at night were significantly superior in the physiotherapy group at 6 wk. The most considerable differences between the groups were observed for the ROM, in favor of the PT group until 12 wk of follow-up. This could imply that PT after an intra-articular corticosteroid injection is of additional clinical value in the treatment of FS. The result of physiotherapy is improved shoulder function, with less limitation in the rehabilitation process of patients with FS up to the first three months after a corticosteroid injection in the shoulder joint.
An initial good improvement is frequently reported in studies using corticosteroid injection for FS[22,35]. The beneficial value of additional physiotherapy was also reported by Carette et al[21]. In his clinical trial, corticosteroid injection followed by physiotherapy provided a faster recovery of shoulder function compared to injection alone, or placebo injection combined with physiotherapy. Ryans et al[22] conducted a RCT comparing four treatment strategies for FS. The authors concluded that corticosteroids were effective for pain relief and shoulder disability in the short-term, and physiotherapy was effective in restoring external rotation. In both studies, the differences were most distinct at the early follow-up and at 6 and 12 wk, but not significant after more than three months. This is quite similar to our findings. A reason for this might be the self-limiting natural course of the disease. Nevertheless, the beneficial effect of physiotherapy in the short-term can be of clinically-relevant value in case the duration of both symptoms and disabilities is shortened with this strategy.
On the contrary, other studies do not support the use of physiotherapy in the treatment of FS[23,24]. In a systematic review, Blanchard et al[24] found inferior results of PT compared to corticosteroid injection. Some even consider physiotherapy to be inappropriate during early (painful) stage of FS[2,36]. A possible explanation for inferior results from physiotherapy in the treatment of FS is inadequacy to take in to account the tissue irritability level. Irritability is a term to reflect the tissue’s ability to handle physical stress, presumably related to the extent of inflammatory activity. Tissue irritability can be categorized into three levels based on: patient reported pain, pain at end ROM, and the difference between active and passive ROM[25]. PT intensity can vary in the length of treatment, frequency of sessions, intensity of mobilization techniques, and types of exercises. Intensive physiotherapy at an early stage of FS without taking into account the tissue irritability level, can potentially worsen the symptoms of FS. For example, Diercks et al[23] reported a negative effect of PT, including passive stretching and manual mobilization, compared to supportive therapy within pain limits. However, no corticosteroid injections were used in the trial of Diercks et al[23]. Intra-articular corticosteroids have an anti-inflammatory effect, which is likely to attenuate tissue irritability[37]. We believe that in order to optimize treatment of early-stage FS, PT intensity should be guided by tissue irritability level. Moreover, PT is preferably started after an intra-articular corticosteroid injection.
In this prospective RCT, the study population was clearly defined according to strict criteria to include patients with idiopathic FS in stage one or two with symptoms lasting at least three months. The corticosteroid injections were administered under ultrasound guidance by experienced radiologists. Rehabilitation was performed according to a uniform physiotherapy protocol and carried out by specialized shoulder physiotherapists. The ROM measurements were assessed by the treating orthopedic surgeon. Although not blinded for the allocated intervention, these measurements were done consistently and by an experienced surgeon.
The major limitation of this study is the relatively small number of included patients. The results of this trial should therefore be interpreted with caution. A sample size of 41 subjects per group with a power of 90%, alpha 0.05 and a 10% drop-out rate was calculated at the beginning of the study. This was based on the primary outcome parameter SPADI, with a minimal clinically important difference of 13 and a standard deviation of 17. Unfortunately, it was impossible to include this number of patients within a reasonable period of time. This was attributable to two factors. Firstly, the costs for physiotherapy were supported by the Slotervaart Center of Orthopedic Research and Education, however this was only available for a limited number of patients. Three separate research grant applications for funding of the trial were declined. Secondly, there was an unexpected amount of unwillingness to participate among eligible patients. We tried to increase the number of inclusions by attracting attention for the trial in several ways. Printed posters were exposed in the waiting rooms of the Orthopaedic Department, an article about the trial was published in the local hospital journal, and an information letter was sent to more than 200 general practitioners in the catchment area. However, even with these small numbers, a positive effect of physiotherapy was observed up to three months of follow-up. It is possible that more significant differences between both treatment groups would have been found with a larger number of included patients.
A control group without corticosteroid injection was not made available in the study design to monitor the true natural course of the condition. This was because of our assumption that this could raise more difficulties persuading patients to participate in the trial. Study patient compliance to physiotherapy sessions was not recorded. However, a high compliance rate was expected, as the provided PT was free of charge. We are not aware of any patient cross-over, i.e., starting physiotherapy on their own once assigned to the non-PT group. A possible explanation for inferior SPADI scores and ROM measurements at 6 wk in the non-PT group could be the confounding role of diabetes in two patients in this group. A prolonged refractory course of FS can be expected with diabetes[38,39]. However, the results from additional analysis that excluded these two diabetic patients did not change the conclusions.
Nevertheless, it is important to note that there is no clear understanding of the exact mechanism responsible for the natural course of FS as well as its improvement over time for most patients. We do agree that an important aspect of treatment is expert advice and the education of patients, with attention paid to the patients’ perspectives regarding their expectations and experiences with FS.
With the results of this trial and the current literature, we suggest to offer patients additional PT after an intra-articular corticosteroid injection in the treatment of early-stage FS. The SPADI scores, ROM and pain at night scores are significantly better in the PT group vs the non-PT group at 6 wk. With time, the positive effect of PT had faded out. There were no significant differences between patients in both groups at the final follow-up at 26 wk. Additional PT can improve shoulder function and shorten the duration of functional limitations during recovery for early-stage FS patients up to the first three months.
Frozen shoulder (FS) is a common cause of shoulder pain and disability. A contracted capsule with a decreased capsular volume leads to a typical loss of passive external rotation seen in FS. Physiotherapy and corticosteroid injections are the most widely used treatment modalities in FS, in both primary and secondary healthcare settings.
Corticosteroid injections demonstrated a positive effect on shoulder pain and range of motion (ROM), at least in the short term. However, the role of physiotherapy in the treatment of FS is more uncertain. For example, supervised neglect, consisting of supportive therapy and exercises within pain limits, has also been advocated as an appropriate treatment for FS.
The objective of this randomized controlled trial was therefore to investigate the additional value of physiotherapy treatment (PT) after an intra-articular corticosteroid injection in the management of early stage idiopathic FSs. It is hypothesized that additional physiotherapy is superior to corticosteroid injection alone with respect to ROM and shoulder function.
A two center prospective randomized controlled trial was undertaken. Patients with painful early-stage idiopathic FS were eligible for inclusion. After written consent, patients were randomly allocated into two groups. All patients received an ultrasound-guided intra-articular corticosteroid injection. One group underwent additional PT and the other group did not (non-PT). The primary outcome measure was the SPADI. Secondary outcomes were pain (NPRS), ROM, quality of life (RAND-36 score), and patient satisfaction. Follow-up was scheduled after 6, 12 and 26 wk.
Twenty-one patients were included, 11 patients in the non-PT and ten in the PT group. Both treatment groups showed a significant improvement at 26 wk for SPADI score. At the 6 wk follow-up, median SPADI score was significantly decreased in the PT group (14 IQR: 6-38) vs the non-PT group (63 IQR: 45-76) (P = 0.01). Significant differences in all three ROM directions were observed after 6 wk in favor of the PT group (P ≤ 0.02 for all directions). At 26 wk, both groups did not differ significantly with respect to any of the outcome parameters. No complications were reported in both groups.
Intra-articular corticosteroid infiltration is effective in the treatment of FS. Additional PT can improve shoulder function and shorten the duration of functional limitations during the recovery of early-stage FS patients up to the first three months. The physiotherapy intensity should be guided on tissue irritability. Future research should focus on the different populations other than idiopathic FSs, like post-operative or post-traumatic FSs. Furthermore, a small subset of patients is not satisfactorily treated with conservative treatment as an injection and physiotherapy. It would be very interesting to investigate if these patients with a prolonged and refractory course of disease could be identified at an early time point.
It would be very interesting to investigate if these patients with a prolonged and refractory course of disease could be identified at an early time point.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: The Netherlands
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hernandez-Sanchez S, Mittal R, Peng B, Scibek J S- Editor: Ji FF L- Editor: Filipodia E- Editor: Song H
| 1. | Tasto JP, Elias DW. Adhesive capsulitis. Sports Med Arthrosc Rev. 2007;15:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Robinson CM, Seah KT, Chee YH, Hindle P, Murray IR. Frozen shoulder. J Bone Joint Surg Br. 2012;94:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 3. | van der Windt DA, Koes BW, de Jong BA, Bouter LM. Shoulder disorders in general practice: incidence, patient characteristics, and management. Ann Rheum Dis. 1995;54:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 601] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 4. | Pietrzak M. Adhesive capsulitis: An age related symptom of metabolic syndrome and chronic low-grade inflammation? Med Hypotheses. 2016;88:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Bunker TD, Anthony PP. The pathology of frozen shoulder. A Dupuytren-like disease. J Bone Joint Surg Br. 1995;77:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 290] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Reeves B. The natural history of the frozen shoulder syndrome. Scand J Rheumatol. 1975;4:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 393] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Jayson MI. Frozen shoulder: adhesive capsulitis. Br Med J (Clin Res Ed). 1981;283:1005-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Griggs SM, Ahn A, Green A. Idiopathic adhesive capsulitis. A prospective functional outcome study of nonoperative treatment. J Bone Joint Surg Am. 2000;82-A:1398-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 246] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Guyver PM, Bruce DJ, Rees JL. Frozen shoulder - A stiff problem that requires a flexible approach. Maturitas. 2014;78:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Binder AI, Bulgen DY, Hazleman BL, Roberts S. Frozen shoulder: a long-term prospective study. Ann Rheum Dis. 1984;43:361-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 194] [Reference Citation Analysis (0)] |
| 11. | Shaffer B, Tibone JE, Kerlan RK. Frozen shoulder. A long-term follow-up. J Bone Joint Surg Am. 1992;74:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 316] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Hand C, Clipsham K, Rees JL, Carr AJ. Long-term outcome of frozen shoulder. J Shoulder Elbow Surg. 2008;17:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 305] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 13. | Rangan A, Hanchard N, McDaid C. What is the most effective treatment for frozen shoulder? BMJ. 2016;354:i4162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Maund E, Craig D, Suekarran S, Neilson A, Wright K, Brealey S, Dennis L, Goodchild L, Hanchard N, Rangan A. Management of frozen shoulder: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2012;16:1-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 15. | Lewis J. Frozen shoulder contracture syndrome - Aetiology, diagnosis and management. Man Ther. 2015;20:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Mun SW, Baek CH. Clinical efficacy of hydrodistention with joint manipulation under interscalene block compared with intra-articular corticosteroid injection for frozen shoulder: a prospective randomized controlled study. J Shoulder Elbow Surg. 2016;25:1937-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | van der Windt DA, Koes BW, Devillé W, Boeke AJ, de Jong BA, Bouter LM. Effectiveness of corticosteroid injections versus physiotherapy for treatment of painful stiff shoulder in primary care: randomised trial. BMJ. 1998;317:1292-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 182] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Winters JC, Sobel JS, Groenier KH, Arendzen HJ, Meyboom-de Jong B. Comparison of physiotherapy, manipulation, and corticosteroid injection for treating shoulder complaints in general practice: randomised, single blind study. BMJ. 1997;314:1320-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 141] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Shah N, Lewis M. Shoulder adhesive capsulitis: systematic review of randomised trials using multiple corticosteroid injections. Br J Gen Pract. 2007;57:662-667. [PubMed] |
| 20. | Song A, Higgins LD, Newman J, Jain NB. Glenohumeral corticosteroid injections in adhesive capsulitis: a systematic search and review. PM R. 2014;6:1143-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Carette S, Moffet H, Tardif J, Bessette L, Morin F, Frémont P, Bykerk V, Thorne C, Bell M, Bensen W. Intraarticular corticosteroids, supervised physiotherapy, or a combination of the two in the treatment of adhesive capsulitis of the shoulder: a placebo-controlled trial. Arthritis Rheum. 2003;48:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 240] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 22. | Ryans I, Montgomery A, Galway R, Kernohan WG, McKane R. A randomized controlled trial of intra-articular triamcinolone and/or physiotherapy in shoulder capsulitis. Rheumatology (Oxford). 2005;44:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Diercks RL, Stevens M. Gentle thawing of the frozen shoulder: a prospective study of supervised neglect versus intensive physical therapy in seventy-seven patients with frozen shoulder syndrome followed up for two years. J Shoulder Elbow Surg. 2004;13:499-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Blanchard V, Barr S, Cerisola FL. The effectiveness of corticosteroid injections compared with physiotherapeutic interventions for adhesive capsulitis: a systematic review. Physiotherapy. 2010;96:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Kelley MJ, Shaffer MA, Kuhn JE, Michener LA, Seitz AL, Uhl TL, Godges JJ, McClure PW. Shoulder pain and mobility deficits: adhesive capsulitis. J Orthop Sports Phys Ther. 2013;43:A1-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 26. | Vermeulen HM, Rozing PM, Obermann WR, le Cessie S, Vliet Vlieland TP. Comparison of high-grade and low-grade mobilization techniques in the management of adhesive capsulitis of the shoulder: randomized controlled trial. Phys Ther. 2006;86:355-368. [PubMed] |
| 27. | Green S, Buchbinder R, Hetrick S. Physiotherapy interventions for shoulder pain. Cochrane Database Syst Rev. 2003;CD004258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 197] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 733] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 29. | Elvers JWH, Oostendorp RAB, Siervelt IN, van der Heijden KWAP. De Nederlandstalige Shoulder pain and Disability Index (SPADI-Dutch Version) bij patiënten na een subacromiale decompressie volgens Neer. Ned Tijdschr voor Fysiother. 2003;113:126-131. |
| 30. | Thoomes-de Graaf M, Scholten-Peeters GG, Duijn E, Karel Y, Koes BW, Verhagen AP. The Dutch Shoulder Pain and Disability Index (SPADI): a reliability and validation study. Qual Life Res. 2015;24:1515-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | VanderZee KI, Sanderman R, Heyink JW, de Haes H. Psychometric qualities of the RAND 36-Item Health Survey 1.0: a multidimensional measure of general health status. Int J Behav Med. 1996;3:104-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 399] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 32. | Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23352] [Cited by in RCA: 23946] [Article Influence: 725.6] [Reference Citation Analysis (0)] |
| 33. | ten Klooster PM, Drossaers-Bakker KW, Taal E, van de Laar MA. Patient-perceived satisfactory improvement (PPSI): interpreting meaningful change in pain from the patient’s perspective. Pain. 2006;121:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Roy JS, MacDermid JC, Woodhouse LJ. Measuring shoulder function: a systematic review of four questionnaires. Arthritis Rheum. 2009;61:623-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 540] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 35. | Griesser MJ, Harris JD, Campbell JE, Jones GL. Adhesive capsulitis of the shoulder: a systematic review of the effectiveness of intra-articular corticosteroid injections. J Bone Joint Surg Am. 2011;93:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Harris G, Bou-Haidar P, Harris C. Adhesive capsulitis: review of imaging and treatment. J Med Imaging Radiat Oncol. 2013;57:633-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Roh YH, Yi SR, Noh JH, Lee SY, Oh JH, Gong HS, Baek GH. Intra-articular corticosteroid injection in diabetic patients with adhesive capsulitis: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2012;20:1947-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | White D, Choi H, Peloquin C, Zhu Y, Zhang Y. Secular trend of adhesive capsulitis. Arthritis Care Res (Hoboken). 2011;63:1571-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Wang K, Ho V, Hunter-Smith DJ, Beh PS, Smith KM, Weber AB. Risk factors in idiopathic adhesive capsulitis: a case control study. J Shoulder Elbow Surg. 2013;22:e24-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |