Published online Apr 18, 2017. doi: 10.5312/wjo.v8.i4.350
Peer-review started: October 11, 2016
First decision: November 30, 2016
Revised: December 13, 2016
Accepted: January 2, 2017
Article in press: January 3, 2017
Published online: April 18, 2017
Processing time: 189 Days and 17.8 Hours
To investigate whether normal thickness cartilage in osteoarthritic knees demonstrate depletion of proteoglycan or collagen content compared to healthy knees.
Magnetic resonance (MR) images were acquired from 5 subjects scheduled for total knee arthroplasty (TKA) (mean age 70 years) and 20 young healthy control subjects without knee pain (mean age 28.9 years). MR images of T1ρ mapping, T2 mapping, and fat suppressed proton-density weighted sequences were obtained. Following TKA each condyle was divided into 4 parts (distal medial, posterior medial, distal lateral, posterior lateral) for cartilage analysis. Twenty specimens (bone and cartilage blocks) were examined. For each joint, the degree and extent of cartilage destruction was determined using the Osteoarthritis Research Society International cartilage histopathology assessment system. In magnetic resonance imaging (MRI) analysis, 2 readers performed cartilage segmentation for T1ρ/T2 values and cartilage thickness measurement.
Eleven areas in MRI including normal or near normal cartilage thickness were selected. The corresponding histopathological sections demonstrated mild to moderate osteoarthritis (OA). There was no significant difference in cartilage thickness in MRI between control and advanced OA samples [medial distal condyle, P = 0.461; medial posterior condyle (MPC), P = 0.352; lateral distal condyle, P = 0.654; lateral posterior condyle, P = 0.550], suggesting arthritic specimens were morphologically similar to normal or early staged degenerative cartilage. Cartilage T2 and T1ρ values from the MPC were significantly higher among the patients with advanced OA (P = 0.043). For remaining condylar samples there was no statistical difference in T2 and T1ρ values between cases and controls but there was a trend towards higher values in advanced OA patients.
Though cartilage is morphologically normal or near normal, degenerative changes exist in advanced OA patients. These changes can be detected with T2 and T1ρ MRI techniques.
Core tip: Magnetic resonance images of eleven healthy knees and five knees with advanced osteoarthritis (OA) were studied using T1ρ and T2 mapping. Histopathologic samples were also taken from the five osteoarthritic knees following total knee arthroplasty. Our results indicate that even though cartilage is morphologically normal or near normal, cartilage degenerative changes exist in advanced OA patients. This suggests that normal thickness cartilage or mild cartilage thinning in the advanced OA knee demonstrates depletion of proteoglycan or collagen content compared with similar appearing cartilage in young healthy knees. These early changes can be detected with T2 and T1ρ MRI techniques.
- Citation: Kester BS, Carpenter PM, Yu HJ, Nozaki T, Kaneko Y, Yoshioka H, Schwarzkopf R. T1ρ/T2 mapping and histopathology of degenerative cartilage in advanced knee osteoarthritis. World J Orthop 2017; 8(4): 350-356
- URL: https://www.wjgnet.com/2218-5836/full/v8/i4/350.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i4.350
Osteoarthritis (OA) is one of the fastest growing medical conditions worldwide, affecting at least 27 million people in the United States alone[1,2]. It is a major contributor to functional disability and loss of autonomy in older adults[3]. These factors represent a significant health and financial burden to the general population[2,4]. Knee and hip OA cause the greatest burden of disability, leading to the need for prosthetic joint replacements in the most severe cases[5]. Decreasing the need for such procedures, and costs to both the patient and society, motivates the need for research into disease prevention and early detection.
OA is characterized by the progressive loss of articular cartilage. However, significant damage to the collagen-proteoglycan matrix and elevation of cartilage water content are believed to precede the loss of cartilage and consequent symptoms of knee OA[6,7]. Magnetic resonance imaging (MRI) techniques are been developed over the past decade that allow for the detection of these early and subtle changes to the cartilage matrix[8-11]. Among these techniques, T1ρ has stood out as a high sensitivity option to detect early changes without the use of contrast agents[7].
Prior studies have already demonstrated increased cartilage T1ρ values, a surrogate of cartilage damage, in patients with knee OA[12-15]. Specifically, T1ρ and T2 values are known to be elevated in asymptomatic, healthy subjects with early stage OA compared to individuals without focal lesions[13]. While severe focal lesions are common indications for total knee replacement, patients may also be considered for joint sparing or cartilage preservation procedures. We aim to determine whether normal appearing cartilage by MRI in the non-symptomatic regions of advanced knee OA demonstrate depletion of proteoglycan and collagen content by T1ρ and T2 mapping and to correlate these measurements with degenerative changes of cartilage by histology. We hypothesize that normal thickness cartilage or mild cartilage thinning (early staged cartilage degeneration) in advanced knee OA will demonstrate depletion of proteoglycan or collagen content, compared with similar appearing cartilage in young healthy knees.
Five advanced OA patients scheduled for total knee arthroplasty (TKA) were enrolled in this study. A board certified orthopaedic surgeon (RS) recruited them (Kellgren-Lawrence score of 3 or 4; mean age 70 years, range 62-90 years; 2 men and 3 women). Twenty knees from 20 healthy volunteers (mean age 28.9 years, range 19-38 years; 13 men and 7 women) without any history of knee symptoms or prior knee surgery were used as an imaging control group. The study protocol was approved by the institutional review board and all subjects provided written informed consent before any study-related procedures were performed.
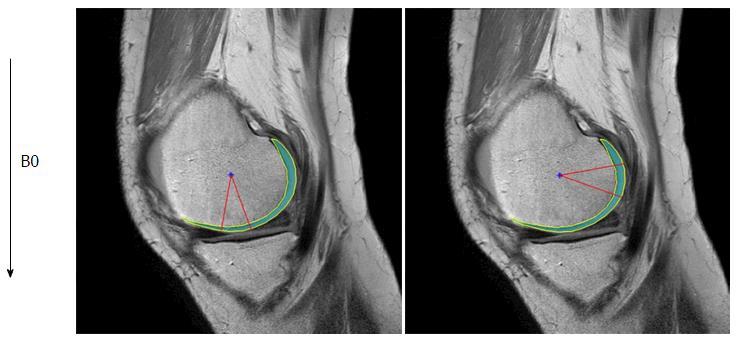
All MR studies were performed on a 3.0-T unit (Achieva, Philips Healthcare, Netherland) utilizing an 8-channel knee receive-only radiofrequency coil. Three sagittal MR images were acquired including fat suppressed (FS) proton density-weighted imaging (PDWI) sequence, T2 mapping sequence, and T1ρ mapping sequence. All sagittal images were obtained without oblique angulation, parallel to the magnetic static field (B0). Parallel imaging was used on all imaging sequences utilizing Sensitivity Encoding for MRI. The acquisition parameters were as follows. FS PDWI: 2D turbo spin-echo; Repetition time (TR)/echo time (TE) = 4311/30 ms, number of excitation (NEX) = 2, and total acquisition time = 3 min 35 s. T2 mapping: 2D turbo spin-echo; TR/TE = 2700/13, 26, 39, 52, 65, 78, 91 ms, NEX = 1 and total acquisition time = 13 min 26 s. T1ρ: 3D FS PROSET (Principle of Selective Excitation Technique); TR/TE = 6.4/3.4 ms, flip angle = 10°, echo train length = 64, NEX = 1, spin-lock frequency = 575 Hertz, time of spin-lock (TSL), 20, 40, 60 and 80 ms, and acquisition time = 4 min 9 s × 4. All images were obtained with field of view = 140 × 140 mm, slice thickness/gap = 3/0 mm, image matrix = 512 × 512, number of slices = 31 and effective in-plane spatial resolution = 0.27 × 0.27 mm. Each femoral condyle was divided into 4 areas: The medial distal condyle (MDC), medial posterior condyle (MPC), lateral distal condyle (LDC), and lateral posterior condyle (LPC). Therefore, a total of 20 areas of MRI of the femoral condyle from 5 patients with advanced OA were reviewed.
TKA was conducted as scheduled on each operative candidate. Surgically resected condyles were recovered intraoperatively and divided into 4 parts (MDC, MPC, LDC, LPC). A total of 20 specimens (bone and cartilage blocks) were histopathologically examined.
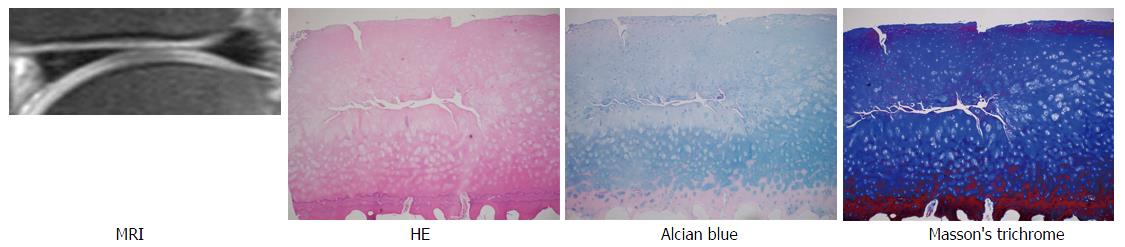
The MDP, MPC, LDC and LDP of the distal femur removed at surgery were fixed in 10% neutral buffered formalin for at least 72 h, decalcified using dilute hydrochloric acid (Rapid Bone Decalcifier, American Master Tech Inc., Lodi CA) for two days, and post fixed in formalin for at least 2 more days. Sagittal sections across the entire mid portion of each of the condyles underwent routine paraffin embedding and staining with hematoxylin and eosin. In this way, the same region was sampled for each of the specimens, and maximum extent of the lesion could be assessed in the mid sagittal plane of each of the condyles. Additional paraffin sections were stained with Masson’s trichrome and Alcian blue. For each joint, the degree and extent of cartilage destruction was determined using the Osteoarthritis Research Society International cartilage histopathology assessment system[16] by a pathologist with experience in bone and soft tissue pathology. For this system, the degree of cartilage destruction (OA grade) and the extent of destruction (OA stage) are multiplied to determine the OA score. The surgical edges were not assessed to avoid possible over-interpretation of surgical artifacts.
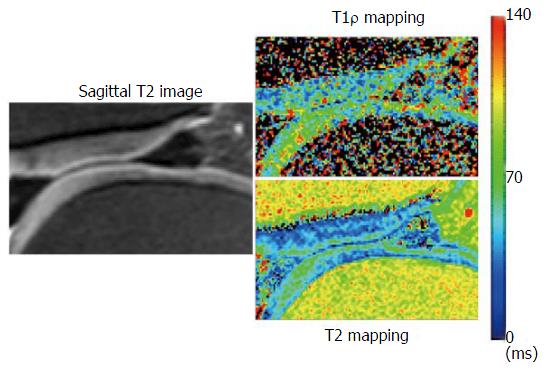
Images were transferred in Digital Imaging and Communications in Medicine format to a personal computer (Windows 7), which was used to perform all post-processing and analyses. T2 and T1ρ analyses were performed using in-house developed and implemented software in MatLab (MathWorks, Natick, MA) (Figure 1). Manual cartilage extraction of the femoral condyle in healthy volunteers (n = 20) and advanced OA patients (n = 5) was performed on both T2 and T1ρ images by a board-certified orthopaedic surgeon with 14 years of experience and a board-certified radiologist with 13 years of experience, independently. Images with TE = 26 in T2 and TSL = 20 in T1ρ were chosen for segmentation due to high signal-to-noise ratio compared to the other images, based on prior studies[17,18]. T2 and T1ρ values were measured in a range of -10 to 20 degrees for the distal condyle and 70 to 100 degrees for the posterior condyle (Figure 2). The angle 0 is defined along B0. We calculated average T2 and T1ρ values of two observers at each femoral condyle, and average thickness of the cartilage as pixel numbers in the segmented area at each condyle.
Differences in T2/T1ρ values and thickness of the cartilage between normal cartilage and advanced degenerative cartilage do not conform to normal distributions. These differences were assessed using a nonparametric Mann-Whitney U test. Statistical review of the study was performed by a researcher with training in biomedical statistics. SPSS Statistics version 22 (IBM, Armonk, New York) was used for calculations. In all cases, a P value of 0.05 or less was deemed statistically significant.
A total of 20 areas on MRI of the femoral condyles from 5 advanced OA patients were reviewed. Eleven areas including normal or near normal cartilage thickness (2 MDCs, 2 MPCs, 4 LDCs, 3 LPCs) were selected. The average OA grade, stage, and, scores of corresponding specimens (bone blocks and cartilage) were 3.82 (range: 3-4.5), 3.45 (range: 2-4), and 13.1 (range: 7-16), respectively, compatible with mild to moderate OA (Table 1). Examples of FS PDWI, hematoxylin and eosin stain, Alcian blue stain, and Masson’s trichrome stain are demonstrated in Figure 3.
| No. | Location | Grade | Stage | Score | Pathology comments |
| 1 | MDC | 4 | 4 | 16 | Superficial erosion, prominent vertical fissures and depletion of more than the upper 2/3 of proteoglycans by alcian blue staining |
| 2 | MPC | 4 | 3 | 12 | Focal erosion, a few small vertical clefts, depletion of the upper 1/2 to 2/3 of proteoglycans by alcian blue staining |
| 3 | LDC | 3 | 4 | 12 | Superficial fibrillation, small vertical clefts, minimal superficial depletion of proteoglycans by alcian blue staining |
| 4 | LDC | 4.5 | 3 | 13.5 | Deep erosion extending almost to bone and almost complete depletion of proteoglycans by alcian blue staining |
| 5 | MDC | 4 | 3 | 12 | Superficial erosion, prominent vertical and horizontal fissures and depletion of more than the upper 2/3 of proteoglycans by alcian blue staining |
| 6 | LPC | 3.5 | 2 | 7 | Focal superficial erosion almost complete depletion of proteoglycans by alcian blue staining |
| 7 | MPC | 4 | 4 | 16 | Erosion, focally deep and superficial depletion of proteoglycans by alcian blue staining over most of the surface, with complete depletion at region of deep erosion |
| 8 | LDC | 3.5 | 4 | 14 | Focal erosion and vertical fissures extending to mid zone with complete depletion of proteoglycans by alcian blue staining at site of fissures |
| 9 | LPC | 3 | 4 | 12 | Focal vertical fissures extending to mid zone with minimal depletion of proteoglycans by alcian blue staining |
| 10 | LDC | 4.5 | 3 | 13.5 | Focal deep erosion and superficial depletion of proteoglycans by alcian blue staining |
| Mean | 3.82 | 3.45 | 13.09 |
Table 2 shows the T2/T1ρ values and thickness of the cartilage in normal volunteers and advanced osteoarthritis patients. Although the difference of each cartilage thickness between normal volunteers and advanced OA patients was not observed, T2/ T1ρ values were significantly higher at the MPC in advanced OA patients compared to normal volunteers (P < 0.05). T2/T1ρ values also tended to be higher in advanced OA patients compared to normal volunteers at the MDC, LDC and LPC without significant difference.
| Control (n = 20) | AOA (n = 5) | P1 | ||
| MDC | T2-value2 | 52.23 | 64.9 | 0.524 |
| T1ρ-value | 52.5 | 52.98 | 0.642 | |
| T2-cartilage thickness3 | 6.13 | 5.38 | 0.461 | |
| T1ρ-cartilage thickness | 5.7 | 6.8 | 0.97 | |
| MPC | T2-value | 46.83 | 59.3 | 0.016 |
| T1ρ-value | 57.15 | 73.5 | 0.043 | |
| T2-cartilage thickness | 8.6 | 10.05 | 0.352 | |
| T1ρ-cartilage thickness | 8.7 | 8.93 | 0.938 | |
| LDC | T2-value | 48.93 | 53.7 | 0.067 |
| T1ρ-value | 55.85 | 62.55 | 0.371 | |
| T2-cartilage thickness | 6.58 | 6.4 | 0.654 | |
| T1ρ-cartilage thickness | 6.2 | 5.75 | 0.587 | |
| LPC | T2-value | 44.2 | 50.8 | 0.218 |
| T1ρ-value | 48.53 | 68.5 | 0.055 | |
| T2-cartilage thickness | 8.93 | 7.95 | 0.55 | |
| T1ρ-cartilage thickness | 8.5 | 9.15 | 0.601 |
Knee OA is a multifactorial disease with a significant population burden[1]. Novel strategies in the management of knee OA are based on early detection and minimally invasive procedures[11,19]. Certain patients with focal advanced knee OA may benefit from joint preservation strategies if remaining articular cartilage is healthy. In our study we aimed to assess whether normal appearing cartilage in advanced knee OA patients demonstrate depletion of proteoglycan and collagen content by T2/T1ρ analysis, markers of early OA. We have demonstrated that although non-osteoarthritic portions of the femoral condyle in patients with advanced knee OA have similar morphologic characteristics compared to controls in routine MRI, there are significant changes on T2/T1ρ mapping that can measure differences on the biomolecular level.
Many in vivo studies have demonstrated an association between increased T2/T1ρ values and various stages of OA about the knee[12-15]. T1ρ values have been seen to increase with age, but are also higher in middle-aged populations with isolated patellofemoral and tibiofemoral compartment knee OA[13,14,20]. T1ρ relaxation times in particular may be elevated by as much as 30%-40% in patients with early knee OA[14]. Furthermore, Stahl et al[13] demonstrated that patients with asymptomatic knee OA have increased T2/T1ρ values in some compartments compared to healthy controls. These data are consistent with our findings that T2/T1ρ values are consistently higher in multiple compartments in patients with advanced OA compared to asymptomatic controls. By isolating pathologic samples with mild or near normal pathologic changes of articular cartilage we have demonstrated a subset of patients with mild arthritic changes. Li et al[12] have already shown significantly elevated T1ρ relaxation times in subcompartments of knee OA subjects where no prior morphologic changes were observed. This type of study demonstrates the utility of quantitative MRI sequences in detecting early biochemical changes within the articular cartilage matrix, but is limited to radiographic assessments alone. We have isolated not only radiographically similar, but pathologically similar cartilage samples to be used in this type of analysis.
This study agrees with multiple other publications that demonstrate the use of T2/T1ρ relaxation times for the early detection of knee OA[13,21,22]. The unique contribution is the comparison of normal or near normal imaging samples between cases and controls. Thuillier et al[23] examined patients with patellar-femoral pain but without radiographic evidence of knee OA and found significantly elevated T1ρ values in the lateral patellar cartilage compared to controls. Several other studies have also showed that focal cartilage defects identified on arthroscopy are correlated with elevated MRI relaxation times[22,24,25]. We have similarly shown that morphologically normal articular cartilage, though adjoining osteoarthritic compartments of the knee, exhibit early changes in cartilage degeneration. These early changes include articular cartilage hydration, loss of proteoglycan content, thinning and loosening of collagen fibrils. While statistically significant changes were not observed in all compartments for this small sample size, the trends are readily apparent in all groups and notably significant in the MPC.
The utility of these sequences in joint preservation or replacement remains to be seen. T2 and T1ρ mapping have increasingly been applied with high fidelity to track outcomes after articular cartilage repair[26]. Studies have shown significant improvements in T1ρ relaxation times following microfracture and mosaicplasty, but values do not appear to ever return to baseline[26-28]. The question stands as to whether focal articular cartilage defects about the knee are amenable to preservation therapies if surrounding articular cartilage exhibits degenerative changes. No doubt there is a spectrum and diversity of cartilage injuries, and only a subset are arthritic in nature, but our data suggest that patients should be closely examined for early articular changes prior to such therapies. T2 and T1ρ mapping may have an important role in identifying which patients may benefit from preservation strategies and which are better candidates for joint replacement. Furthermore, these strategies may be used to develop personalized, systematic recommendations for patients with articular cartilage injuries.
This study is not without limitations. Only five patients undergoing TKA were recruited for the OA arm of the study. Although T2 and T1ρ were significantly higher in the posteromedial condylar segments, this study was underpowered to demonstrate statistically significant differences in the remaining condyles. We believe that a larger sample size would bolster our conclusions. Of note, there was a marked difference in age between the OA group and controls (70 years vs 28.9 years). Differences in T2 and T1ρ mapping may be confounded by physiologic changes with age alone, as previously mentioned. Although the concept of morphologically normal but biochemically impaired cartilage is valid, this observation may weaken the validity of our argument regarding joint preservation options. Furthermore, this is a cross-sectional design with no long-term follow-up as all OA patients underwent TKA. They also were not recruited according to degree or radiographic severity of disease and there is no long-term follow-up regarding symptom development in control subjects. However, there are lessons to be learned from this work that may help in the development of personalized treatments for OA and cartilage injuries.
In conclusion, our findings lend additional support to the use of T2 and T1ρ mapping in the diagnosis and management of OA of the knee. We have uniquely shown that even though cartilage is morphologically normal or near normal, cartilage degenerative changes exist in advanced OA patients. These early changes can be detected with T2 and T1ρ MRI techniques and consideration should be given to the use of these sequences in the early detection of OA.
Contract grant sponsor: National Center for Research; Resources; Contract grant sponsor: National Center for Advancing Translational Sciences; Contract grant sponsor: National Institutes of Health; Contract grant number: UL1TR000153.
Characterized by the progressive loss of articular cartilage, osteoarthritis (OA) is one of the largest and fastest growing medical conditions worldwide. Significant damage to the collagen-proteoglycan matrix is believed to precede the loss of cartilage and consequent symptoms of knee OA. Among imaging techniques, magnetic resonance T1ρ has stood out as a high sensitivity option to detect these early changes in otherwise young, healthy joints.
Prior studies have demonstrated increased cartilage T1ρ values, a surrogate of cartilage damage, in patients with knee OA. Specifically, T1ρ and T2 values are known to be elevated in asymptomatic, healthy subjects with early stage OA compared to individuals without focal lesions. The basic science foundation for the use of these techniques is now understood, but translating them into clinical practice is an area of current interest.
In recent years, novel strategies have been explored for the early detection of OA. Magnetic resonance T1ρ and T2 mapping has emerged as an excellent candidate for this endeavor. The authors have uniquely shown that even though cartilage is morphologically normal or near normal, cartilage degenerative changes exist in advanced OA patients. These early changes can be detected with T2 and T1ρ magnetic resonance imaging techniques and consideration should be given to the use of these sequences in the early detection of OA.
The authors’ findings lend support to the use of T2 and T1ρ mapping in the diagnosis and management of OA of the knee. The results of this study suggest that asymptomatic individuals under consideration for knee joint preservation strategies may benefit from pre-procedure T2 and T1ρ analysis. Future studies should build upon their results to determine specific T2 and T1ρ parameters whereby joint preservation strategies are likely to fail.
Standard T2 and lesser-known T1ρ magnetic resonance pulse sequences can be used as surrogates of cartilage damage in patients with knee OA. Specifically, T1ρ and T2 values are known to be elevated in asymptomatic, healthy subjects with early stage OA compared to individuals without focal lesions.
It is a well-written paper.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hasegawa M, Peng BG, Razek AAKA, Sakkas LI S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3206] [Cited by in RCA: 2983] [Article Influence: 175.5] [Reference Citation Analysis (0)] |
| 2. | Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD, Merkel PA. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1536] [Cited by in RCA: 1594] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 3. | Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, Aliabadi P, Levy D. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995;38:1500-1505. [PubMed] |
| 4. | Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, Dragomir A, Kalsbeek WD, Luta G, Jordan JM. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207-1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 778] [Cited by in RCA: 709] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 5. | Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 822] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 6. | Mankin HJ, Brandt KD. Pathogenesis of arthritis. Philadelphia W.B. Saunders. 1993;. |
| 7. | Wáng YX, Zhang Q, Li X, Chen W, Ahuja A, Yuan J. T1ρ magnetic resonance: basic physics principles and applications in knee and intervertebral disc imaging. Quant Imaging Med Surg. 2015;5:858-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 8. | Potter HG, Black BR, Chong le R. New techniques in articular cartilage imaging. Clin Sports Med. 2009;28:77-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Radcliff KE, Sidhu GD, Kepler CK, Gruskay J, Anderson DG, Hilibrand A, Albert TJ, Vaccaro AR. Complications of Flat Bed Rest After Incidental Durotomy. Clin Spine Surg. 2016;29:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Matzat SJ, van Tiel J, Gold GE, Oei EH. Quantitative MRI techniques of cartilage composition. Quant Imaging Med Surg. 2013;3:162-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 11. | Link TM, Stahl R, Woertler K. Cartilage imaging: motivation, techniques, current and future significance. Eur Radiol. 2007;17:1135-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Li X, Han ET, Ma CB, Link TM, Newitt DC, Majumdar S. In vivo 3T spiral imaging based multi-slice T(1rho) mapping of knee cartilage in osteoarthritis. Magn Reson Med. 2005;54:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Stahl R, Luke A, Li X, Carballido-Gamio J, Ma CB, Majumdar S, Link TM. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients--a 3.0-Tesla MRI study. Eur Radiol. 2009;19:132-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Regatte RR, Akella SV, Wheaton AJ, Lech G, Borthakur A, Kneeland JB, Reddy R. 3D-T1rho-relaxation mapping of articular cartilage: in vivo assessment of early degenerative changes in symptomatic osteoarthritic subjects. Acad Radiol. 2004;11:741-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Wang L, Chang G, Xu J, Vieira RL, Krasnokutsky S, Abramson S, Regatte RR. T1rho MRI of menisci and cartilage in patients with osteoarthritis at 3T. Eur J Radiol. 2012;81:2329-2336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1445] [Cited by in RCA: 1731] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 17. | Kaneko Y, Nozaki T, Yu H, Chang A, Kaneshiro K, Schwarzkopf R, Hara T, Yoshioka H. Normal T2 map profile of the entire femoral cartilage using an angle/layer-dependent approach. J Magn Reson Imaging. 2015;42:1507-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Nozaki T, Kaneko Y, Yu HJ, Kaneshiro K, Schwarzkopf R, Hara T, Yoshioka H. T1rho mapping of entire femoral cartilage using depth- and angle-dependent analysis. Eur Radiol. 2016;26:1952-1962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Cole BJ, Pascual-Garrido C, Grumet RC. Surgical management of articular cartilage defects in the knee. Instr Course Lect. 2010;59:181-204. [PubMed] |
| 20. | Kumar D, Souza RB, Subburaj K, MacLeod TD, Singh J, Calixto NE, Nardo L, Link TM, Li X, Lane NE. Are There Sex Differences in Knee Cartilage Composition and Walking Mechanics in Healthy and Osteoarthritis Populations? Clin Orthop Relat Res. 2015;473:2548-2558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, Carballido-Gamio J, Ries M, Majumdar S. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15:789-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 386] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 22. | Nishioka H, Hirose J, Nakamura E, Okamoto N, Karasugi T, Taniwaki T, Okada T, Yamashita Y, Mizuta H. Detecting ICRS grade 1 cartilage lesions in anterior cruciate ligament injury using T1ρ and T2 mapping. Eur J Radiol. 2013;82:1499-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Thuillier DU, Souza RB, Wu S, Luke A, Li X, Feeley BT. T1ρ imaging demonstrates early changes in the lateral patella in patients with patellofemoral pain and maltracking. Am J Sports Med. 2013;41:1813-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Lozano J, Li X, Link TM, Safran M, Majumdar S, Ma CB. Detection of posttraumatic cartilage injury using quantitative T1rho magnetic resonance imaging. A report of two cases with arthroscopic findings. J Bone Joint Surg Am. 2006;88:1349-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Witschey WR, Borthakur A, Fenty M, Kneeland BJ, Lonner JH, McArdle EL, Sochor M, Reddy R. T1rho MRI quantification of arthroscopically confirmed cartilage degeneration. Magn Reson Med. 2010;63:1376-1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Jungmann PM, Baum T, Bauer JS, Karampinos DC, Erdle B, Link TM, Li X, Trattnig S, Rummeny EJ, Woertler K. Cartilage repair surgery: outcome evaluation by using noninvasive cartilage biomarkers based on quantitative MRI techniques? Biomed Res Int. 2014;2014:840170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Holtzman DJ, Theologis AA, Carballido-Gamio J, Majumdar S, Li X, Benjamin C. T(1ρ) and T(2) quantitative magnetic resonance imaging analysis of cartilage regeneration following microfracture and mosaicplasty cartilage resurfacing procedures. J Magn Reson Imaging. 2010;32:914-923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Theologis AA, Schairer WW, Carballido-Gamio J, Majumdar S, Li X, Ma CB. Longitudinal analysis of T1ρ and T2 quantitative MRI of knee cartilage laminar organization following microfracture surgery. Knee. 2012;19:652-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |