Published online Sep 18, 2017. doi: 10.5312/wjo.v8.i9.681
Peer-review started: February 2, 2017
First decision: May 23, 2017
Revised: May 26, 2017
Accepted: July 14, 2017
Article in press: July 15, 2017
Published online: September 18, 2017
Processing time: 232 Days and 7.8 Hours
To develop methods of articular cartilage preparation for X-ray-electron probe microanalysis and to study its elements content in experimental osteoarthrosis.
Twenty dogs aged 2-8 years were divided in research (aged 2 years, induction of osteoarthrosis - IOA) and intact group. Intact group included three subgroups (aged 2, 5 and 8 years). Samples of cartilage after araldite saturation and pouring were partially cut into semithin sections stained with methylene blue and with methylene blue-basic fuchsin. Their smooth surfaces were investigated by X-ray-electron probe microanalysis. Spatial distribution of sulfur, calcium and phosphorus and their concentrations (weight %) were investigated.
X-ray electron probe microanalysis revealed non-uniform sulfur distribution in cartilage of intact animals: Its content increases from superficial zone to deep one, this regularity was preserved in animals with IOA. Differences of IOA with spontaneous chondropathy were revealed. Spontaneous aging was characterized by calcium and phosphorus storage in deep and calcified zones and compensatory increase of sulfated glycosaminoglycans in intermediate and deep cartilage zones as evidenced by the metachromatic reaction and microanalysis data. Unlike spontaneous chondropathy connected with aging in experimentally stimulated osteoarthrosis more intensive storage of calcium but minor phosphorus in intermediate zone were marked. In IOA the calcified cartilage thinning and osteoclastic resorption are apparent with few changes of elements composition; the only difference from control is minority phosphorus content.
The obtained results demonstrate specific tricks of X-ray electron probe microanalysis and its possibility in the research of mechanisms of articular cartilage alterations in osteoarthrosis.
Core tip: In this basic study we present the development of methods of articular cartilage preparation for X-ray-electron probe microanalysis and elements content in articular cartilage in animal experimentally induced (IOA) osteoarthrosis and during spontaneous animal aging (SA). SA was characterized by calcium and phosphorus storage in deep and calcified articular cartilage zones and compensatory increase of sulfated glycosaminoglycans in intermediate and deep zones. In IOA more intensive storage of calcium but few phosphorus in intermediate zone were marked. As for Sulphur content, all zones of uncalcified cartilage in two-year-old animals with IOA were comparable with cartilage of five-year-old intact animals.
- Citation: Stupina T, Shchudlo M, Stepanov M. Electron probe microanalysis оf experimentally stimulated osteoarthrosis in dogs. World J Orthop 2017; 8(9): 681-687
- URL: https://www.wjgnet.com/2218-5836/full/v8/i9/681.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i9.681
Osteoarthrosis (OA) has great social impact in terms of disability, pain, illness and treatment costs. Pathogenesis of OA involves the structures of the whole joint especially subchondral bone and synovium but is characterized predominantly by articular cartilage destruction[1]. Cartilaginous tissues possess relatively few amounts cells (chondrocytes) that account 1%-5% of volume[2] and large amounts of extracellular matrix that constitutes the bulk of tissue and protects chondrocytes from mechanical overloading.
Cartilage destruction in osteoarthrosis is multifactorial cascade process in which participate both cells and extracellular matrix, especially sulfated glycosaminoglycans. These last are included in proteoglycans and play the leading role in support of tissue homeostasis, architectonics and mechanical stability, cellular mitogenic activity, receptive functions and intercellular relations[3].
Mineralization of cartilage has been associated with OA progression and cartilage destruction[4] but some authors consider it as primarily an effect of aging[5].
A lot of biochemical and microscopical methods are used in modern researches of cartilage content and structure but X-ray-microprobe analysis was performed very rare though it permit to evaluate glycosaminoglicans and mineral content by corresponding chemical elements.
To develop the optimal methods of articular hyaline cartilage sample preparation for X-ray-electron probe microanalysis and to compare its elements content in intact animals and in animals with experimentally induced osteoarthrosis.
Twenty healthy adult mongrel dogs of either sex, aged 2-8 years, weighing 18-25 kg, were used in this study. All animals have received human care in compliance with the protocol approved by the Institutional Ethical Committee. The animals were acclimatized to laboratory conditions for one month prior experimentation. Dogs were housed in individual cages (floor area 4.5 m2) and were provided water three times daily and food two times daily with a measured volume. Animals were divided in research (aged 2 years) and intact group (control). Intact group included three subgroups (aged 2, 5 and 8 years). In research group modelling of primary gonarthrosis was performed by reduction of limb blood supply and knee immobilization[6]. So, dogs of research group were operated. Anesthesia was first induced with intramuscular injections of atropine, dimedrol and xylazine and then maintained with intravenous injection sodium pentobarbital (30 mg/kg i.v.). Briefly, each femoral artery was freed from surrounding tissues and after proximal and distal ligation was resected. Each knee joint were immobilized with external fixation apparatus. After 28 d of immobilization dogs of research group were euthanized. Dogs of intact group were also euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection.
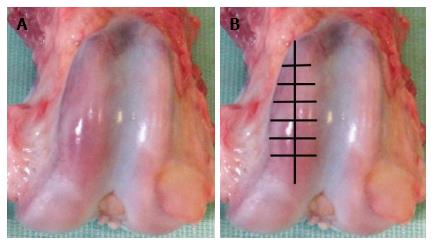
Samples of cartilage were harvested from lateral femoral condyles - presented in Figure 1A. Samples consisted of slices 2-3 mm thick, 5-6 mm long and 2-3 mm wide were cut with a scalpel tangentially within articular cartilage - presented in Figure 1B. Samples were fixed in glutaraldehyde-paraformaldehyde mixture excluding postfixation in tetraoxide osmium because X-ray peaks of heavy metals may mask and overlap on rays of analyzed elements[7,8]. Taking into consideration very high water content in cartilage[2] the specimens were dehydrated with smooth transition from ethanol to aceton according to known methods[9] but with increasing of exposition in 70%-96% ethanol to within the hour. Stages of araldite saturation and pouring were performed according to recently described method[10]. Increased density of cartilage matrix in araldite in comparison with native material decrease volume of X-ray excitation and provide the increase of microanalysis sensitivity.
Further investigation included two stages. At the first stage epoxy blocks were partially cut into semithin (1 mkm) sections with a glass knife using ultramicrotome “Nova” (LKB, Sweden). Slices were stained with methylene blue (metachromatic reaction for sulfated glycosaminoglycans) and with methylene blue-basic fuchsin (for detection of matrix basophily). Histology slides were examined using the “Opton-3” photomicroscope (Germany).
At the second stage smooth surfaces of epoxy blocks resulting from semithin slices cutting were investigated by X-ray-electron probe microanalysis. This technique prevents artifacts and saves the sample preparation time excluding the stage of grinding and polishing.
Three blocks was selected randomly from each animal. They were attached to polished clean aluminium discs with current-conducting adhesive. Surfaces of epoxy blocks were exposed to silver deposition using Eico IB-6 ion coater and JEOL JEE-4X vacuum evaporator. The investigation of element composition was performed using scanning electron microscope JSM-840 (JEOL, Japan) equipped with energy dispersive X-ray analyser (INCA 200, Oxford Instruments).
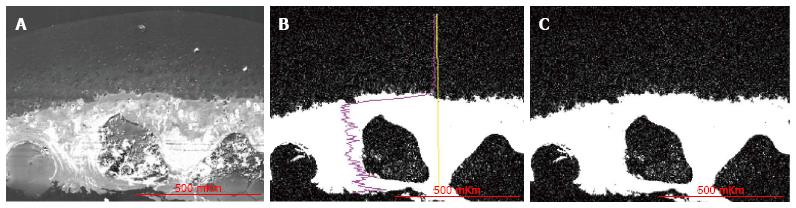
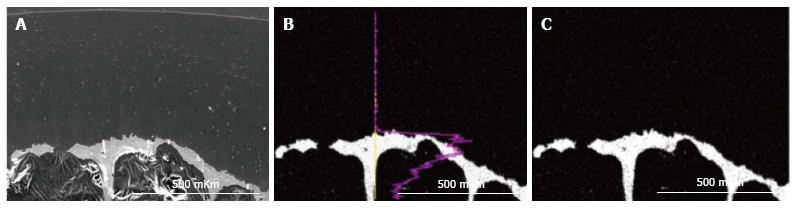
Results were obtained as smart maps, showing spatial distribution of elements and quantitative data in weight per cents. Spatial distribution of sulfur, calcium and phosphorus and their concentrations (ωS, ωСа, ωP - weight %) were investigated. For standard results equipment was calibrated by comparison templet made of wollastonite (СаО: SiO2). Two regimens were used for collecting X-ray spectra: Scanning line and scanning area - presented in Figure 2. In the second regimen strict longitudinal alignment of parallel scanning rows without overlapping fields provided the most representative total sample.
For quantitative data analysis the unpaired Student t test and Mann-Whitney U test were used (software package Attestat Program, version 9.3.1, developed by I.P. Gaidyshev, Certificate of Rospatent official registration No. 2002611109). If the P-value was less than 0.05, the data was considered statistically significant.
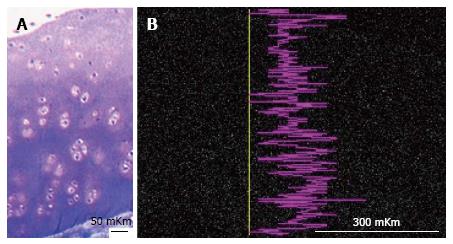
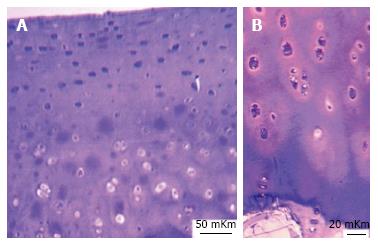
In 2 years-aged intact group metachromatic reaction in interterritorial matrix of superficial zone was moderate but in territorial matrix of intermediate and deep zones it was highly intensive - presented in Figure 3A. X-ray electron probe microanalysis revealed nonuniformity of Sulphur zonal distribution - presented in Figure 3B.
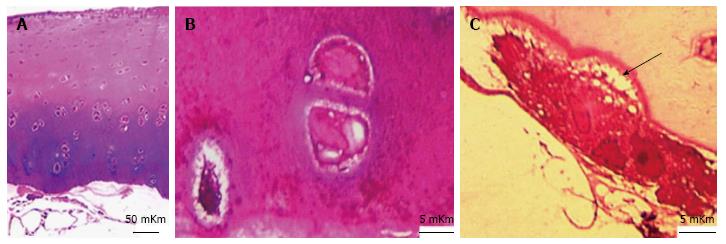
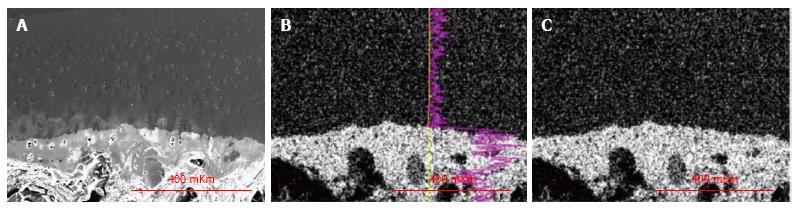
In IOA (induced osteoarthrosis) group metachromatic reaction was localized, its intensity was lowered and accompanied with fibrillation of matrix in superficial zone. Intensively basophilic matrix was revealed in intermediate and deep zones and presented in Figure 4A and B. The calcified cartilage zone was thinned or absolutely absent in some areas (Figures 4A and 5). At the borderline between calcified cartilage and subchondral bone the osteoclastic resorption was marked - presented in Figure 4C.
In 5 years-aged intact group histochemical reaction for sulfated glycosaminoglycans was weak, metachromasy was focal (Figure 6A), matrix of superficial zone was basophilic, many of cells had signs of destruction.
In 8 years-aged intact group metachromatic reaction was also focal, locuses of intensive metachromasy were revealed in intermediate and deep zones (Figure 6B), cartilaginous cells had increased sizes, light homogeneous nuclei and basophilic cytoplasm. Territorial matrix of deep zone was basophilic.
In IOA group ωS were decreased in all of cartilage zones besides calcified, but its distribution with maximal meanings in intermediate and deep zones was the same as in two-year-old intact group. In five-year-old intact group in comparison with two-year-old intact group ωS was decreased in superficial, intermediate and deep zones, but increased in calcified cartilage. In eight-year-old intact group in comparison with two-year-old intact group ωS was increased in intermediate, deep and calcified cartilage zones, but not in superficial zone - presented in Table 1.
| Cartilage zones/groups | Superficial zone | Intermediate zone | Deep zone | Zone of calcified cartilage |
| ωS (mean ± SD, weight%) | ||||
| IOA in 2 yr | 0.18 ± 0.04a | 0.23 ± 0.02a | 0.28 ± 0.03a | 0.13 ± 0.02 |
| Intact | ||||
| 2 yr (control) | 0.35 ± 0.01 | 0.42 ± 0.04 | 0.52 ± 0.02 | 0.14 ± 0.02 |
| 5 yr | 0.20 ± 0.02a | 0.28 ± 0.04a | 0.34 ± 0.02a | 0.28 ± 0.04a |
| 8 yr | 0.33 ± 0.03 | 0.52 ± 0.01a | 0.63 ± 0.04a | 0.41 ± 0.07a |
| ωCa (mean ± SD, weight%) | ||||
| IOA in 2 yr | 0.04 ± 0.02 | 0.08 ± 0.02a | 0.15 ± 0.02a | 9.72 ± 0.03 |
| Intact | ||||
| 2 yr (control) | < 0.01 | 0.04 ± 0.01 | 0.09 ± 0.01 | 13.93 ± 0.02 |
| 5 yr | 0.04 ± 0.01 | 0.05 ± 0.03 | 0.10 ± 0.02 | 16.16 ± 1.04a |
| 8 yr | 0.03 ± 0.02 | 0.05 ± 0.03 | 0.22 ± 0.02a | 21.16 ± 3.04a |
| ωP (mean ± SD, weight%) | ||||
| IOA in 2 yr | < 0.02 | 0.03 ± 0.01 | 0.08 ± 0.01 | 4.74 ± 0.02a |
| Intact | ||||
| 2 yr (control) | < 0.01 | < 0.01 | < 0.01 | 6.89 ± 0.01 |
| 5 yr | < 0.02 | 0.07 ± 0.01a | 0.09 ± 0.02a | 7.44 ± 1.47a |
| 8 yr | 0.04 ± 0.02a | 0.09 ± 0.02a | 0.11 ± 0.02a | 9.44 ± 1.29a |
In IOA group in comparison with two-year-old intact group ωCa was increased in all zones of cartilage besides the calcified. In five-year-old intact group ωCa was increased only in superficial zone and in calcified cartilage. In eight-year-old intact group more expressed increase in deep and calcified zones were marked (Table 1).
Changes of ωP were differently directed. In IOA it was increased in intermediate and deep zones but decreased in calcified cartilage. In 5 years-aged and especially in 8 years-aged intact groups ωP was increased in all zones including calcified cartilage - presented in Figure 7 and Table 1.
So, X-ray electron probe microanalysis revealed non-uniform serum distribution in cartilage of intact animals: its content increases from superficial to deep zone, this regularity was preserved in animals with induced experimental osteoarthrosis. The obtained data are in agreement with literature: The aggrecan content in superficial zone is lower than in others[11]. It is known that metabolism of sulfated glycosaminoglycans changes in the early stages of articular cartilage damage[12].
Recently we obtained histological characteristics of articular cartilage in experimentally induced osteoarthrosis[13,14] corresponding to grade 1-3 according to OARSI classification[15]. It was revealed[14] that in this experimental model chondrocytes of intermediate zone were the most vulnerable: More than 50% cells had signs of necrosis or apoptosis. It is known that apoptotic bodies contain alkaline phosphatase and precipitate calcium promoting cartilage calcification[16].
According to other authors hypertrophic chondrocytes of osteoarthrotic cartilage produce large amounts of collagen X, matrix proteinase 12 and alkaline phosphatase influencing calcification[17]. Ohira and Ishikawa[18] (1986) found precipitates of hydroxiapatite crystals around degenerated chondrocytes.
In current research substantial difference of experimentally induced osteoarthrosis from spontaneous chondropathy were revealed. Spontaneous aging characterizes by calcium and phosphorus storage in deep and calcified zones and compensatory increase of sulfated glycosaminoglycans in intermediate and deep cartilage zones as evidenced by the metachromatic reaction and microanalysis data. Unlike spontaneous chondropathy connected with aging in experimentally reduced osteoarthrosis more intensive storage of calcium but minor phosphorus in intermediate zone were marked. The revealed contradistinction is in agreement with research of human aging, which leads to suggest that phosphorus exuded from bones storages in arteries and cartilage tissue[19].
In experimentally induced osteoarthrosis the calcified cartilage thinning and osteoclastic resorption are apparent with few changes of elements composition; the only difference from control is minority phosphorus content.
Preparation of biologic samples for X-ray electron probe microanalysis perform according the same principles as preparation for electron microscopy but possess its own specific tricks; peculiar properties of research object also must be taken into consideration. The obtained results demonstrate definitely the possibility of X-ray electron probe microanalysis in the research of mechanisms of articular cartilage alteration in osteoarthrosis, these changes is documented and assessed quantitatively.
The authors would like to thank the members of the engineering group for their technical support and I.P. Gaidyshev for his help in statistical analysis.
Multiple biochemical and microscopic methods are used in modern researches of cartilage content and structure in normal and diseased human and animal beings but X-ray-microprobe analysis was performed very rare though it permit to evaluate glycosaminoglicans and mineral content by corresponding chemical elements. The optimal methods of articular hyaline cartilage sample preparation for X-ray-electron probe microanalysis and comparison of its elements content in intact animals of different ages and in animals with experimentally induced osteoarthrosis were not investigated.
Previous research have already proved that in this experimental model of primary osteoarthrosis chondrocytes of intermediate zone were the most vulnerable and that apoptotic bodies promote cartilage calcification.
This is the first study evaluating substantial difference of experimental gonarthrosis induced by reduction of limb blood supply and knee immobilization from spontaneous age-related chondropathy. The optimized methods of articular hyaline cartilage sample preparation for X-ray-electron probe microanalysis are described.
In experimentally induced osteoarthrosis the uncalcified cartilage was characterized with minority Sulphur content, calcium and phosphorus storage in comparison with intact animals of corresponding age. The calcified cartilage thinning and osteoclastic resorption in induction of osteoarthrosis are apparent with few changes of elements composition; the only difference from control is minority phosphorus content.
Experimentally stimulated osteoarthrosis in used biological model histologically corresponds to grade 1-3 according to OARSI classification.
Stupina et al developed methods of articular cartilage preparation for X-ray-electron probe microanalysis and to study its elements content in experimental osteoarthrosis. It is well designed and written manuscript. It is a potential important study to the fields of osteoarthrosis research, diagnosis and therapy.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Russia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cui Q, Zhou S S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Hunter DJ, Felson DT. Osteoarthritis. BMJ. 2006;332:639-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 422] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 2. | Jerosch J. Effects of Glucosamine and Chondroitin Sulfate on Cartilage Metabolism in OA: Outlook on Other Nutrient Partners Especially Omega-3 Fatty Acids. Int J Rheumatol. 2011;2011:969012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Seidman AM, Korel AV. Structural and functional features of the plate human vertebral body growth during critical periods of growth. Surg Vertebral. 2004;2:113-120. |
| 4. | Fuerst M, Bertrand J, Lammers L, Dreier R, Echtermeyer F, Nitschke Y, Rutsch F, Schäfer FK, Niggemeyer O, Steinhagen J. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum. 2009;60:2694-2703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 235] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Mitsuyama H, Healey RM, Terkeltaub RA, Coutts RD, Amiel D. Calcification of human articular knee cartilage is primarily an effect of aging rather than osteoarthritis. Osteoarthritis Cartilage. 2007;15:559-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Makushin VD, Stepanov MA, Stupina TA, Inventors . Method of Simulating Osteoarthrosis of Knee. Russia patent RF 2452999. 2012. Iun 10. |
| 7. | Goldstein J, Newbury D, Echlin P, Dzhoy D, Fiori C, Lifshin E. Raster electronic microscopy and x-rayed Microprobe Analysis. М: World. 1984;1: 348. |
| 8. | Shahlamov VA, Buravkov SV. Application of X-ray local microanalysis in biology and medicine. Arkh Anat Histol Embriol. 1983;4:95-107. |
| 10. | Stupina TA, Shchudlo MM. A method for making preparations from nondecalcified articular cartilage with sublying subchondral bone for multipurpose studies. Bull Exp Biol Med. 2014;157:388-390. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Anderson HC, Hodges PT, Aguilera XM, Missana L, Moylan PE. Bone morphogenetic protein (BMP) localization in developing human and rat growth plate, metaphysis, epiphysis, and articular cartilage. J Histochem Cytochem. 2000;48:1493-1502. [PubMed] |
| 12. | Pavlova VN, Pavlov GG, Shostak NA, Slutsky LI. Joint: The morphology, clinic, diagnostics, treatment. M: OOO “Publisher” Medical News Agency” 2011; 552. |
| 13. | Stupina TA, Shchudlo NA, Stepanov MA. Structural reorganization of the main joint components during the experimental modeling of osteoarthrosis with reduced blood supply. Morfologiia. 2014;146:61-65. |
| 14. | Shevtsov VI, Makushin VD, Stepanov MA, Stupina TA. The problem of the knee osteoarthrosis modeling in dogs for the purpose of pathogenesis studying (experimental morphological study). Genius Orthop. 2012;1:38-42. |
| 15. | Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1445] [Cited by in RCA: 1731] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 16. | Adams CS, Shapiro IM. The fate of the terminally differentiated chondrocyte: evidence for microenvironmental regulation of chondrocyte apoptosis. Crit Rev Oral Biol Med. 2002;13:465-473. [PubMed] |
| 17. | Tchetina EV. The mechanisms of embryogenesis in osteoarthritis: the role of differentiation of chondrocytes in articular cartilage resorption. Sci Practical Rheumatol. 2010;3:65-77. |
| 18. | Ohira T, Ishikawa K. Hydroxyapatite deposition in articular cartilage by intra-articular injections of methylprednisolone. A histological, ultrastructural, and x-ray-microprobe analysis in rabbits. J Bone Joint Surg Am. 1986;68:509-520. [PubMed] |
| 19. | Tohno Y, Tohno S, Minami T, Moriwake Y, Utsumi M, Yamada M. A possible balance of phosphorus accumulations among bone, cartilage, artery, and vein in single human individuals. Biol Trace Elem Res. 1999;69:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |