Published online Sep 18, 2018. doi: 10.5312/wjo.v9.i9.130
Peer-review started: March 29, 2018
First decision: May 9, 2018
Revised: May 14, 2018
Accepted: May 31, 2018
Article in press: May 31, 2018
Published online: September 18, 2018
Processing time: 171 Days and 17.7 Hours
To investigate the structural and functional characteristics of palmar hypodermal tissue vascularization in Dupuytren’s contracture patients of different age groups.
Eighty-seven Dupuytren’s contracture patients underwent partial fasciectomy. Twenty-two of them were less than 55 years old (Y-group, n = 22); the others were 55 and older (O-group, n = 65). In surgically excised representative tissue samples, a histomorphometric analysis of the perforating arteries of the palmar aponeurosis and stereologic analysis of hypodermis vascularity were performed. The method of laser flowmetry estimated the microcirculation of the skin of the palm.
Frequency of cases with rapid development of contracture (less than 5 years) was 13.6% in the Y-group and 40% in the O-group, P < 0.05. The external and luminal diameters of perforating arteries in palmar fascia were decreased more severely in Y. The thickness of intima increased three times compared with healthy control, and the intima/media relation also increased, especially in O. Increased numerical and volumetric micro-vessel densities in hypodermis, percentage of large vessels (more than 12 μm in diameter), and percentage of vessels with signs of periadventitial inflammatory infiltration were noted in Y. The percentage of vessels with adventitial fibrosis was greater in O than in Y. Base capillary flow in Y was increased compared to healthy control subjects and to O, and peak capillary flow was increased in comparison with control.
Compared to the O-group, Y-group patients exhibited more severe constrictive remodeling of palmar fascia perforating arteries supplying hypodermis but more expressed compensatory changes of its capillarization.
Core tip: This is a retrospective study comparing the incidence of quick development of Dupuytren’s contracture (less than 5 years) and peculiarities of palmar hypodermal tissue vascularization and microcirculation in two age groups of patients undergoing partial fasciectomy. The quantitative characteristics of palmar fascia perforating arteries remodeling, the hypodermal adipose tissue vascularization, and skin microcirculatory flow in patients with Dupuytren’s contracture depending on age were represented for the first time. In patients younger than 55 years, the incidence of rapidly progressive contracture was two, nine times less than that in the older group. Both age groups were characterized with constrictive arterial remodeling, especially the younger group, but quantitative signs of compensatory capillaro- and arteriologenesis were more prominent in the younger group.
- Citation: Shchudlo N, Varsegova T, Stupina T, Dolganova T, Shchudlo M, Shihaleva N, Kostin V. Assessment of palmar subcutaneous tissue vascularization in patients with Dupuytren’s contracture. World J Orthop 2018; 9(9): 130-137
- URL: https://www.wjgnet.com/2218-5836/full/v9/i9/130.htm
- DOI: https://dx.doi.org/10.5312/wjo.v9.i9.130
Dupuytren’s disease (palmar fascial fibromatosis) relates to fibro-proliferative pathology of unknown cause that affects predominantly palmar and digital fasciae, limits finger extension, and causes a progressive contracture of metacarpophalangeal and interphalangeal joints and persistent hand deformity. The prevalence of the disease in different countries varies significantly and is caused by geographic factors, ethnic peculiarities, age, and gender population composition[1]. In most of countries, it affects mainly men over 40 years old. Risk factors for Dupuytren’s contracture remain a topic of discussion. The independent role of alcohol, smoking and the aggravating effect of their combination, the role of chronic traumatization during manual labor, and effects of vibration have been proven[2,3]. The main mechanisms of pathogeny are expression of genes, controlling fibrosis and tissue remodeling[4], vessel narrowing, tissue hypoxia, and formation of free radicals and active forms of oxygen[5], reactive inflammation[6].
Pathologically changed tissues in Dupuytren’s contracture represent fibromatosis nodules and chords with different microscopic structures. Nodules are characterized by increased vascularity and abundant fibroblasts, and among them there are plenty of myofibrolasts with contractile properties; cords contain less cellular elements and are relatively avascular but rich in collagen. Transforming growth factor β is considered the main modulator of myofibroblast transdifferentiation[7]. Palmar fascial fibromatosis is a kind of reactive proliferation, but not like tumor processes[8], although it was characterized by infiltrative growth[9] in hypodermis, dermis, tendons sheaths, and joints capsules. On the other hand, in the skin and hypodermis of Dupuytren’s contracture patients, high expression of stem cells markers was revealed[10]. Stem cells are considered an additional source of fibroblasts and myofibrolasts proliferation. Laminin-rich blood vessels also act as the centers of myofibrolasts proliferation[11].
Substantial differences in genes expression were noted in the hypodermis but not in skin of Dupuytren’s contracture patients compared to healthy individuals[12]. In type 2 diabetes, the severity of insulin resistance is determined by changes in vascularity of hypodermis[13,14]. A characteristic feature of Dupuytren’s contracture tissues is plenty of young vessels[9], but the influence of tissue vascularization on the course of the disease is unclear. There are contradictory reports regarding the impact of patient’s age on disease onset, progression, and recurrence[15,16]. Vascularization of hypodermis in Dupuytren’s contracture patients of different age groups has not been studied. The aim of our research is to investigate the structural and functional characteristics of palmar hypodermal tissue vascularization in Dupuytren’s contracture patients of different age groups.
At the FSBI Russian Ilizarov Scientific Center, 236 patients with Dupuytren’s contracture were operated on 2013 through 2018. Age of the patients ranged from 27 to 84 years. All of them gave prior informed consent for surgery. The study protocol was approved by the ethics committee of the institution. At the time of surgery, in most cases, the contracture matched the stages 2-3 according to R. Tubiana[9] classification, but four patients had stage 4. The inclusion criteria included histologically confirmed clinical diagnosis of Dupuytren’s contracture and detailed anamnesis morbi in the case report. The exclusion criterion was the acute trauma of the hand in anamnesis. For clinical characteristics, the following data were taken into account: age, sex, duration of clinical signs of fibromatosis, bilateral affection and number of fingers with impaired function, and comorbid conditions.
Tissue samples of pathologically changed palmar aponeurosis with hypodermis from 87 patients were fixed in a mixture of 20 g/L solutions of glutaraldehyde and paraformaldehyde in phosphate buffer (рН 7.4) adding 1 g/L picric acid. Then according to standard protocols, samples were embedded in paraffin. For preparation of histological sections (thickness 5-7 μm), the Reichert microtome (Vienna, Austria) was used. Sections were stained with hematoxylin-eosin, Masson’s trichrome method was used for collagen, and Weigert-van Gieson’s was used for elastic fibers selective evaluation. Digital images of the fields of view of the photomicroscope “Оpton” (Oberkochen, Germany) were obtained using the “DiaMorph” hardware-software complex (Moscow, Russia). Histomorphometry was performed with the PhotoFiltre and “VT-Master-Morphology” programs (VideoTest, St. Petersburg, Russia). From each tissue sample, 30 visual fields were obtained (magnification 200 ×). Using the electronic version of the test grid for point-counting method the percentage of adipocytes, numerical and volumetric vessels densities were assessed. Numerical density is the number of blood vessels profiles per unit area of histological section; volumetric density is their volume fraction in a series of histological sections. The percentages of vessels with signs of periadventitial inflammatory infiltration and periadventitial fibrosis were determined. In digital images of transverse sections of perforating palmar aponeurosis arteries (magnification 500 ×), their outside diameters, lumen diameters, wall thickness, intima thickness, and media thickness were measured and intima/media ratio was counted. Patients with acute hand wounds (n = 3) were used as the source of small tissue samples from normal palmar aponeurosis.
Assessment of microcirculation of skin tissues was carried out using laser doppler flowmetry (LDF) with a BLF 21 monitor (Transonic System Inc., Ithaca, NY, United States), calibrated according to manufacturer’s recommendations. The apparatus was equipped with a standard fiber separation probe (0.25 mm) and laser wavelength of 780 nm with a measurement depth of 0.5-1.0 mm. The method of LDF examines vessels of the microcirculatory bed, located in the dermis and hypodermis. The probe was placed on the palmar surface of the hand (Figure 1) on areas of pronounced fibromatosis (bases of IV-V fingers) and fastened with a double-sided adhesive membrane. Measurements of LDF were expressed in arbitrary units of perfusion (PU). To study the mechanisms involved in the regulation of tissue blood flow, a local ischemic test including the installation of an occlusive cuff on the forearm was performed. After registration of capillary flow at rest (microcirculatory flow base - MFB) and after 3 min of ischemia (microcirculatory flow peak - MFP), the index of peak microcirculatory blood flow was counted: IMFP = MFP/MFB × 100% - percentage of increase in capillary blood flow after cuff release. Time of hyperemic response - from the moment of cuff release to maximal growth of capillary blood flow, velocity of hyperemic response - velocity of growth of microcirculatory flow from the moment of cuff release to maximal capillary blood flow, time of half-recovery, speed of half-recovery, time of full microcirculatory blood flow recovery, duration of hyperemic response, and the intensity (area) of the hyperemic response were also defined.
After obtaining the parameters of descriptive statistics, all samples of quantitative data were evaluated for the normality of distribution using the Shapiro-Wilk test. For some samples, the normality hypothesis was not confirmed. The majority of quantitative characteristics were represented in tables in the form of medians and quartiles [Me (Q1; Q3)]. The hypothesis of differences was tested using non-parametric Wilcoxon and Mann-Whitney criteria and exact Barnard test with a significance level of 0.05. For all statistical treatments, the software package Attestat Program (version 9.3.1, developed by Gaidyshev IP, Certificate of Rospatent official registration No. 2002611109) was used.
General characteristics of Dupuytren’s contracture patients are presented in Table 1. Out of 87 patients, 22 (25.3%) noted the first symptoms of palmar fibromatosis at the age of less than 50 years and were younger than 55 at the time of surgery (Y-group). The remaining 65 patients were aged 50 years and older at disease onset and they were 55 and more years old at the time of surgery (O-group). These groups differed in the frequency of cases, with a rapidly progressive development of contracture (less than 5 years) in Y-group 13.6% and in O-group 40.0% (P = 0.029161528). The incidence of cardiovascular diseases in the Y-group was 27.3%, and in the O-group 61.5% (P = 0.001772739).
| Parameter | Value |
| Demographic characteristics | |
| Age (M ± σ) (min-maх) | (59 ± 10) (24-83) |
| Male:Female relation | 8:01 |
| Status localis | |
| Frequency of bilateral affection | 28 (32, 18) |
| Duration of disease, years – (min-max) - Me (Q1; Q3) | (0.5-20) - 5 (4; 10) |
| Level of contracture – (min-max) - Me (Q1; Q3) | (2-4) - 2.5 (2; 3) |
| Number of fingers with impaired function (Min-max) - Me (Q1; Q3) | (1-8) - 2 (1-2) |
| Comorbid conditions | |
| Arterial hypertension | 41 (47.1) |
| Ischemic heart disease | 5 (5.7) |
| Chronic cardial insufficiency | 3 (3.4) |
| Postinfarction cardiosclerosis | 2 (2.3) |
| Diabetes type 2 | 4 (4.6) |
| Chronic obstructive pulmonary disease | 6 (7.8) |
| Chronic hepatitis | 6 (7.8) |
| Neuritis of the auditory nerve | 4 (4.6) |
| Urolithiasis disease | 2 (2.3) |
| Obesity | 2 (2.3) |
| Extremal active smoking | 7 (8.0) |
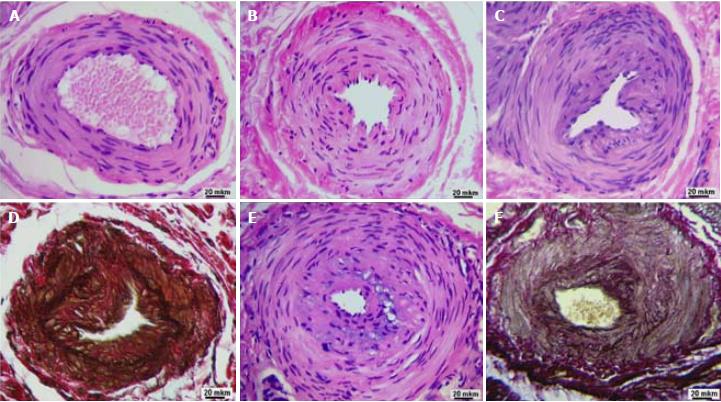
According to microscopic examination of histological preparations, along with arteries of a typical structure (Figure 2A), there are arteries in the state of constrictive remodeling that differ from normal by a significant narrowing of the lumen. In some cases, such arteries are characterized by pronounced fibrosis of adventitia and contraction of the muscular layer (Figure 2B) but preserved integrity of the internal elastic membrane. In the luminal lining of such arteries, there are only single neo-intimal cells - modified smooth muscle cells. In other cases, the narrowing of the lumen of remodeled arteries is due to the presence of a pronounced polymorphic cell neo-intimal layer (Figures 2C and E). The specific coloration of elastin allowed for visualization of damage to the internal elastic membrane (Figure 2D), which were residual fragments of the old (pre-existing) internal elastic membrane, and a network of newly formed elastic fibers in the thickness of the neo-intima (Figure 2F).
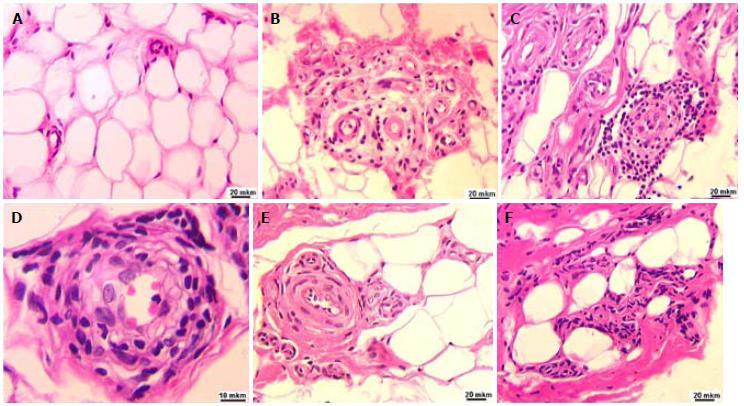
The hypodermal tissue adjacent to the surface layer of the palmar aponeurosis was characterized by increased vascularization. Thick-walled capillaries were located singly (Figure 3A) or in the form of clusters. Perivascular spaces were often infiltrated with inflammatory cells (Figure 3B), which in some cases formed continuous multicellular cuffs (Figure 3C and D). In some loci of the hypodermis, pronounced perivascular fibrosis (Figures 3C, E and F), replacement of fat cells with collagen deposits, and perivascular clusters of fibroblasts were evident (Figure 3F).
With morphometric analysis, it was established that the outer diameter and diameter of the lumen of the perforating arteries of the palmar aponeurosis were reduced in comparison with the control in both Dupuytren’s contracture groups, but this reduction was greater in the Y-group than the O-group (Table 2). The thickness of the artery wall was also increased in comparison with the control, but this increase was greater extent in the O-group. Both groups are characterized by a reliable approximately three-fold increase in intimal thickness, statistically insignificant thinning of the medial layer, and an increased intima/media thickness ratio compared to control. Intima thickness and intima/media thickness ratio were significantly greater in the O-group than in the Y-group.
| Group/parameters | Healthy control n = 3 | Y-group (< 55 yr of age) n = 8 | O-group (≥ 55 yr of age) n = 19 |
| Diameter | 412.8 | 31566 (306.96/326.91)ac | 334.85 (309.06/441.19)a |
| of artery (μm) | (351.43/506.31) | P2 = 0.000332; P3 = 0.136006 | P1 = 0.010485 |
| Lumen | 243.15 | 151.56 (94.72/153.90)ac | 178.19 (113.78/246.52)a |
| diameter (μm) | (223.07/326.52) | P2 = 0.000736; P3 = 0.249671 | P2 = 0.007253 |
| Wall thickness (μm) | 89.03 | 92.16 (80.88/106.12)c | 117.98 (98.37/133.13)a |
| (65.97/88.88) | P2 = 0.49959; P3 = 0.010846 | P2 = 0.004483 | |
| Thickness | 8.41 | 22.26 (14.79/33.42)a | 25.37 (21.16/41.75)a |
| of intima (μm) | (7.80/0.82) | P2 = 0.004998; P3 = 0.355126 | P2 = 0.000463 |
| Thickness | 58.45 | 46.52 (38.08/60.30) | 46.77 (38.26/64.03) |
| of media (μm) | (44.01/60.28) | P2 = 0.37436; P3 = 0.924826 | P2 = 0.17192 |
| Intima/media thickness ratio | 0.18 | 0.53 (0.25/0.55)a | 0.68 (0.33/0.96)a |
| (0.12/0.24) | P2 = 0.001992; P3 = 0.282078 | P2 = 0.000198 |
The Y-group was characterized by a higher content of adipocytes in the hypodermis and aponeurosis tissue than the O-group (Table 3). The numerical and volumetric density of the vessels in the hypodermis, as well as the percentage of vessels with signs of peri-adventitial inflammatory infiltration, was also larger in the Y-group. The O-group was characterized by a greater degree of perivascular fibrosis. The average diameter of the vessels was significantly higher in the Y-group. The percentage of vessels with a diameter greater than 12 μm in the Y-group was 47.1%, and in the O-group it was 41.2% (P = 0.039384).
| Group/parameters | Y-group (< 55 yr of age) n = 15 | O-group (≥ 55 yr of age) n = 14 |
| Percentage of adipoсytes in serial sections of Dupuytren’s tissue sample [Me (Q1/Q3), %] | 35.05 (23.40/40.09)aP1 = 0.000338 | 27.79 (19.37/32.82) |
| Numerical microvessels density [Me (Q1/Q3), μm-2] | 5 (4/7)aP2 = 0 | 3 (2/4) |
| Volumetric microvessels density [Me (Q1/Q3), %] | 2.48 (1.44/4.13)aP2 = 8.88 × 10-16 | 1.16 (0.52/2.12) |
| Relative number of microvessels with signs of periadventitial inflammatory infiltration | 21.84aP2 = 1.69 × 10-5 | 12.93 |
| Relative number of microvessels with signs of adventitial fibrosis | 4.48aP2 = 0.002358 | 8.19 |
| Microvessels diameters [Me (Q1/Q3), μm] | 12.55 (9.26/18.05)aP2 = 0.019425 | 11.58 (8.67/16.84) |
The base microcirculatory flow in the Y-group was significantly increased in comparison with the norm and the O-group (Table 4). Peak microcirculatory flow in the Y-group was also increased but only in comparison with corresponding norm. In the O-group, the index of peak microcirculatory flow was increased compared to norm and the Y-group. Both patients’ groups differed from healthy controls, with more pronounced variability of post-occlusive hyperemia as well as a more pronounced difference in their right and left arms.
| Parameters | Norm (25-54 yr of age)n = 22 | DCY (< 55 yr of age)n = 6 | Norm (55-70 yr of age)n = 6 | DCO (≥ 55 yr of age)n = 18 |
| Age | 33 (23/54) | 52 (45/54) | 59 (55/63) | 66 (59/69) |
| Microcirculatory flow base (PU) | 4 (3.5/5.7) | 15.45c (8.4/20.6) P3 = 0.020 | 3.5 (2.55/4.7) | 4.15a (3.1/7.8) P2 = 0.019 |
| Microcirculatory flow peak (PU) | 14.7 (11.8/18.3) | 27.5c (22.2/44.4) P3 = 0.002 | 18.2 (8.15/18.7) | 20.1 (16.7/24.6) |
| Microcirculatory flow peak index (%) | 340 (232/443) | 218.5 (125/488) | 373 (223/449) | 508 (336/544) P2 = 0.045 |
| Time of hyperemic response (s) | 15 (10/25) | 17.5 (10/30) | 20 (20/25) | 20 (15/90) |
| Velocity of hyperemic response (PU/s) | 0.62 (0.50/0.81) | 0.56 (0.14/1.19) | 0.52 (0.15/0.55) | 0.81 (0.21/1.09) |
| Time of half-recovery (s) | 45 (15/75) | 30 (5/60) | 35 (20/70) | 40 (30/130) |
| Speed of half-recovery (PU/s) | 0.18 (0.14/0.34) | 0.96 (0.16/1.54) | 0.2 (0.11/0.28) | 0.28 (0.09/0.49) |
| Time of full recovery (s) | 247 (205/380) | 212 (40/600) | 147 (125/190) | 400 (90/600) |
| Duration of hyperemic response (s) | 270 (180/300) | 242 (55/660) | 165 (150/210) | 515 (120/700) |
| Intensity (area) of hyperemic response (relative units) | 1127 (705/1830) | 2799 (193/6216) | 1575 (474/2202) | 3804 (1110/5084) |
The role of adipose tissue in the pathogenesis of Dupuytren’s contracture has been extensively studied. The prevalence of the disease in older age groups was associated with less content of adipose tissue in fascial structures and hypodermis and corresponding reduction of its protective-amortization role during chronic hand traumatization[17,18]. Besides peculiarities of the lipid composition in adipose tissue, the similarity of gene expression in adipose tissue and in fibromatous nodes was established in patients with Dupuytren’s contracture[12]. The role of adipose tissue in angiogenesis is known[19], and the presence of markers of angiogenesis in Dupuytren’s contracture palmar aponeurosis was proved[20]. The composition of the luminal lining of microvessels revealed potential precursors of myofibroblasts[21].
In this study, the quantitative characteristics of the hypodermal adipose tissue vascularization in patients with Dupuytren’s contracture were represented for the first time. It has been established that the perforating arteries of the palmar aponeurosis, which give rise to the vascular plexus of the hypodermis, are in a state of constrictive remodeling. Such a remodeling was characterized by a reduction of arteries outside and luminal diameters, and in patients older than 55 years it was accompanied with thickening of the arterial wall.
In both age groups, the thickness of the intima and intima/media thickness ratio were increased compared to control. In the older age group, a significantly higher incidence of arterial hypertension and other cardiovascular diseases were noted. A more pronounced decrease in the external diameter and lumen of the perforating arteries in the younger age group suggests that constrictive remodeling of arteries is mainly associated with the development of fascial fibromatosis rather than cardiovascular diseases. This assumption is consistent with the results of a study of normotensive patients with another rheumatic disease - systemic lupus erythematosus[22].
A limitation of our study was the small sample size of patients in the post-occlusive hyperemia test. However, the increased variability of its characteristics in comparison with the norm and the large difference in the parameters of the right and left hands suggest that this sample, and in particular the area of the hyperemic response, may reflect the severity of pathological and compensatory-adaptive changes. In patients with ischemic heart disease, the area of hyperemic response was relevant to endothelial dysfunction[23]. We assumed that a study with a large sample of patients with Dupuytren’s contracture before and after the operation will clarify the role of microcirculation in the development of individually oriented treatment protocols.
The larger numerical and volumetric densities of microvessels in the hypodermal tissue in patients younger than 55 years of age compared to those in the older age group are consistent with larger microcirculatory blood flow. These data, combined with a significantly greater proportion of vessels with a diameter of more than 12 μm, indicate more pronounced processes of capillary and arteriologenesis. We have not, however, used angiogenesis markers, and this is another limitation of our study. The incidence of quick progression of contracture was higher in the older group. The secondary hypothesis of our study is that the use of therapeutic angiogenesis in patients operated on for Dupuytren’s contracture will slow the recurrence and spread of fibromatosis.
In conclusion, more severe constrictive remodeling of palmar fascia perforating arteries supplying the hypodermis was revealed in patients younger than 55, but the increased vascularization of the hypodermis serves as a compensatory-adaptive mechanism that slows the development of fibromatosis and contracture.
Patients with Dupuytren’s contracture are numerous in hand clinics. In most cases, conservative treatment is not curable. Surgical excision of diseased tissue and correction of contracture are effective but the risks of recurrence and extension of pathological changes are high. Measures of successful influence on course of the disease are absent.
Multiple studies of Dupuytren’s disease deal with transformations of connective tissue, its molecular mechanisms, peculiarities of cells immuno-histochemical phenotypes, and gene expression. Though vascular remodeling mediates pathogenesis of various diseases, little is known about changes in palmar fascia and hypodermis vascularity in Dupuytren’s contracture patients - especially its quantitative characteristics.
We wanted to analyze histological morphometric parameters of palmar fascia arteries and microvascularity of palmar hypodermis of Dupuytren’s contracture patients and to assess the functional parameters of microcirculation of palmar skin tissues. The main purpose was to present peculiarities of structural and functional characteristics of palmar hypodermal tissue vascularization in different age groups and to determine whether these groups differ in the rate of progression of the hand deformity.
We identified clinical characteristics of Dupuytren’s contracture patients (n = 87), morphometric characteristics of palmar fascia perforating arteries (n = 30), stereologic characteristics of hypodermis, parameters of microcirculatory flow, and post-occlusive hyperemia test (n = 52) in Dupuytren’s contracture patients in younger and older groups and healthy controls in different age groups. Methods were standard, but to the best of our knowledge, they had not been used for Dupuytren’s contracture research.
Cases with rapid progression of hand deformity (less than 5 years) were more common in older patients (55 and more years old). Signs of arterial constrictive remodeling were more prominent in the younger group. Adipocytes content, microvessel density, and per cent of microvessels with signs of inflammatory infiltration were bigger in the younger group. Vessels with adventitial fibrosis were seen more often in the older group. Base capillary flow in the younger group was bigger than the healthy control subjects and the older group; peak capillary flow was increased in comparison with control.
Constrictive remodeling of palmar fascia arteries in studied groups of patients was associated with the development of fascial fibromatosis rather than cardiovascular disease. In patients younger than 55 years, old compensatory changes of the microcirculatory bed in palmar hypodermis contributed to more benign course of the disease.
Further analysis of functional and structural characteristics of blood vessels in different age and comorbidity groups of Dupuytren’s contracture patients are necessary for development of effective vasogenic therapy, which may effectively influence disease progression and extending.
The authors would like to thank the members of the engineering group for their technical support and Gaidyshev IP for his help with statistical analysis.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Russia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Shao R S- Editor: Ji FF L- Editor: Filipodia E- Editor: Song H
| 1. | Rayan GM. Dupuytren’s disease: anatomy, pathology, presentation, and treatment. Instr Course Lect. 2007;56:101-111. [PubMed] |
| 2. | Godtfredsen NS, Lucht H, Prescott E, Sørensen TI, Grønbaek M. A prospective study linked both alcohol and tobacco to Dupuytren’s disease. J Clin Epidemiol. 2004;57:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Descatha A, Jauffret P, Chastang JF, Roquelaure Y, Leclerc A. Should we consider Dupuytren’s contracture as work-related? A review and meta-analysis of an old debate. BMC Musculoskelet Disord. 2011;12:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Forrester HB, Temple-Smith P, Ham S, de Kretser D, Southwick G, Sprung CN. Genome-wide analysis using exon arrays demonstrates an important role for expression of extra-cellular matrix, fibrotic control and tissue remodelling genes in Dupuytren’s disease. PLoS One. 2013;8:e59056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Murrell GA. The role of the fibroblast in Dupuytren’s contracture. Hand Clin. 1991;7:669-680; discussion 681. [PubMed] |
| 6. | Mayerl C, Del Frari B, Parson W, Boeck G, Piza-Katzer H, Wick G, Wolfram D. Characterisation of the inflammatory response in Dupuytren’s disease. J Plast Surg Hand Surg. 2016;50:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Rehman S, Goodacre R, Day PJ, Bayat A, Westerhoff HV. Dupuytren’s: a systems biology disease. Arthritis Res Ther. 2011;13:238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Wang L, Zhu H. Clonal analysis of palmar fibromatosis: a study whether palmar fibromatosis is a real tumor. J Transl Med. 2006;4:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Warren RF. The pathology of Dupuytren’s contracture. Br J Plast Surg. 1953;6:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Hindocha S, Iqbal SA, Farhatullah S, Paus R, Bayat A. Characterization of stem cells in Dupuytren’s disease. Br J Surg. 2011;98:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Viil J, Maasalu K, Mäemets-Allas K, Tamming L, Lõhmussaar K, Tooming M, Ingerpuu S, Märtson A, Jaks V. Laminin-rich blood vessels display activated growth factor signaling and act as the proliferation centers in Dupuytren’s contracture. Arthritis Res Ther. 2015;17:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Shih B, Brown JJ, Armstrong DJ, Lindau T, Bayat A. Differential gene expression analysis of subcutaneous fat, fascia, and skin overlying a Dupuytren’s disease nodule in comparison to control tissue. Hand (NY). 2009;4:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Spencer M, Unal R, Zhu B, Rasouli N, McGehee RE Jr, Peterson CA, Kern PA. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab. 2011;96:E1990-E1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 14. | Robciuc MR, Kivelä R, Williams IM, de Boer JF, van Dijk TH, Elamaa H, Tigistu-Sahle F, Molotkov D, Leppänen VM, Käkelä R. VEGFB/VEGFR1-Induced Expansion of Adipose Vasculature Counteracts Obesity and Related Metabolic Complications. Cell Metab. 2016;23:712-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 187] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 15. | Reilly RM, Stern PJ, Goldfarb CA. A retrospective review of the management of Dupuytren’s nodules. J Hand Surg Am. 2005;30:1014-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Hindocha S, Stanley JK, Watson JS, Bayat A. Revised Tubiana’s staging system for assessment of disease severity in Dupuytren’s disease-preliminary clinical findings. Hand (NY). 2008;3:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Tubiana R. Dupuytren’s disease of the radial side of the hand. Hand Clin. 1999;15:149-159. [PubMed] |
| 18. | Flint M. The genesis of the palmar lesion. Dupuytren’s disease: biology and treatment. New York: Churchill Livingstone 1990; 140-145. |
| 19. | Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 270] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 20. | Holzer LA, Cör A, Pfandlsteiner G, Holzer G. Expression of VEGF, its receptors, and HIF-1α in Dupuytren’s disease. Acta Orthop. 2013;84:420-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | On N, Koh SP, Brasch HD, Dunne JC, Armstrong JR, Tan ST, Itinteang T. Embryonic Stem Cell-Like Population in Dupuytren’s Disease Expresses Components of the Renin-Angiotensin System. Plast Reconstr Surg Glob Open. 2017;5:e1422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Morreale M, Mulè G, Ferrante A, D’ignoto F, Cottone S. Early Vascular Aging in Normotensive Patients With Systemic Lupus Erythematosus: Comparison With Young Patients Having Hypertension. Angiology. 2016;67:676-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Stiefel P, Moreno-Luna R, Vallejo-Vaz AJ, Beltrán LM, Costa A, Gómez L, Ordóñez A, Villar J. Which parameter is better to define endothelial dysfunction in a test of postocclusive hyperemia measured by laser-Doppler flowmetry? Coron Artery Dis. 2012;23:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |