Peer-review started: December 6, 2017
First decision: December 18, 2017
Revised: December 22, 2017
Accepted: February 4, 2018
Article in press: February 5, 2018
Published online: March 18, 2018
Processing time: 100 Days and 11.1 Hours
To analyze how various implants placement variables affect sacroiliac (SI) joint range of motion.
An experimentally validated finite element model of the lumbar spine and pelvis was used to simulate a fusion of the SI joint using various placement configurations of triangular implants (iFuse Implant System®). Placement configurations were varied by changing implant orientation, superior implant length, and number of implants. The range of motion of the SI joint was calculated using a constant moment of 10 N-m with a follower load of 400 N. The changes in motion were compared between the treatment groups to assess how the different variables affected the overall motion of the SI joint.
Transarticular placement of 3 implants with superior implants that end in the middle of the sacrum resulted in the greatest reduction in range of motion (flexion/extension = 73%, lateral bending = 42%, axial rotation = 72%). The range of motions of the SI joints were reduced with use of transarticular orientation (9%-18%) when compared with an inline orientation. The use of a superior implant that ended mid-sacrum resulted in median reductions of (8%-14%) when compared with a superior implant that ended in the middle of the ala. Reducing the number of implants, resulted in increased SI joint range of motions for the 1 and 2 implant models of 29%-133% and 2%-39%, respectively, when compared with the 3 implant model.
Using a validated finite element model we demonstrated that placement of 3 implants across the SI joint using a transarticular orientation with superior implant reaching the sacral midline resulted in the most stable construct. Additional clinical studies may be required to confirm these results.
Core tip: Minimally invasive fusion of the sacroiliac (SI) joint is a potential treatment for patients suffering with symptoms related to the SI joint. This study used finite element analysis to investigate how implant orientation, superior implant length, and implant number affect SI joint range of motion. The results of this study demonstrate that placement of 3 implants across the SI joint using a transarticular orientation with superior implant reaching the sacral midline resulted in the most stable construct.
- Citation: Lindsey DP, Kiapour A, Yerby SA, Goel VK. Sacroiliac joint stability: Finite element analysis of implant number, orientation, and superior implant length. World J Orthop 2018; 9(3): 14-23
- URL: https://www.wjgnet.com/2218-5836/full/v9/i3/14.htm
- DOI: https://dx.doi.org/10.5312/wjo.v9.i3.14
Minimally invasive fusion of the sacroiliac (SI) joint is a potential treatment for patients suffering with symptoms related to the SI joint. Although diagnosis of the primary pain generator in low back pain is challenging[1], proper diagnosis allows for the most effective treatment. Of patients dealing with low back pain, between 15% and 30% have the SI joint as a pain generator[2-4].
Recently, minimally invasive fusion of the SI joint has been shown to be an effective method for reducing SI joint pain[5]. In addition, minimally invasive procedures have been shown to reduce blood loss, length of stay, and surgical time, while resulting in more positive outcomes for the patient compared with traditional open fusion procedures[5].
There are many factors that influence the choice and placement of implants placed across the SI joint. The sacral anatomy allows for placement of iliosacral hardware within sacral safe zones, although differences in anatomy have a significant effect on the location and size of the safe zones[6]. There is evidence that placement of multiple implants in unstable pelvic fracture models results in the greatest biomechanical stability[7-9]. Additional studies have demonstrated that placement of iliosacral screws within regions of higher bone density result in higher extraction forces[10,11].
Previous ex vivo experimental studies have investigated the biomechanical effects of placing SI joint fusion devices[12,13]. These studies have shown that placement of 3 triangular titanium plasma spray (TPS) coated titanium implants significantly reduced motion of the treated SI joint. A comparison of two lateral placement variations, inline (posterior) and transarticular, showed that both variations significantly reduced motion, and suggested that the transarticular orientation may provide more initial stability.
Finite element modeling is another technique used to investigate the biomechanics of the SI joint and pelvis[14-17]. Ivanov et al[14,15] validated an SI joint FE model by comparing the FEA model ROM with experimental data for the intact and sequential ligament sectioning conditions from Simonian et al[18]. This SI joint model was later confirmed[17] to demonstrate that SI joint treatment using implants resulted in comparable reductions in motion to those reported in cadavers by Soriano-Baron et al[13].
Although, clinical and experimental evidence shows that placing 3 triangular TPS coated implants has successful clinical and biomechanical results, questions remain concerning the optimal parameters for implant placement. The objective of this study was to investigate and quantify the effect of implant orientation, superior implant length, and implant number on SI joint range of motion.
A finite element model of the lumbar spine, pelvis, and both femurs was used to simulate SI joint motion; this model has previously been used to evaluate the effects of leg length discrepancy, effects of lumbar spine fusion on the SI joint, and effects of SI joint fusion on the lumbar spine[14-17]. The femoral head was fixed into the acetabular cup to ensure loading, but that no motion occurred at the hip joint. Briefly, a pelvis was scanned using computed tomography (CT) and material properties for bones, ligaments, and joints were assigned[14,15]. The material properties of the sacral cancellous bone were assumed to be isotropic and varied in accordance to the apparent bone mineral density from a normal sacrum (t-score > -1)[19] using a power law distribution (α = 2)[20]. For treated models, the core of the titanium plasma spray (TPS) coated implants (iFuse Implant 7.0 mm; SI-BONE, Inc., San Jose, CA, United States) was assigned the material properties of Ti6Al4V ELI (E = 115 GPa), the interface between the implant core and adjacent bone can be found in Lindsey et al[17].
The intact and instrumented model loads were simulated using a compressive follower load of 400 N, and a 10 N-m bending moment applied at the superior surface of the L1 vertebra[21,22]. The compressive follower load was extended to the sacrum level and the angle of the connector elements defined such that the entire lumbo-pelvic segment did not go into any rotational motion following contraction of the connector elements. Loading was simulated in flexion-extension, lateral bending (left and right), and axial rotation (left and right) during double-leg stance. The range of motion of the SI joint was determined for each loading direction[14].
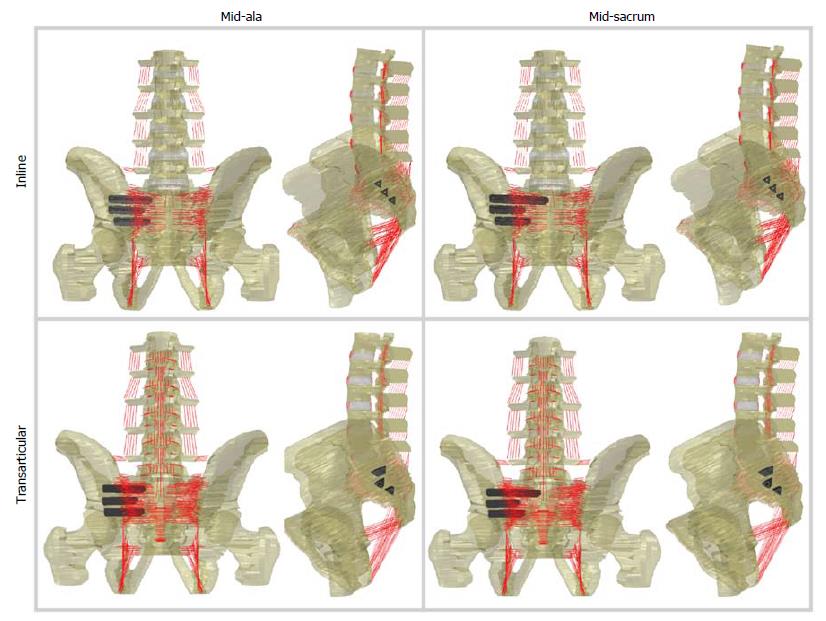
Three treatment variables were investigated: Implant orientation, superior implant length, and implant number. Two potential implant orientations, inline (posterior) and transarticular, have been previously investigated[13] and both were further investigated here (Figure 1). Clinically, the superior implant length is often chosen to end within the middle of the ala (i.e., directly above the S1 foramen); based upon previous trauma literature[11], we also investigated a longer superior implant that extended to the midline of the sacrum (Figure 1). Typically, three implants are placed[23], for this investigation either 1, 2, or 3 implants were placed. All potential instrumented combinations were simulated, resulting in 22 unique models (Table 1). The superior implant was either 55 mm long (mid-ala) or 75 mm long (mid-sacrum) for both the inline and transarticular orientation (placement of the superior implant is identical for the two orientations). The middle implants were 45 mm long for both the inline and transarticular orientations, while the inferior implant was 45 mm long for the inline orientation and 50 mm long for the transarticular orientation.
| Treatment/orientation | Implant placed | Superior implant ending point | SI joint ROM (°) [Reduction in ROM (%)] | ||||
| Superior | Middle | Inferior | Flexion-extension | Lateral bending | Axial rotation | ||
| Intact | - | - | - | -1 | 1.94° (-) | 0.66° (-) | 1.11° (-) |
| Inline orientation | X | X | X | Mid-ala | 0.7° (64%) | 0.45° (32%) | 0.41° (63%) |
| Mid-sacrum | 0.63° (68%) | 0.41° (38%) | 0.38° (66%) | ||||
| X | - | X | Mid-ala | 0.82° (58%) | 0.47° (29%) | 0.44° (60%) | |
| Mid-sacrum | 0.69° (64%) | 0.43° (35%) | 0.4° (64%) | ||||
| X | X | - | Mid-ala | 0.97° (50%) | 0.5° (24%) | 0.49° (56%) | |
| Mid-sacrum | 0.76° (61%) | 0.45° (32%) | 0.43° (61%) | ||||
| - | X | X | -1 | 0.91° (53%) | 0.53° (20%) | 0.55° (50%) | |
| X | - | - | Mid-ala | 1.36° (30%) | 0.58° (12%) | 0.67° (40%) | |
| Mid-sacrum | 1.21° (38%) | 0.58° (12%) | 0.61° (45%) | ||||
| - | X | - | -1 | 1.32° (32%) | 0.65° (2%) | 0.73° (34%) | |
| - | - | X | -1 | 1.25° (36%) | 0.69° (-5%) | 0.79° (29%) | |
| Transarticular orientation | X | X | X | Mid-ala | 0.59° (70%) | 0.41° (38%) | 0.34° (69%) |
| Mid-sacrum | 0.52° (73%) | 0.38° (42%) | 0.31° (72%) | ||||
| X | - | X | Mid-ala | 0.69° (64%) | 0.42° (36%) | 0.36° (68%) | |
| Mid-sacrum | 0.58° (70%) | 0.39° (41%) | 0.32° (71%) | ||||
| X | X | - | Mid-ala | 0.81° (58%) | 0.45° (32%) | 0.4° (64%) | |
| Mid-sacrum | 0.64° (67%) | 0.41° (38%) | 0.35° (68%) | ||||
| - | X | X | -1 | 0.76° (61%) | 0.47° (29%) | 0.46° (59%) | |
| X | - | - | Mid-ala | 1.36° (30%) | 0.58° (12%) | 0.67° (40%) | |
| Mid-sacrum | 1.21° (38%) | 0.58° (12%) | 0.61° (45%) | ||||
| - | X | - | -1 | 1.11° (43%) | 0.6° (9%) | 0.7° (37%) | |
| - | - | X | -1 | 1.05° (46%) | 0.62° (6%) | 0.73° (34%) | |
The effect of treatment was assessed by calculating the difference in ROM between the intact and treated configurations for each combination of implant orientation, superior implant length, and number of implants in flexion-extension, lateral bending, and axial rotation. The percent change was calculated in comparison with the intact ROM. The median and range for the difference in ROM and percent change were determined for each motion. Effects of individual treatment variables are described below.
Implant orientation: The effect of implant orientation was assessed by calculating the difference between the inline and transarticular (TA) configurations. Differences in ROM were calculated as a function of superior implant length (SIL) (mid-ala, mid-sacrum, or none) and number of implants (3-superior/middle/inferior, 2- superior/inferior, 2-superior/middle, 2-middle/inferior, 1-middle, and 1-inferior), for a total of 9 combinations. The percent change was calculated in comparison with the inline ROM. The treatment of one implant in the superior position was not compared between orientations since the configurations are identical for the inline and transarticular orientations. The median and range for the difference in ROM and percent change were determined.
Superior implant length: The effect of the superior implant length was assessed by calculating the difference between the mid-sacrum (MS) and mid-ala (MA) configurations. Differences in ROM were calculated as a function of orientation (Inline, Transarticular) and number of implants (3-superior/middle/inferior, 2-superior/inferior, 2-superior/middle, and 1-superior), for a total of 8 combinations. The percent change was calculated in comparison with the ROM of mid-ala superior implant length. The median and range for the difference in ROM and percent change were determined.
Implant number: The effect of implant number was assessed by calculating the difference in ROM between all single or dual implant configurations and the corresponding 3 implant configuration as a function of orientation (Inline, Transarticular) and superior implant length (SIL) (mid-ala, mid-sacrum) for a total of 18 combinations, and normalizing by the corresponding 3 implant configuration (implants without a superior implant were normalized to the mid-ala configuration). The median and range for the difference in ROM and percent change were determined for each single or dual implant configuration.
The article does not contain any studies with human participants or animals performed by any of the authors.
Placement of 3 implants using the inline and transarticular orientations resulted in reductions in motion of 64%, 32%, 63%, and 70%, 38%, 69%, in flexion-extension, lateral bending, and axial rotation, respectively (Table 1). These reductions are consistent with the range of reductions reported by Soriano-Baron et al[13] and provided confidence that this FE model is sufficient to make comparisons between treatment variables that have not previously been investigated in cadaver studies (i.e., implant number, placement technique, and superior implant length).
Transarticular placement of 3 implants with a mid-sacrum length superior implant resulted in the greatest reduction in range of motion (Table 1). One superior implant (mid-ala length) has the least reduction in range of motion in flexion-extension; one inferior implant placed using the inline orientation has the least reduction in range of motion in lateral bending and axial rotation. Transarticular placement of a superior (mid-sacrum length) and inferior implant has the most reduction in range of motion for a 2 implant configuration.
Altering the implant orientation from the inline to the transarticular placement technique resulted in median reductions in motion of 16%, 9% and 18%, in flexion-extension, lateral bending, and axial rotation, respectively (Table 2).
| Orientation | Implants (positions) | Superior implant ending point | Reduction in SI joint ROM (°) (%) | ||
| Flexion-extension | Lateral bending | Axial rotation | |||
| Transarticular vs inline | 3 (S, M, I) | Mid-ala | 0.11° (16%) | 0.04° (9%) | 0.07° (17%) |
| Mid-sacrum | 0.11° (17%) | 0.03° (7%) | 0.07° (18%) | ||
| 2 (S, -, I) | Mid-ala | 0.13° (16%) | 0.05° (11%) | 0.08° (18%) | |
| Mid-sacrum | 0.11° (16%) | 0.04° (9%) | 0.08° (20%) | ||
| 2 (S, M, -) | Mid-ala | 0.16° (16%) | 0.05° (10%) | 0.09° (18%) | |
| Mid-sacrum | 0.12° (16%) | 0.04° (9%) | 0.08° (19%) | ||
| 2 (-, M, I) | -1 | 0.15° (16%) | 0.06° (11%) | 0.09° (16%) | |
| 1 (-, M, -) | -1 | 0.21° (16%) | 0.05° (8%) | 0.03° (4%) | |
| 1 (-, -, I) | -1 | 0.20° (16%) | 0.07° (10%) | 0.06° (8%) | |
| Median (°) (Range) | 0.13° (0.11-0.21) | 0.05° (0.03-0.07) | 0.08° (0.03-0.09) | ||
| Median (%) (Range) | 16% (16-17) | 9% (7-11) | 18% (4-20) | ||
Extending the superior implant to the midline of the sacrum resulted in median reductions in motion of 14%, 8% and 9%, in flexion-extension, lateral bending, and axial rotation, respectively (Table 3).
| Reduction in SI joint ROM (°) (%) | |||||
| Superior implant ending point | Orientation | Implants (position) | Flexion-extension | Lateral bending | Axial rotation |
| Mid-sacrum vs mid-ala | Inline | 3 (S, M, I) | 0.07° (10%) | 0.04° (9%) | 0.03° (7%) |
| 2 (S, -, I) | 0.13° (16%) | 0.04° (9%) | 0.04° (9%) | ||
| 2 (S, M, -) | 0.21° (22%) | 0.05° (10%) | 0.06° (12%) | ||
| 1 (S, -, -) | 0.15° (11%) | 0.00° (0%) | 0.06° (9%) | ||
| Trans-articular | 3 (S, M, I) | 0.07° (12%) | 0.03° (7%) | 0.03° (9%) | |
| 2 (S, -, I) | 0.11° (16%) | 0.03° (7%) | 0.04° (11%) | ||
| 2 (S, M, -) | 0.17° (21%) | 0.04° (9%) | 0.05° (13%) | ||
| 1 (S, -, -) | 0.15° (11%) | 0.00° (0%) | 0.06° (9%) | ||
| Median (°) (Range) | 0.14° (0.07-0.21) | 0.035° (0.00-0.05) | 0.045° (0.03-0.06) | ||
| Median (%) (Range) | 14% (10-22) | 8% (0-10) | 9% (7-13) | ||
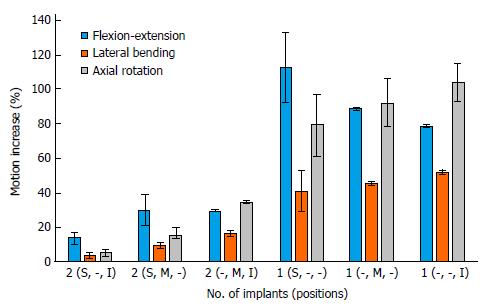
The two implant models with superior and inferior implants resulted in increased motions of 10%-17%, 2%-5% and 3%-7% compared with the 3 implant model, in flexion-extension, lateral bending, and axial rotation, respectively (Figure 2). Two implant models configurations with the implants placed close together (superior/middle, and middle/inferior) resulted in increased motions when compared with the 3 implant configuration of 21%-39%, 8%-18%, and 13%-35% in flexion-extension, lateral bending, and axial rotation, respectively (Figure 2; Tables 4-6). For single implant models, the motion increases ranged from 78% to 133%, 29% to 53% and 61% to 115%, in flexion-extension, lateral bending, and axial rotation, respectively (Figure 2).
| Treatment | Implants (positions) | Superior implant ending point | Range of motion (°) | Motion increase (°) | % 3 Implant motion |
| Intact | - | -1 | 1.94 | - | - |
| Inline | 3 (S, M, I) | Mid-ala | 0.7 | Reference configuration | |
| Inline | 2 (S, M, -) | Mid-ala | 0.97 | 0.27 | 39 |
| Inline | 2 (S, -, I) | Mid-ala | 0.82 | 0.12 | 17 |
| Inline | 2 (-, M, I) | -1 | 0.91 | 0.21 | 30 |
| Inline | 1 (S, -, -) | Mid-ala | 1.36 | 0.66 | 94 |
| Inline | 1 (-, M, -) | -1 | 1.32 | 0.62 | 89 |
| Inline | 1 (-, -, I) | -1 | 1.25 | 0.55 | 79 |
| Inline | 3 (S, M, I) | Mid-sacrum | 0.63 | Reference configuration | |
| Inline | 2 (S, M, -) | Mid-sacrum | 0.76 | 0.13 | 21 |
| Inline | 2 (S, -, I) | Mid-sacrum | 0.69 | 0.06 | 10 |
| Inline | 1 (S, -, -) | Mid-sacrum | 1.21 | 0.58 | 92 |
| Transarticular | 3 (S, M, I) | Mid-ala | 0.59 | Reference configuration | |
| Transarticular | 2 (S, M, -) | Mid-ala | 0.81 | 0.22 | 37 |
| Transarticular | 2 (S, -, I) | Mid-ala | 0.69 | 0.1 | 17 |
| Transarticular | 2 (-, M, I) | -1 | 0.76 | 0.17 | 29 |
| Transarticular | 1 (S, -, -) | Mid-ala | 1.36 | 0.77 | 131 |
| Transarticular | 1 (-, M, -) | -1 | 1.11 | 0.52 | 88 |
| Transarticular | 1 (-, -, I) | -1 | 1.05 | 0.46 | 78 |
| Transarticular | 3 (S, M, I) | Mid-sacrum | 0.52 | Reference configuration | |
| Transarticular | 2 (S, M, -) | Mid-sacrum | 0.64 | 0.12 | 23 |
| Transarticular | 2 (S, -, I) | Mid-sacrum | 0.58 | 0.06 | 12 |
| Transarticular | 1 (S, -, -) | Mid-sacrum | 1.21 | 0.69 | 133 |
| Treatment | Implants (positions) | Superior implant ending point | % 3 Implant motion | Implants (positions) | Median (%) [range] |
| Inline | 2 (S, -, I) | Mid-ala | 17 | 2 (S, -, I) | 14.5% (10-17) |
| Inline | 2 (S, -, I) | Mid-sacrum | 10 | ||
| Transarticular | 2 (S, -, I) | Mid-ala | 17 | ||
| Transarticular | 2 (S, -, I) | Mid-sacrum | 12 | ||
| Inline | 2 (S, M, -) | Mid-ala | 39 | 2 (S, M, -) | 30% (21-39) |
| Inline | 2 (S, M, -) | Mid-sacrum | 21 | ||
| Transarticular | 2 (S, M, -) | Mid-ala | 37 | ||
| Transarticular | 2 (S, M, -) | Mid-sacrum | 23 | ||
| Inline | 2 (-, M, I) | -1 | 30 | 2 (-, M, I) | 29.5% (29-30) |
| Transarticular | 2 (-, M, I) | -1 | 29 | ||
| Inline | 1 (S, -, -) | Mid-ala | 94 | 1 (S, -, -) | 112.5% (92-133) |
| Inline | 1 (S, -, -) | Mid-sacrum | 92 | ||
| Transarticular | 1 (S, -, -) | Mid-ala | 131 | ||
| Transarticular | 1 (S, -, -) | Mid-sacrum | 133 | ||
| Inline | 1 (-, M, -) | -1 | 89 | 1 (-, M, -) | 88.5% (88-89) |
| Transarticular | 1 (-, M, -) | -1 | 88 | ||
| Inline | 1 (-, -, I) | -1 | 79 | 1 (-, -, I) | 78.5% (78-79) |
| Transarticular | 1 (-, -, I) | -1 | 78 | ||
| Treatment | Implants (positions) | Superior implant ending point | Range of motion (°) | Motion increase (°) | % 3 implant motion |
| Intact | - | -1 | 0.66 | - | - |
| Inline | 3 (S, M, I) | Mid-ala | 0.45 | Reference configuration | |
| Inline | 2 (S, M, -) | Mid-ala | 0.5 | 0.05 | 11 |
| Inline | 2 (S, -, I) | Mid-ala | 0.47 | 0.02 | 4 |
| Inline | 2 (-, M, I) | -1 | 0.53 | 0.08 | 18 |
| Inline | 1 (S, -, -) | Mid-ala | 0.58 | 0.13 | 29 |
| Inline | 1 (-, M, -) | -1 | 0.65 | 0.2 | 44 |
| Inline | 1 (-, -, I) | -1 | 0.69 | 0.24 | 53 |
| Inline | 3 (S, M, I) | Mid-sacrum | 0.41 | Reference configuration | |
| Inline | 2 (S, M, -) | Mid-sacrum | 0.45 | 0.04 | 10 |
| Inline | 2 (S, -, I) | Mid-sacrum | 0.43 | 0.02 | 5 |
| Inline | 1 (S, -, -) | Mid-sacrum | 0.58 | 0.17 | 41 |
| Transarticular | 3 (S, M, I) | Mid-ala | 0.41 | Reference configuration | |
| Transarticular | 2 (S, M, -) | Mid-ala | 0.45 | 0.04 | 10 |
| Transarticular | 2 (S, -, I) | Mid-ala | 0.42 | 0.01 | 2 |
| Transarticular | 2 (-, M, I) | -1 | 0.47 | 0.06 | 15 |
| Transarticular | 1 (S, -, -) | Mid-ala | 0.58 | 0.17 | 41 |
| Transarticular | 1 (-, M, -) | -1 | 0.6 | 0.19 | 46 |
| Transarticular | 1 (-, -, I) | -1 | 0.62 | 0.21 | 51 |
| Transarticular | 3 (S, M, I) | Mid-sacrum | 0.38 | Reference configuration | |
| Transarticular | 2 (S, M, -) | Mid-sacrum | 0.41 | 0.03 | 8 |
| Transarticular | 2 (S, -, I) | Mid-sacrum | 0.39 | 0.01 | 3 |
| Transarticular | 1 (S, -, -) | Mid-sacrum | 0.58 | 0.2 | 53 |
| Treatment | Implants (positions) | Superior implant ending point | % 3 Implant motion | Implants (positions) | Median (%) [range] |
| Inline | 2 (S, -, I) | Mid-ala | 4 | 2 (S, -, I) | 3.5% (2-5) |
| Inline | 2 (S, -, I) | Mid-sacrum | 5 | ||
| Transarticular | 2 (S, -, I) | Mid-ala | 2 | ||
| Transarticular | 2 (S, -, I) | Mid-sacrum | 3 | ||
| Inline | 2 (S, M, -) | Mid-ala | 11 | 2 (S, M, -) | 10% (8-11) |
| Inline | 2 (S, M, -) | Mid-sacrum | 10 | ||
| Transarticular | 2 (S, M, -) | Mid-ala | 10 | ||
| Transarticular | 2 (S, M, -) | Mid-sacrum | 8 | ||
| Inline | 2 (-, M, I) | -1 | 18 | 2 (-, M, I) | 16.5% (15-18) |
| Transarticular | 2 (-, M, I) | -1 | 15 | ||
| Inline | 1 (S, -, -) | Mid-ala | 29 | 1 (S, -, -) | 41% (29-53) |
| Inline | 1 (S, -, -) | Mid-sacrum | 41 | ||
| Transarticular | 1 (S, -, -) | Mid-ala | 41 | ||
| Transarticular | 1 (S, -, -) | Mid-sacrum | 53 | ||
| Inline | 1 (-, M, -) | -1 | 44 | 1 (-, M, -) | 45% (44-46) |
| Transarticular | 1 (-, M, -) | -1 | 46 | ||
| Inline | 1 (-, -, I) | -1 | 53 | 1 (-, -, I) | 52% (51-53) |
| Transarticular | 1 (-, -, I) | -1 | 51 | ||
| Treatment | Implants (positions) | Superior implant ending point | Range of motion | Motion increase (°) | % 3 implant motion |
| Intact | - | 1 | 1.11 | - | - |
| Inline | 3 (S, M, I) | Mid-ala | 0.41 | Reference configuration | |
| Inline | 2 (S, M, -) | Mid-ala | 0.49 | 0.08 | 20 |
| Inline | 2 (S, -, I) | Mid-ala | 0.44 | 0.03 | 7 |
| Inline | 2 (-, M, I) | 1 | 0.55 | 0.14 | 34 |
| Inline | 1 (S, -, -) | Mid-ala | 0.67 | 0.26 | 63 |
| Inline | 1 (-, M, -) | 1 | 0.73 | 0.32 | 78 |
| Inline | 1 (-, -, I) | 1 | 0.79 | 0.38 | 93 |
| Inline | 3 (S, M, I) | Mid-sacrum | 0.38 | Reference configuration | |
| Inline | 2 (S, M, -) | Mid-sacrum | 0.43 | 0.05 | 13 |
| Inline | 2 (S, -, I) | Mid-sacrum | 0.4 | 0.02 | 5 |
| Inline | 1 (S, -, -) | Mid-sacrum | 0.61 | 0.23 | 61 |
| Transarticular | 3 (S, M, I) | Mid-ala | 0.34 | Reference configuration | |
| Transarticular | 2 (S, M, -) | Mid-ala | 0.4 | 0.06 | 18 |
| Transarticular | 2 (S, -, I) | Mid-ala | 0.36 | 0.02 | 6 |
| Transarticular | 2 (-, M, I) | 1 | 0.46 | 0.12 | 35 |
| Transarticular | 1 (S, -, -) | Mid-ala | 0.67 | 0.33 | 97 |
| Transarticular | 1 (-, M, -) | 1 | 0.7 | 0.36 | 106 |
| Transarticular | 1 (-, -, I) | 1 | 0.73 | 0.39 | 115 |
| Transarticular | 3 (S, M, I) | Mid-sacrum | 0.31 | Reference configuration | |
| Transarticular | 2 (S, M, -) | Mid-sacrum | 0.35 | 0.04 | 13 |
| Transarticular | 2 (S, -, I) | Mid-sacrum | 0.32 | 0.01 | 3 |
| Transarticular | 1 (S, -, -) | Mid-sacrum | 0.61 | 0.3 | 97 |
| Treatment | Implants (positions) | Superior implant ending point | % 3 Implant motion | Implants (positions) | Median (%) [range] |
| Inline | 2 (S, -, I) | Mid-ala | 7 | 2 (S, -, I) | 5.5% (3-7) |
| Inline | 2 (S, -, I) | Mid-sacrum | 5 | ||
| Transarticular | 2 (S, -, I) | Mid-ala | 6 | ||
| Transarticular | 2 (S, -, I) | Mid-sacrum | 3 | ||
| Inline | 2 (S, M, -) | Mid-ala | 20 | 2 (S, M, -) | 15.5% (13-20) |
| Inline | 2 (S, M, -) | Mid-sacrum | 13 | ||
| Transarticular | 2 (S, M, -) | Mid-ala | 18 | ||
| Transarticular | 2 (S, M, -) | Mid-sacrum | 13 | ||
| Inline | 2 (-, M, I) | 1 | 34 | 2 (-, M, I) | 34.5% (34-35) |
| Transarticular | 2 (-, M, I) | 1 | 35 | ||
| Inline | 1 (S, -, -) | Mid-ala | 63 | 1 (S, -, -) | 80% (61-97) |
| Inline | 1 (S, -, -) | Mid-sacrum | 61 | ||
| Transarticular | 1 (S, -, -) | Mid-ala | 97 | ||
| Transarticular | 1 (S, -, -) | Mid-sacrum | 97 | ||
| Inline | 1 (-, M, -) | 1 | 78 | 1 (-, M, -) | 92% (78-106) |
| Transarticular | 1 (-, M, -) | 1 | 106 | ||
| Inline | 1 (-, -, I) | 1 | 93 | 1 (-, -, I) | 104% (93-115) |
| Transarticular | 1 (-, -, I) | 1 | 115 | ||
The finite element model used in this study resulted in intact and treated SI joint motions that are consistent with previous experimental studies[13]. The combination of the current results and the previous validations confirm that both the intact and treated models in this study are functioning in a physiologic manner.
The current study demonstrated that the implant orientations across the SI joint can alter the range of motion. The SI joint contains both cartilaginous and fibrocartilaginous portions, with the cartilaginous portion exhibiting greater subchondral sacral bone density[19]. The transarticular orientation positions the middle and inferior implants more ventrally (approximately 15°-20°) and across the cartilaginous portion of the SI joint (Figure 1). Soriano-Baron et al[13] reported that the transarticular orientation had larger average reduction in SI joint ROM, although this was not determined to be significant.
The current study also demonstrated that placement of a longer superior implant resulted in reduced SI joint range of motion. Kraemer et al[11] demonstrated that iliosacral screws had a higher pullout force when the threads were positioned in the sacral midbody compared with those positioned in the ala. The results from Kraemer et al[11] are consistent with later anatomical studies that have reported reduced bone mineral density within the ala[24]. The current study demonstrated that increasing the length of the superior implant to the higher density bone of the sacral midline reduces the range of motion of the SI joint in flexion-extension, lateral bending, and axial rotation. Clinically, anatomic constraints must be considered prior to placement of a longer first implant.
The current study also demonstrated that placement of 3 implants resulted in greater motion reduction than any combination of two implants. Multiple studies have demonstrated that the use of a single SI screw results in less stability when compared with 2 SI screws[7-9]. The current study investigated treatment with 1, 2, or 3 implants to evaluate the treated SI joint range of motion as a function of implant number. Clinically, a prospective randomized trial documented 3 implants being placed in 91% of cases; with the rest of the cases using either 2 implants (5% cases) or 4 implants (4% cases)[23]. Although a small portion of clinical cases used 4 implants, this condition was not investigated in this study as placement is highly dependent on the size of the sacrum. The results from the current study demonstrate that reducing the number of placed implants results in increased initial SI joint range of motion. Two implants with increased separation, however, are more stable than 2 implants placed close together.
The current study is not without limitations. As with all finite element models, certain assumptions must be made to model the system. As previously noted, the current model is based on a single patient and did not simulate SI joint dysfunction, therefore generalizing the results to the general patient population should be made with care[17]. The current study assumed sacral cancellous bone material properties based on those found in normal cancellous bone (t-score > -1). Although the reported bone mineral densities are different in the normal, osteopenic, and osteoporotic sacra, the distribution of low and high density locations are consistent in all three cases[19,24]; as such, we expect that the findings in the different bone quality groups will be consistent. The current model and previous experimental study had consistent intact ROM and motion reductions after treatment, but there are some differences for the loading conditions simulated in this study (double-leg stance, follower load, and larger applied moment). Although the loading conditions were different, the consistency in intact ROM suggested that these disparities were counteracting each (e.g., follower load and double-leg stance increase stability; higher applied moment increase ROM)[17], and demonstrated that the SI joint and treatment were being effectively modeled. Lastly, the theoretical model used in this study did not model all in vivo characteristics (e.g., biological healing response after surgery); as such, additional clinical studies may be required to confirm these results.
While the minimum biomechanical requirements for clinically successful fixation of the SI joint are currently unknown, the current study investigated 3 clinical implant placement parameters and compared the resulting SI joint reduction in range of motion with a baseline model. The baseline model investigated here (inline orientation, mid-ala superior implant length, 3 implants) is a common technique that has positive clinical outcomes[23]. The range of motion of the SI joint in the current study was assessed in 3 anatomical loading directions, of which flexion-extension demonstrated both the largest intact range of motion (1.94°) and, after treatment, overall reductions in motion (0.58°-1.42°). Lateral bending and axial rotation resulted in small median reductions in motion (< 0.1°) when the variables were investigated, which may not be clinically significant by themselves. In contrast, flexion-extension was more sensitive to altering the variables with median reductions in motion > 0.1°. Although the 3 motions investigated had varying sensitivity, they consistently (i.e., positively/negatively) altered the reductions in motion. These results demonstrate that in flexion-extension, when compared with the baseline model, placement of the implants in areas of thicker cortical bone (transarticular orientation) and higher bone density (longer superior implant) leads to similar median increased reductions in motion of 16% and 14%, respectively. This study suggests that a surgeon can optimize implant placement in 3 ways: (1) Longer superior implants; (2) transarticular placement; and (3) using 3 implants (and/or increasing implant separation). Although the long-term clinical outcomes from these placement variations is unknown, the current study provides clinicians with insight and rationale into determining optimal implant placement.
Minimally invasive fusion of the sacroiliac (SI) joint is a potential treatment for patients suffering with symptoms related to the SI joint. The use of a lateral procedure for SI joint fusion has been shown to be an effective method for reducing SI joint pain. Previous anatomical studies have demonstrated significant variability in sacral anatomy and the resultant location and size of safe zones for implant placement.
A surgeon has options regarding the number of implants, length of implants, and their orientation; the optimal placement parameters for SI joint fixation are currently unknown. Quantification of the changes in SI joint motion as a result of varying the potential implant placement variables will provide a surgeon input when performing an SI joint fusion procedure.
The objective of this study was to investigate and quantify the effect of implant orientation, superior implant length, and implant number on SI joint range of motion.
This study used a previously validated finite element analysis to investigate how implant orientation, superior implant length, and implant number affect SI joint range of motion. Implant orientation was simulated using either an inline or a transarticular placement. The length of the superior implant was varied to end either in the middle of the ala or at the sacral midline. The number of implants was 1, 2, or 3 implants. The SI joint range of motion was calculated using a constant moment of 10 N-m with a follower load of 400 N in flexion-extension, lateral bending, and axial rotation. A total of 23 model configurations were tested and the difference in SI joint range of motion compared.
The use of a transarticular placement with a mid-sacrum length superior implant resulted in the greatest reduction in SI joint ROM. The use of transarticular placement resulted in median reductions in motion of 16%, 9%, and 18%, in flexion-extension, lateral bending, and axial rotation, respectively. Extending the superior implant to the sacral midline resulted in median reductions in motion of 14%, 8%, and 9%, in flexion-extension, lateral bending, and axial rotation, respectively. Reducing the number of implants (i.e., 1 or 2 implants) resulted in increased motions in all directions. Implant configurations with 2 implants placed farthest apart had the smallest increases.
This study demonstrates that the treated SI joint range of motion is affected by implant orientation, superior implant length, and implant number. These results show that the optimal placement investigated was 3 implants placed using a transarticular placement with a superior implant that reaches the sacral midline. This study suggests that a surgeon can optimize implant placement in 3 ways: (1) Longer superior implants; (2) transarticular placement; and (3) using 3 implants (and/or increasing implant separation).
The use of a finite element model to simulate the SI joint and treatment effects allows for investigation of many variables and provides valuable insight regarding how each variable effects SI joint stability. These results allow for more detailed investigation using either in vitro or in vivo studies.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Angoules A, Cui QJ, Emara KM, Guerado E S- Editor: Cui LJ L- Editor: A E- Editor: Li D
| 1. | Dreyfuss P, Dreyer SJ, Cole A, Mayo K. Sacroiliac joint pain. J Am Acad Orthop Surg. 2004;12:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Bernard TN Jr, Kirkaldy-Willis WH. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop Relat Res. 1987;266-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 92] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976). 1995;20:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 537] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 4. | Sembrano JN, Polly DW Jr. How often is low back pain not coming from the back? Spine (Phila Pa 1976). 2009;34:E27-E32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 250] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 5. | Heiney J, Capobianco R, Cher D. A systematic review of minimally invasive sacroiliac joint fusion utilizing a lateral transarticular technique. Int J Spine Surg. 2015;9:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Gardner MJ, Morshed S, Nork SE, Ricci WM, Chip Routt ML Jr. Quantification of the upper and second sacral segment safe zones in normal and dysmorphic sacra. J Orthop Trauma. 2010;24:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Yinger K, Scalise J, Olson SA, Bay BK, Finkemeier CG. Biomechanical comparison of posterior pelvic ring fixation. J Orthop Trauma. 2003;17:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | van Zwienen CM, van den Bosch EW, Snijders CJ, Kleinrensink GJ, van Vugt AB. Biomechanical comparison of sacroiliac screw techniques for unstable pelvic ring fractures. J Orthop Trauma. 2004;18:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | van Zwienen CM, van den Bosch EW, Hoek van Dijke GA, Snijders CJ, van Vugt AB. Cyclic loading of sacroiliac screws in Tile C pelvic fractures. J Trauma. 2005;58:1029-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Smith SA, Abitbol JJ, Carlson GD, Anderson DR, Taggart KW, Garfin SR. The effects of depth of penetration, screw orientation, and bone density on sacral screw fixation. Spine (Phila Pa 1976). 1993;18:1006-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 77] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Kraemer W, Hearn T, Tile M, Powell J. The effect of thread length and location on extraction strengths of iliosacral lag screws. Injury. 1994;25:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Lindsey DP, Perez-Orribo L, Rodriguez-Martinez N, Reyes PM, Newcomb A, Cable A, Hickam G, Yerby SA, Crawford NR. Evaluation of a minimally invasive procedure for sacroiliac joint fusion - an in vitro biomechanical analysis of initial and cycled properties. Med Devices (Auckl). 2014;7:131-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Soriano-Baron H, Lindsey DP, Rodriguez-Martinez N, Reyes PM, Newcomb A, Yerby SA, Crawford NR. The effect of implant placement on sacroiliac joint range of motion: posterior versus transarticular. Spine (Phila Pa 1976). 2015;40:E525-E530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Ivanov AA, Alexander A. Development, validation and clinical application of finite element human pelvis model. 2008;. |
| 15. | Ivanov AA, Kiapour A, Ebraheim NA, Goel V. Lumbar fusion leads to increases in angular motion and stress across sacroiliac joint: a finite element study. Spine (Phila Pa 1976). 2009;34:E162-E169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Kiapour A, Abdelgawad AA, Goel VK, Souccar A, Terai T, Ebraheim NA. Relationship between limb length discrepancy and load distribution across the sacroiliac joint--a finite element study. J Orthop Res. 2012;30:1577-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Lindsey D, Kiapour A, Yerby S, Goel V. Sacroiliac Joint Fusion Minimally Affects Adjacent Lumbar Segment Motion: A Finite Element Study. Int J Spine Surg. 2015;[cited 2015 Nov 17] Available from: http://ijssurgery.com/10.14444/2064. |
| 18. | Simonian PT, Routt ML Jr, Harrington RM, Mayo KA, Tencer AF. Biomechanical simulation of the anteroposterior compression injury of the pelvis. An understanding of instability and fixation. Clin Orthop Relat Res. 1994;245-256. [PubMed] |
| 19. | Richards AM, Coleman NW, Knight TA, Belkoff SM, Mears SC. Bone density and cortical thickness in normal, osteopenic, and osteoporotic sacra. J Osteoporos. 2010;2010:pii: 504078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Rice JC, Cowin SC, Bowman JA. On the dependence of the elasticity and strength of cancellous bone on apparent density. J Biomech. 1988;21:155-168. [PubMed] |
| 21. | Patwardhan AG, Havey RM, Meade KP, Lee B, Dunlap B. A follower load increases the load-carrying capacity of the lumbar spine in compression. Spine (Phila Pa 1976). 1999;24:1003-1009. [PubMed] |
| 22. | Panjabi M, Malcolmson G, Teng E, Tominaga Y, Henderson G, Serhan H. Hybrid testing of lumbar CHARITE discs versus fusions. Spine (Phila Pa 1976). 2007;32:959-966; discussion 967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Polly DW, Cher DJ, Wine KD, Whang PG, Frank CJ, Harvey CF, Lockstadt H, Glaser JA, Limoni RP, Sembrano JN; INSITE Study Group. Randomized Controlled Trial of Minimally Invasive Sacroiliac Joint Fusion Using Triangular Titanium Implants vs Nonsurgical Management for Sacroiliac Joint Dysfunction: 12-Month Outcomes. Neurosurgery. 2015;77:674-690; discussion 690-691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 24. | Peretz AM, Hipp JA, Heggeness MH. The internal bony architecture of the sacrum. Spine (Phila Pa 1976). 1998;23:971-974. [PubMed] |