Published online Aug 18, 2017. doi: 10.5312/wjo.v8.i8.612
Peer-review started: December 31, 2016
First decision: February 20, 2017
Revised: March 6, 2017
Accepted: May 3, 2017
Article in press: May 5, 2017
Published online: August 18, 2017
Processing time: 225 Days and 21.1 Hours
Rotator cuff repair (RCR) is one of the most commonly performed surgical procedures in orthopaedic surgery. The reported incidence of deep soft-tissue infections after RCR ranges between 0.3% and 1.9%. Deep shoulder infection after RCR appears uncommon, but the actual incidence may be higher as many cases may go unreported. Clinical presentation may include increasing shoulder pain and stiffness, high temperature, local erythema, swelling, warmth, and fibrinous exudate. Generalized fatigue and signs of sepsis may be present in severe cases. Varying clinical presentation coupled with a low index of suspicion may result in delayed diagnosis. Laboratory findings include high erythrocyte sedimentation rate and C-reactive protein level, and, rarely, abnormal peripheral blood leucocyte count. Aspiration of glenohumeral joint synovial fluid with analysis of cell count, gram staining and culture should be performed in all patients suspected with deep shoulder infection after RCR. The most commonly isolated pathogens are Propionibacterium acnes, Staphylococcus epidermidis, and Staphylococcus aureus. Management of a deep soft-tissue infection of the shoulder after RCR involves surgical debridement with lavage and long-term intravenous antibiotic treatment based on the pathogen identified. Although deep shoulder infection after RCR is usually successfully treated, complications of this condition can be devastating. Prolonged course of intravenous antibiotic treatment, extensive soft-tissue destruction and adhesions may result in substantially diminished functional outcomes.
Core tip: Rotator cuff repair (RCR) has become one of the most frequently performed orthopaedic procedures during the last two decades. Paralleling the exponential increase in the number of RCRs, uncommon complications such as postoperative deep shoulder infections may be seen more frequently. Patients who are suspected to have a post-RCR infection require a thorough diagnostic evaluation, including clinical signs and symptoms, laboratory workups and cultures. Although appropriate management of this condition with surgical debridement and lavage, and long-term IV antibiotics usually results in eradication of the infection, complications can be disabling and functional outcomes poor. The majority of the patients with deep infections after RCR report unsatisfactory outcomes with permanent functional limitations.
- Citation: Atesok K, MacDonald P, Leiter J, McRae S, Stranges G, Old J. Postoperative deep shoulder infections following rotator cuff repair. World J Orthop 2017; 8(8): 612-618
- URL: https://www.wjgnet.com/2218-5836/full/v8/i8/612.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i8.612
Rotator cuff pathology is one of the most commonly encountered orthopaedic problems, with an estimated prevalence of 17% to 35% including asymptomatic patients[1]. In symptomatic patients, rotator cuff repair (RCR) usually provides good to excellent clinical outcomes[1-4]. As a result, RCR has become one of the most frequently performed orthopaedic procedures during the last two decades[5,6]. Paralleling the exponential increase in the number of RCRs, associated complications such as postoperative deep shoulder infections may be seen more frequently[7]. Published literature indicates that the incidence of deep shoulder infection after open or mini-open RCR ranges between approximately 0.3% and 1.9%, and the condition is more common in male patients[7-9]. While the rate of infection is generally thought to be lower after arthroscopic RCR, current high level evidence supporting this assertion is limited. In a recently published retrospective study including 3294 all-arthroscopic RCRs, Pauzenberger et al[10] reported an infection rate of 0.85%. In another retrospective case series, Vopat et al[11] studied the effects of surgical technique on infection rate. Out of 1824 RCRs performed by a single surgeon, 14 had an early deep postoperative shoulder infection that required surgical irrigation and debridement. Of these 14 patients who developed deep infections, primary RCR surgery was performed arthroscopically in only three of them, while 11 patients received open or mini-open repairs. The authors stated that “The most important finding in this study was that patients with non-arthroscopic RCR (open/mini open surgeries) had a greater risk [odds ratio (OR) = 8.63, P < 0.001] of infection compared with patients who had an all-arthroscopic RCR”. It must be noted that all the available data in the literature consists of retrospective case series and that the patient records were reviewed based on follow-up notes or a re-operation registry for debridement[7-11]. Hence, the actual incidence of infection may be higher, as many cases may go unreported due to patients choosing to seek treatment at different institutions than where the primary repair had been performed.
Risk factors for the development of suppurative infections of the shoulder joint can be summarized under three main categories: Anatomic, patient-related, and surgical technique or operating room (OR) environment-related risk factors.
The axillary area has been shown to provide an enriched colonization environment for various bacteria due to the presence of numerous sebaceous glands and hair follicles[12-14]. Surgical incisions and entry portals for open, mini-open, and arthroscopic RCR are near the axilla, which may increase the possibility of inadvertent transmission of colonized microorganisms into the joint during surgery. Furthermore, precautions, such as clipping the axillary hair or preparing axillary skin with various solutions have not been proven to be successful in reducing infection rates or bacterial load[15,16].
There are various patient characteristics that can adversely affect the body’s defense against infections, including diabetes mellitus, immunosuppression, chronic diseases, advanced age, smoking, intravenous drug use, malnutrition, obesity, kidney and/or liver failure, and malignancies[13,17,18]. In a retrospective comparative study from Chen et al[18], three out of 30 (10%) type I diabetic patients developed infections following RCR. However, no patients in the non-diabetic group had infections after RCR. Pauzenberger et al[10] found that age over 60 was an independent risk factor for post-RCR infections. In addition to systemic conditions, local factors, such as previous shoulder surgery and local corticosteroid injections, may also increase the risk of deep shoulder infection after RCR[19,20].
Pauzenberger et al[10] indicated that males are more prone to infection after RCR. The authors showed that out of 28 patients with deep infections after arthroscopic RCR, 27 (96.4%) were male and only 1 (3.6%) was female (OR = 21.41, P = 0.003). Likewise, Vopat et al[11] reported that 92% of the patients in the infected group after RCR were male, compared to 58% of the control group patients who did not develop infections after RCR (OR = 9.52, P = 0.042). Although more male patients undergo RCR than female patients, this difference does not appear to be large enough to explain the significant difference in infection rates between men and women[5]. Interestingly, there is evidence in the literature showing that Propionibacterium acnes’ superficial skin colonization rate around arthroscopy portal sites was 81.6% in male patients and 46.1% in female patients[21]. This colonization rate difference between men and women may be attributed to the significantly higher serum testosterone levels in the male population vs the female population[21,22].
The risk of postoperative deep shoulder infection appears to be higher in open or mini-open RCR techniques compared with arthroscopic techniques[1,11]. It is conceivable that the likelihood of bacterial contamination increases as the operation time and the size of the surgical incision increases. Another point worth considering is that open or mini-open RCR techniques have been performed much longer than arthroscopic repair techniques, which have only been performed for the last few decades. This fact, along with the improvement in disinfection and OR safety protocols, may have also influenced the decrease in infection rates for arthroscopic techniques[23,24]. Nonetheless, there can be differences in the incidence of infection between various arthroscopic procedures. Yeranosian et al[17] reported that out of 165820 arthroscopic shoulder surgeries, 450 required additional surgery due to infections. The authors have noted that the incidence of infection was highest after RCR (0.29%) when compared with other arthroscopic procedures (P < 0.01). This finding underlines the significance of RCR as a procedure that increases the risk of infection, even when performed arthroscopically.
At the early stages, patients with deep infections after RCR usually present with increasing shoulder pain, stiffness, and, in some cases, loss of previously achieved postoperative range of motion (ROM)[1,11,25-27]. There may be noticeable fibrinous exudate or pus drainage from the surgical wound and/or arthroscopy portals, with local swelling, erythema, and warmth around the shoulder joint[7,8]. However, systemic signs and symptoms, such as fever, chills, low blood pressure, and generalized fatigue, are not common but they may be seen when there is a delay in presentation or when patients have immunosuppressive diseases.
Diagnostic lab workups to rule out or confirm deep shoulder infection after RCR include standard peripheral white blood cell (WBC) count with differential, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels. Peripheral WBC count is usually within normal limits; however, ESR and CRP levels can be elevated. ESR and CRP levels are sensitive but not specific markers for deep shoulder infection. Athwal et al[8] studied 39 cases of deep shoulder infections after RCR and detected an elevated WBC count in only five patients (approximately 13%); ESR was available for 30 cases and was elevated in 18 cases (60%). The authors checked CRP levels in 10 cases, and was elevated in five of them (50%). Kwon et al[7] reported similar results in a study including 14 patients with deep shoulder infections after RCR. In this study, the WBC count was measured in 10 patients, and nine of them had normal levels, averaging 7.6 × 103/μL (range, 4.9-10.8 × 103/μL). The ESR was measured in eight patients and found to be elevated at least two-fold in seven patients (mean ESR value, 69 mm/h ± 32 mm/h). CRP was measured for two patients, and both were found to be increased, measuring 1.1 mg/dL and 7.7 mg/dL (normal, < 0.8 mg/dL).
Glenohumeral joint aspiration and analysis with a cell count should be routinely performed in all patients presenting with a suspected deep shoulder infection. Aspirated fluid should also be examined microscopically for crystals, and a gram stain must be performed. As in most cases of septic arthritis, analysis of infected shoulder joint aspiration usually reveals a WBC count above 50000/μL, with more than 75% polymorphonuclear leukocytes. These values indicate a deep infection of the shoulder, even if the gram stain returns negative and crystals are detected in the joint fluid. Aspiration fluid should be cultured in all cases, regardless of the results of cell count and the microscopic fluid analysis. Even though joint aspiration and synovial fluid analysis should be part of standard diagnostic work up for every case with a suspected joint infection, such data from patients with a reported deep shoulder infection after RCR is lacking in the literature.
The most commonly isolated pathogens are, not surprisingly, the main species in sebaceous areas of normal skin flora, including Propionibacterium acnes, coagulase-negative Staphylococci (e.g., Staphylococcus epidermidis), and Staphylococcus aureus[7-9,13]. However, infections due to various other microorganisms, such as Corynebacterium species, Proteus mirabilis, Enterococcus faecalis, Peptostreptococcus magnus, Bacillus species, Streptococcus viridans, Actinomyces species, and poly-microbial culture results, were also reported (Table 1)[7-10,27].
| Ref. | Patient No. | No. of isolated organisms | P. acnes | S. aureus | Coagulase-negative Staph1 | Other microorganisms |
| Kwon et al[7] | 14 (11 mono, 3 poly-microbial) | 19 | 7 | 4 | 6 | 2 (Proteus mirabilis and Enterococcus faecalis) |
| Athwal et al[8] | 38 (39 shoulders: 33 mono, 6 poly-microbial) | 45 | 20 | 8 | 12 | 5 (Coryebacterium species × 2, Peptostreptococcus magnus, Bacillus species, Streptococcus viridans) |
| Settecerri et al[9] | 16 (15 mono, 1 poly-microbial) | 15 | 6 | 4 | 4 | 1 (Peptostreptococcus) |
| Pauzenberger et al[10] | 28 (mono-microbial isolation in 23 patients) | 23 | 8 | 2 | 12 | 1 (Actinomyces species) |
| Mirzayan et al[27] | 13 (7 mono, 3 poly-microbial, 3 no growth) | 15 | 3 | 5 | 5 | 2 (Diptheroids and Streptococcal species) |
Shoulder surgeons have recently focused on Propionibacterium acnes as a causative agent in many cases of deep shoulder infections after arthroscopic and open shoulder procedures. P. acnes is an anaerobic gram-positive bacillus densely colonized in the dermal skin layers around the head and shoulders. Despite routine preoperative antibiotic prophylaxis and skin preparation in shoulder arthroscopy, the rate of surgical site deep tissue inoculation with P. acnes can be as high as 19.6%[21]. Furthermore, these patients were also found to have positive P. acnes superficial skin colonization that may indicate contamination by means of surgical instruments[21]. Interestingly, Pauzenberger et al[10] reported that although administration of prophylactic antibiotics reduced the rate of infection from 1.54% to 0.28% (P < 0.001), there was no significant reduction in the rate of infections due to P. acnes. Further research is needed to study the correlation between superficial skin colonization and deep surgical tissue inoculation with P. acnes and postoperative deep shoulder infections following RCR.
Radiographic evaluation in patients with deep shoulder infections after RCR is rarely necessary and usually reveals normal findings, particularly in acute cases. In subacute or delayed cases, ultrasonography and magnetic resonance imaging (MRI) with an intravenous contrast agent may be valuable to detect abscess formation around the shoulder joint or to identify complications, such as osteomyelitis[13].
In rare cases, when the joint aspiration and culture results remain negative but the patient has clinical symptoms of infection, an indium 111-labeled WBC scan can be considered. Although this imaging modality might be helpful in localizing inflammation, it does not clearly distinguish between infectious and noninfectious inflammatory processes. Furthermore, reported sensitivity of indium 111-labeled WBC scans for the diagnosis of infectious conditions ranges from 60% to 100%, and specificity ranges from 69% to 92%[28].
Deep soft-tissue infections of the shoulder after RCR require a thorough and meticulous management that involves surgical debridement with copious lavage and long-term intravenous (IV) antibiotic treatment. Although this approach is universally accepted, the literature mostly provides evidence regarding open debridement due to the fact that the great majority of published studies are retrospective case series including patients who had either open RCRs or arthroscopically-assisted mini-open RCRs[7-9,11,27]. However, studies indicate that arthroscopic RCRs have increased by 600%, while open repairs have increased by only 34% during the time interval between 1996 and 2006[5]. It is highly possible that the percentage of arthroscopic RCRs have increased even further since 2006 compared with open repairs, as arthroscopic RCR has become the procedure of choice for the surgical treatment of rotator cuff pathologies. Hence, arthroscopic debridement and lavage needs to be emphasized as the primary surgical procedure to address acute deep shoulder infections after RCR.
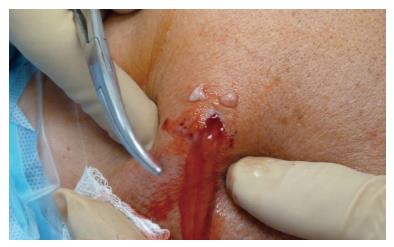
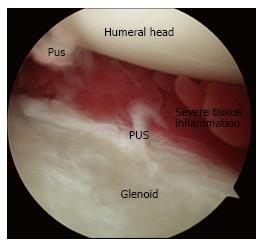
Any antibiotic treatment prior to surgery should be discontinued at least five to seven days before surgery. The importance of withholding lavage and IV antibiotics until obtaining cultures from the pus and debrided deep tissues cannot be overemphasized. In general, after the operative site is prepared and draped, incisions along the infected portals are made, and the pus from deeper tissues is drained through these portals. Cultures are then taken from the drained pus, and the swabs are used to obtain deep tissue cultures through the portals (Figure 1). It is advisable to start with a dry arthroscopy of the glenohumeral joint to achieve a better visualization of the infection and to obtain more tissues for culturing before the joint is washed (Figure 2). The glenohumeral joint and the previously repaired rotator cuff is assessed intraoperatively following the initial lavage. Ideally, all the suture material and anchors are removed, and the joint is washed profusely using a minimum of 10 L of fluid, with the last liter containing 100000 units of bacitracin[27]. Although there could be variations in approach among individual surgeons, a re-repair of the rotator cuff during the same arthroscopic debridement and lavage procedure is not suggested before completing a long-term IV antibiotic therapy and confirming that the infection has been eradicated. In selected patients, based on intraoperative assessment, the sutures and anchors can be retained if the infection is not extensive and there is no loosening of the repaired cuff tissue.
Deep infection usually involves the surgical wound and forms a tract that connects the deep tissues to the superficial layers of the surgical incision. Hence, open surgical debridement and lavage is the mainstay of treatment in patients who had open RCR as the initial procedure. Athwal et al[8] treated 39 patients with post-RCR deep shoulder infections by means of an open irrigation and debridement (30 patients), and a combined arthroscopic and open debridement (nine patients). They reported that a mean of 3.3 surgical debridements were necessary for eradicating the infection. Between surgical debridements, the wound was left open and packed with sterile gauze in 18 shoulders and closed over a drain in 21. They used antibiotic-laden cement beads in five patients. In a series including 16 patients with deep infections after an open RCR, Settecerri et al[9] did an average of 3.5 open debridements (range two to eight debridements), and the wound was left open and packed with sterile gauze between the procedures. Other studies report a similar treatment approach to deep shoulder infection after open RCR[7,27].
This evidence shows a tendency for multiple open debridement and lavage procedures in patients with deep infections after open RCR. Furthermore, leaving the wound open and packed with sterile gauze between debridements may also mean extended hospital stays for these patients that can negatively influence their quality of life and increase the economic burden on the healthcare system. Of note, patients with deep shoulder infections who underwent arthroscopic RCR as the initial procedure should also be treated with open debridement and lavage if complications such as osteomyelitis, abscess formation, or tissue necrosis exist.
It is imperative to approach post-RCR deep shoulder infection as septic arthritis and to consider IV antibiotic treatment for a minimum of 4 to 6 wk to successfully eradicate the infection and to minimize the risk of complications, such as osteomyelitis, that may occur with a higher incidence due to the suture anchors placed in the humeral head. The antibiotic should be chosen based on the culture and susceptibility results and after a consultation with the infectious disease specialist who will be involved in the patient’s management through the course of the initial treatment and the follow-up. A peripherally inserted central catheter must be placed and managed appropriately by regular flushing with normal saline between antibiotic doses to maintain patency. Depending on the causative microorganism and the patient’s response to the initial treatment, IV antibiotic treatment can be supplemented or extended by oral antibiotics, as supported in the literature[7-9].
The assessment of therapeutic response and follow-up of infection is mainly done by clinically evaluating the patient and monitoring infection markers, such as ESR and CRP. The duration of antibiotic treatment and the confirmation of infection eradication requires shared decision making between the orthopaedic surgeon and the infectious disease specialist.
Deep infections of the shoulder after RCR are usually successfully eradicated with debridement and lavage, and long-term IV antibiotics[7-9]. Nevertheless, devastating complications, such as osteomyelitis, abscess formation, post-infectious glenohumeral arthritis, and insufficient soft tissue coverage can be encountered, and functional outcomes may be far less than optimal. Kwon et al[7] reported a 67% dissatisfaction rate in a group of 12 patients treated for deep infection after RCR, with a mean UCLA score of 23.6 (excellent and good ≥ 28, fair and poor ≤ 27). Among these patients, two needed rotational muscle flaps due to insufficient deltoid tissue after repeated open debridements. Athwal et al[8] reported a complication rate of 32% during the medical and surgical treatment of deep infection after RCR. Their series included one patient who required arthrodesis and two patients who underwent shoulder arthroplasty due to glenohumeral arthrosis within 66 mo of eradicating the deep infection. Mirzayan et al[27] studied 13 patients with chronic deep infections following open RCR. Seven patients had osteomyelitis of the humeral head, two had osteomyelitis of the humeral head and the glenoid, two had osteomyelitis of the clavicle and the acromion, and two had no osteomyelitis but had a subdeltoid abscess. Seven patients required a rotational flap to allow for joint coverage. The results of the Simple Shoulder Test in this study revealed that only three patients could lift a one-pound weight (0.5 kg) and none could lift an eight-pound weight (3.6 kg) to shoulder level without bending the elbow. Eight patients could throw underhand; however, only one could throw overhand.
Although these studies clearly indicate that outcomes after an infected open or mini-open rotator cuff repair can be permanently disabling, no studies to date have reported the effects of deep infections after arthroscopic RCRs on functional outcomes.
Deep shoulder infections after rotator cuff repair are not frequently encountered. Patients who are suspected to have a post-RCR infection require a thorough diagnostic evaluation, including clinical signs and symptoms, laboratory workups and cultures. Although appropriate management of this condition with surgical debridement and lavage, and long-term IV antibiotics usually results in eradication of the infection, complications can be disabling and functional outcomes poor. Abscess formation, osteomyelitis, post-infectious glenohumeral arthritis, and loss of the soft tissue envelope are among the most devastating complications resulting from post-RCR deep infections. The majority of the patients with deep infections after RCR report unsatisfactory outcomes with permanent functional limitations.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cui Q, Fukuchi RK, Nikolopoulos D S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Roberson TA, Azar FM, Miller RH, Smith RA, Throckmorton TW. Predictors of Early Complications After Rotator Cuff Repair. Tech Should Elb Surg. 2016;17:88-92. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Nicholas SJ, Lee SJ, Mullaney MJ, Tyler TF, Fukunaga T, Johnson CD, McHugh MP. Functional Outcomes After Double-Row Versus Single-Row Rotator Cuff Repair: A Prospective Randomized Trial. Orthop J Sports Med. 2016;4:2325967116667398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Jung HJ, Sim GB, Bae KH, Kekatpure AL, Chun JM, Jeon IH. Rotator cuff surgery in patients older than 75 years with large and massive tears. J Shoulder Elbow Surg. 2017;26:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Antoni M, Klouche S, Mas V, Ferrand M, Bauer T, Hardy P. Return to recreational sport and clinical outcomes with at least 2years follow-up after arthroscopic repair of rotator cuff tears. Orthop Traumatol Surg Res. 2016;102:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 553] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 6. | Paloneva J, Lepola V, Äärimaa V, Joukainen A, Ylinen J, Mattila VM. Increasing incidence of rotator cuff repairs--A nationwide registry study in Finland. BMC Musculoskelet Disord. 2015;16:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 7. | Kwon YW, Kalainov DM, Rose HA, Bisson LJ, Weiland AJ. Management of early deep infection after rotator cuff repair surgery. J Shoulder Elbow Surg. 2005;14:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Athwal GS, Sperling JW, Rispoli DM, Cofield RH. Deep infection after rotator cuff repair. J Shoulder Elbow Surg. 2007;16:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Settecerri JJ, Pitner MA, Rock MG, Hanssen AD, Cofield RH. Infection after rotator cuff repair. J Shoulder Elbow Surg. 1999;8:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Pauzenberger L, Grieb A, Hexel M, Laky B, Anderl W, Heuberer P. Infections following arthroscopic rotator cuff repair: incidence, risk factors, and prophylaxis. Knee Surg Sports Traumatol Arthrosc. 2017;25:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Vopat BG, Lee BJ, DeStefano S, Waryasz GR, Kane PM, Gallacher SE, Fava J, Green AG. Risk Factors for Infection After Rotator Cuff Repair. Arthroscopy. 2016;32:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Yang ES, Tan J, Eells S, Rieg G, Tagudar G, Miller LG. Body site colonization in patients with community-associated methicillin-resistant Staphylococcus aureus and other types of S. aureus skin infections. Clin Microbiol Infect. 2010;16:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Saltzman MD, Marecek GS, Edwards SL, Kalainov DM. Infection after shoulder surgery. J Am Acad Orthop Surg. 2011;19:208-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Crossley KB, Ross J. Colonization of hospitalized patients by Staphylococcus aureus, Staphylococcus epidermidis and enterococci. J Hosp Infect. 1985;6:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Marecek GS, Weatherford BM, Fuller EB, Saltzman MD. The effect of axillary hair on surgical antisepsis around the shoulder. J Shoulder Elbow Surg. 2015;24:804-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Boonyasiri A, Thaisiam P, Permpikul C, Judaeng T, Suiwongsa B, Apiradeewajeset N, Fakthongphan T, Suddee S, Laoagtipparos W, Thamlikitkul V. Effectiveness of Chlorhexidine Wipes for the Prevention of Multidrug-Resistant Bacterial Colonization and Hospital-Acquired Infections in Intensive Care Unit Patients: A Randomized Trial in Thailand. Infect Control Hosp Epidemiol. 2016;37:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Chen AL, Shapiro JA, Ahn AK, Zuckerman JD, Cuomo F. Rotator cuff repair in patients with type I diabetes mellitus. J Shoulder Elbow Surg. 2003;12:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Hiemstra LA, Macdonald PB, Froese W. Subacromial infection following corticosteroid injection. J Shoulder Elbow Surg. 2003;12:91-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Armstrong RW, Bolding F. Septic arthritis after arthroscopy: the contributing roles of intraarticular steroids and environmental factors. Am J Infect Control. 1994;22:16-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Chuang MJ, Jancosko JJ, Mendoza V, Nottage WM. The Incidence of Propionibacterium acnes in Shoulder Arthroscopy. Arthroscopy. 2015;31:1702-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Király CL, Alén M, Korvola J, Horsmanheimo M. The effect of testosterone and anabolic steroids on the skin surface lipids and the population of Propionibacteria acnes in young postpubertal men. Acta Derm Venereol. 1988;68:21-26. [PubMed] |
| 23. | Seavey R. High-level disinfection, sterilization, and antisepsis: current issues in reprocessing medical and surgical instruments. Am J Infect Control. 2013;41:S111-S117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Hackett DJ Jr, Crosby LA. Infection Prevention in Shoulder Surgery. Bull Hosp Jt Dis (2013). 2015;73 Suppl 1:S140-S144. [PubMed] |
| 25. | Randelli P, Spennacchio P, Ragone V, Arrigoni P, Casella A, Cabitza P. Complications associated with arthroscopic rotator cuff repair: a literature review. Musculoskelet Surg. 2012;96:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Brislin KJ, Field LD, Savoie FH 3rd. Complications after arthroscopic rotator cuff repair. Arthroscopy. 2007;23:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 234] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 27. | Mirzayan R, Itamura JM, Vangsness CT Jr, Holtom PD, Sherman R, Patzakis MJ. Management of chronic deep infection following rotator cuff repair. J Bone Joint Surg Am. 2000;82-A:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Lewis SS, Cox GM, Stout JE. Clinical utility of indium 111-labeled white blood cell scintigraphy for evaluation of suspected infection. Open Forum Infect Dis. 2014;1:ofu089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |