INTRODUCTION
Glenohumeral instability is a frequent problem encountered within high-risk patient populations[1,2], and it poses important short- and long-term implications for shoulder function. While numerous previous studies have focused on the ideal management, risk stratification, and/or surgical technique for addressing patients with shoulder instability, few have examined the natural history of instability events as it relates to secondary arthrosis. The presence of concomitant shoulder instability and premature degenerative wear creates a difficult clinical scenario, and optimal treatment requires a fundamental knowledge of underlying anatomy and current, evolving treatment options.
With the largest range of motion of any articulation and minimal osseous constraint, the glenohumeral joint is largely stabilized by rotator cuff compression and its inherent concavity, which is deepened by its circumferential labral attachment. In addition to the anterior and posterior inferior labrum, the inferior glenohumeral ligament (IGHL) complex provides vital restraint to humeral translation. The anterior band of the IGHL limits anterior instability in elevated positions of abduction and external rotation, whereas the posterior band acts in forward flexion and internal rotation. Additionally, the rotator interval, which contains the coracohumeral, middle glenohumeral, and superior glenohumeral ligaments, has been a focal area of research. However, its contributions to inferior and anterioposterior glenohumeral restraint remains a continuing source of debate[3,4].
Shoulder instability is classically defined by the directionality of excessive glenohumeral translation in association with a provocative examination. However, this must be differentiated from patients with asymptomatic laxity, particularly those with multidirectional involvement. Anterior instability accounts for the vast majority of patients with shoulder dislocations, with a reported incidence rate ranging from 0.08 to 0.24 patients per 1000 person-years in civilian populations[5-7]. Within a higher risk military demographic, the incidence may rise to nearly 3% per year when subluxation events are also considered[1]. Conversely, posterior instability has historically accounted for only a smaller fraction of all unstable shoulders, with approximately 2% to 10% reported[8-10]. More recent series indicate that isolated posterior instability may comprise over a quarter of patients with shoulder stabilization, and approximately 20% of additional patients will undergo surgery for combined or bidirectional instability[11]. Especially within young active patients, circumferential labral pathology may also be common with isolated or recurrent anterior shoulder instability[12,13], so there must be a high index of suspicion in the evaluation of these at-risk individuals.
MANAGEMENT OF GLENOHUMERAL INSTABILITY
Initial management
The initial management of an acute shoulder dislocation includes reduction of the glenohumeral joint followed by a period of immobilization in a sling with rest, ice, and anti-inflammatory medications. After the acute phase, initiation of passive range of motion and guided physical therapy can begin. Traditionally, patients were placed in a sling, which typically puts the shoulder into internal rotation. Duration in a sling varies according to the treating physician, and can range from very brief initial sling use to 2-3 wk of immobilization in variable positions[14].
The indications for surgical management include recurrent instability following a trial of nonoperative treatment, ongoing pain and dysfunction from recurring subluxations, or selected, higher patient demographics with shoulder instability. There has also been focus on primary surgical stabilization in the young first-time dislocator, especially among young athletes and military servicemembers. A study in West Point cadets demonstrated the importance of age as a predictor for redislocation, with patients less than 20 years old having a 92% rate of redislocation with non-operative treatment[15]. Similarly, a retrospective review of young athletes, predominantly rugby players with average age of 21 years, demonstrated that 94.5% of the patients treated non-operatively sustained recurrent dislocation vs only one patient (3.5%) in the operative group[16].
In another prospective randomized controlled trial in active duty service members, Bottoni et al[17] compared 14 patients treated with four weeks of immobilization with 10 patients treated initially with acute arthroscopic Bankart repair. Of the 12 patients in the non-operative group available for follow-up, 9 (75%) developed recurrent instability. Comparatively, only one patient (11.1%) in the operative group developed recurrent instability. As a result, the authors advocated acute arthroscopic stabilization for young, high risk patients with a first-time anterior dislocation in order to reduce the rate of recurrent instability and worsening secondary pathology, including glenohumeral arthropathy.
Open vs arthroscopic
Bankart repair and capsulorraphy were traditionally performed via an open deltopectoral approach, and historically considered the gold standard for repair. However, over the past three decades, clinical outcomes with an arthroscopic technique have improved dramatically, and many surgeons view this as an equivalent procedure to the classic open Bankart procedure. Variations of the open approach largely differ according to management of the subscapularis, with complete and partial tendon takedown described, as well as horizontal splitting techniques[18,19]. With the evolution of contemporary suture anchor technology, the arthroscopic Bankart repair has become increasingly more common, and advocates highlight its enhanced cosmesis, avoidance of surgical site morbidity (i.e., subscapularis compromise), ability to treat all associated intra-articular pathology, and improved external rotation. This debate has continued in the existent literature, and most studies have preferentially focused on recurrence rates and patient outcomes scores among comparative series. In a 2004 meta-analysis, the documented recurrence rate (subluxation or dislocation) for arthroscopic repair was 20%, whereas use of an open technique demonstrated a significantly lower rate (10%) of subsequent instability[20]. Additionally, the authors found that a higher proportion of patients in the open group had a good or excellent Rowe score postoperatively. However, this study encompassed older techniques for arthroscopic labral repair, including trans-glenoid sutures and bioabsorbable suture tacks, which may introduce selection bias. A subsequent prospective randomized controlled trial of open vs arthroscopic Bankart repairs with modern suture anchor technique demonstrated a significantly lower recurrence rate at two years in the open group (11%) compared with the arthroscopic group (23%)[21]. Furthermore, the authors found age less than 25, male gender and presence of a Hill-Sachs lesions as risk factors for recurrent instability. Conversely, a large scale study from the United States military demonstrated that open anterior stabilization had nearly two-fold higher rate of short-term revision surgery than those with arthroscopic procedures after multivariate analysis, although this failed to control for degree of occult bone loss.
Augmentation
Variations of the original Bankart repair have been introduced over the years in an attempt to further mitigate rates of recurrent instability. These methods have included a number of different bone and soft tissue transfers used in the setting of bony deficiency and/or irreparable soft tissue damage. Historically, the Magnuson-Stack procedure was a non-anatomic transfer of the subscapularis from its native insertion on the lesser tuberosity to the greater tuberosity in an attempt to increase tension and improve stability[22,23]. Similarly, the Putti-Platt procedure provided a longitudinal, “pants over vest” shortening of the subscapularis and underlying capsule in order to tighten the anterior soft tissue restraints[24]. However, clinical outcomes with both of these non-anatomic procedures significantly disrupted the length-tension relationship of the subscapularis, leading to anterior overtensioning, loss of external rotation, and premature glenohumeral arthritis[25-28].
Alternatively, Latarjet described a technique of transfer of the coracoid process to the anterior inferior glenoid as a bony augmentation[29]. A slightly different type of coracoid transplantation procedure was described by Helfet and named in honor of his mentor, Walter Bristow[30]. The Latarjet has become increasing popular as an option for the treatment of glenoid or bipolar bone loss, which is a significant predictor of recurrent instability after arthroscopic repair[31]. Modifications to the initial description were made by Young et al[32], which included fixation with two screws, repairing the anterior capsule to the coracoacromial ligament retained on the coracoid graft and placing the graft through a split in the subscapularis to provide a “sling” effect of the conjoined tendon. Further adaptations, such as the congruent arc technique, have also been proposed. In this circumstance, the inferior aspect of the coracoid, as opposed to the lateral surface described in the original technique, is rotated to match the contour of the glenoid neck and extend the potential articular surface[33,34].
Other alternatives may be considered for complex shoulder instability with critical bone loss or engaging bipolar lesions. Potential graft sources for anterior glenoid reconstruction may include autograft or allograft iliac crest, allograft distal tibia, allograft glenoid, and/or local distal clavicular autograft[35-37]. All of these procedures are predicated on the goal of extending the articular surface of the glenoid without providing a bony constraint to anterior humeral translation[38]. On the humeral side, the “remplissage” procedure provides a capsulotenodesis of the posterior capsule and infraspinatus tendon into the Hill-Sachs lesion to prevent its engagement with the glenoid[39]. This non-anatomic, arthroscopic procedure can be combined with a labral repair to provide stability even in the setting of glenoid bone loss[40].
POST-INSTABILITY ARTHROPATHY
In an attempt to describe and characterize the natural history of the development of dislocation arthropathy after a primary shoulder dislocation, Hovelius et al[41] followed 257 shoulders initially treated with non-operative management at long-term follow-up. At 25-year follow-up, 227 shoulders met criteria for inclusion. Of those, 29% had developed mild arthropathy, 9% moderate, and 17% severe, and less than half (44%) were classified as normal. Risk factors for development of secondary arthropathy included age greater than 25 at time of initial dislocation, high energy mechanism of injury during sporting activity, and history of alcohol abuse. When evaluating the same patient cohort requiring subsequent stabilization surgery, Hovelius et al[42] concluded that approximately two-thirds of patients under the age of 25 with surgery for first-time anterior dislocation developed at least mild arthropathy by final follow-up.
In addition to assessing the long term results of primary dislocations treated with initial non-operative management, the rates of secondary arthropathy have also been quantified for other methods of treating anterior shoulder stabilizations. To this end, Hovelius et al[43] performed a retrospective comparative analysis of 26 shoulders with open Bankart repair and 30 shoulders with Bristow-Latarjet procedure at greater than 15-year follow-up. Of the shoulders that underwent Bankart repair, 16 (61.5%) went on to develop arthropathy (14 mild, 2 moderate), as compared to 9 (30%) shoulders in the Latarjet group (5 mild, 3 moderate, 1 severe). Interestingly, all patients who developed moderate or severe arthropathy, from either treatment group, reported being very satisfied with their outcome. This may reflect a disconnect between radiographic outcomes and patient-reported function after symptomatic instability has resolved, and this has been documented in other prior series as well[27,44].
In a separate long term study assessing both the clinical and radiographic outcomes after Laterjet procedure performed for recurrent instability, Allain et al[45] found that none of the 58 shoulders had recurrent dislocation at 10-23 years postoperatively, although 6 had apprehension, and 1 had intermittent subluxations. However, only 22 shoulders (38%) demonstrated no evidence of glenohumeral osteoarthritis. Thirty-four shoulders (59%) had radiographic evidence of osteoarthritis, with the majority (25 shoulders) being grade 1 with no detrimental effects on upper extremity function. Two additional patients had severe, grade 4 changes with eccentric wear present, and there was a significant correlation between degree of secondary arthropathy and functional scores on both Constant and Rowe outcome measures. Recurrent dislocation, as opposed to subluxation, was significantly associated with a higher rate of secondary arthrosis, whereas number of shoulder dislocations and time to surgery were not significant predictors.
With failed primary stabilization procedures, patients with revision surgery may also be at heightened risk for instability arthropathy due to attritional bone loss and increasing injury complexity. Tauber et al[46] investigated the reasons for failure after index stabilization surgery, but also assessed for the subsequent development and/or progression of arthritic changes after revision surgery. The authors found no significant difference in the progression of arthritis between bony glenoid augmentation procedures and soft tissue repairs at time of revision surgery, although only 14 of 41 (34%) had no radiographic signs of arthritis at the time of revision surgery. At mean 49 mo follow-up, only 5 shoulders (12%) continued to demonstrate no evidence of glenohumeral arthritis. The authors suggest that once the development of arthritis has been initiated, subsequent surgery for revision anterior stabilization may not mitigate the onset or progression of secondary arthropathy.
The notion that the natural history of dislocation arthropathy is unchanged by surgical stabilization is echoed in a more recent study by Hovelius’ group[47]. At 33-35 years from transfer of the coracoid for shoulder stabilization, 31 shoulders were available for follow-up. Of these shoulder, 39% were normal, 27% had mild OA, 23% moderate and 11% severe. These findings were similar to their previous study (outlined above) on the long term outcome of shoulder dislocations treated non operatively[41]. Interestingly, the majority of patients remained as satisfied with their outcomes at long-term follow-up as they were at 2-4 years from surgery, and only one patient required re-operation for recurrent instability.
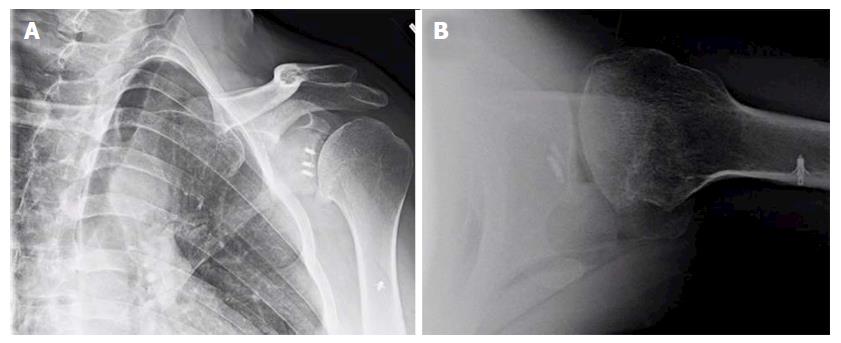
Despite these long-term nature of these studies, most investigations do not control or evaluate for the role of surgical technique or patient-specific factors on the development of dislocation arthropathy[34,41,43,45,47]. It is well established that the overtensioning of the capsular and/or subscapularis, as in the historical non-anatomic procedures, may overconstrain the glenohumeral joint and contribute to premature onset of arthritis with asymmetric, anterior glenoid wear. Additionally, non-absorbable or metallic implants, prominent anchor placement, and/or malpositioned arthroscopic knots can abrade the articular surface of the humeral head, thus increasing the risk for the arthritic change (Figure 1). Most series also emphasize that lateral placement of coracoid bone graft can hasten the development of dislocation arthropathy, or at least significantly increase the rate of secondary chondral damage[45,48]. While some authors theorize that intra-articular coracoid graft placement (i.e., capsule repaired to the lateral vs medial edge of the graft used) may increase the chances of humeral head abrasion[48,49], there is no clear data correlating this technique with clinical or radiographic endpoints.
Figure 1 Anteroposterior (A) and lateral (B) X-rays of a 39-year-old male with dislocation arthropathy status post instability procedure with metal anchors.
CONSERVATIVE TREATMENT
Periarticular injections
Injections for management of shoulder arthropathy are considered a treatment alternative as they represent a less invasive treatment method to surgery. Although there are several types of injections, the efficacy of these injections is not well articulated in the existent literature.
Diagnostic injections may also have utility when clinical examination alone does not yield a clear diagnosis or primary source of pathology. While also offering potential therapeutic value, diagnostic injections can be performed with short acting local anesthetics, particularly xylocaine or lidocaine, into the specific areas of interest, such as the biceps sheath and acromioclavicular joint. Particularly given the association between infusion pain pumps and irreversible chondrolysis[50], longer acting medications (e.g., bupivacaine) should be avoided over shorter acting agents due to greater potential for chondrotoxicity[51-56].
Cortisone injections are also frequently used for the nonoperative treatment of glenohumeral arthritis; however, their efficacy has not been well studied and the majority of scientific evidence is based on limited case series[57-59]. Corticosteroids interrupt the inflammatory cascade and typically result in a transient decrease in secondary pain. Unfortunately, the efficacy and duration of symptomatic relief is not known and often patient-dependent, typically ranging from weeks and months. Therefore, a limitation of cortisone injections is lack of consistent durability. Additional concerns exist regarding the potential systemic side effects and increased risk of infection when performed within 3 mo of an arthroplasty procedure[60].
While used less frequently, injectable non-steroidal anti-inflammatory drugs (NSAIDs), such as Ketorolac, may result in fewer side effects and may have similar efficacy to cortisone[61]. As with cortisone, NSAIDs may also lack effect durability and represent a temporizing treatment option. Further research is required to better ascertain the role and relative efficacy of local NSAID injections in young patients with post-instability arthritis.
Viscosupplementation/hyaulronic acid injections and orthobiologics: Viscosupplementation, while FDA-approved for knee osteoarthritis, is considered off-label for use in the shoulder. A prior meta-analysis performed evaluating the use of hyaulronic acid (HA) injections in the shoulder for a variety of disorders, including osteoarthritis, revealed an absence of evidence for clinically significant improvement[62]. More recently, a subsequent meta-analysis of 8 studies evaluating osteoarthritis of the glenohumeral joint, including two randomized prospective trials, suggested a lack of convincing evidence for the efficacy of HA injections[63-67].
Given the lack of definitive efficacy, reimbursement for HA injections is limited. Theoretically it should behave in the shoulder similar to the knee as both are synovial joints. Recently, scientific support for viscosupplementation in the knee was challenged by guidelines published by the American Academy of Orthopaedic Surgeons (AAOS), making application in general less likely to be supported by third party payers. Nevertheless, its safety profile and ease of administration make it a safe and reasonable alternative to more invasive treatment options.
Further innovations in orthobiologics represent the vanguard of nonoperative treatment for many musculoskeletal conditions, although clinical trials are lacking. Specifically, platelet-rich plasma (PRP) or stem cell injections may have potential as viable treatment option for glenohumeral arthritis, but no literature currently exists to support their use. Recent literature indicates that there may be benefit for PRP injections in the setting of knee arthritis[68]. Biologic alternative injections that modulate cartilage repair processes and regulate inflammatory mediators are a principle area of current research but no definitive treatment options have been developed to date.
Physical therapy
Little to no literature exists regarding the use of physical therapy as a form of treatment for glenohumeral osteoarthritis. A recent clinical practice guideline from the AAOS stated that there was “no evidence for or against” the use of physical therapy or other modalities such as “massage, joint mobilization, joint manipulation, exercise, phonophoresis, iontophoresis, ultrasound, laser, acupuncture, and/or electrical stimulation”[69]. Given the low risks associated and limited alternatives, physical therapy may be a reasonable consideration in patients desiring non-surgical management. However, it may also exacerbate symptoms related to painful, advanced arthritis. If restoration of motion or dynamic control of residual instability are among the primary goals, then a physical therapist or self-directed home exercise program may provide value among available treatment options[70].
Oral medication
A variety of oral analgesics are available for treatment of osteoarthritis including NSAIDs, oral corticosteroids, acetaminophen, topical analgesics, and various non-regulated supplements such as chondroitin sulfate, vitamins, and herbal supplements. Non-narcotic analgesics are routinely recommended for the treatment of pain associated with glenohumeral arthritis, but as with other conservative interventions, there is no specific evidence documenting their effectiveness. Nonethless, these medications are considered a first-line alternative to more invasive interventions, must be balanced with the potential side effects. Specifically, NSAIDs may be contraindicated for patients with known kidney disorders, gastritis or a history of peptic ulcer disease, hypertension, coronary artery diseases, and/or other medical problems that impair drug metabolism.
SURGICAL MANAGEMENT
Treatment decision making
When conservative treatment fails to ameliorate symptoms in patients with instability arthropathy, a multitude of potential surgical options exist. Ultimately, treatment should be decided through a shared decision-making process and tailored to their unique anatomic factors, physiological age, comorbidities, and functional demands. In contrast to patients with osteoarthritis, individuals with early arthropathy due to glenohumeral instability are often younger, more active, and involved in both athletics or intense occupational duties. Accordingly, the potential clinical benefits of a given procedure must be balanced with its efficacy, durability, and impact on future surgical treatment.
Relevant clinical variables such as size or extent of glenohumeral chondral lesion(s), residual instability, concomitant motion loss, and presence of associated pathology.
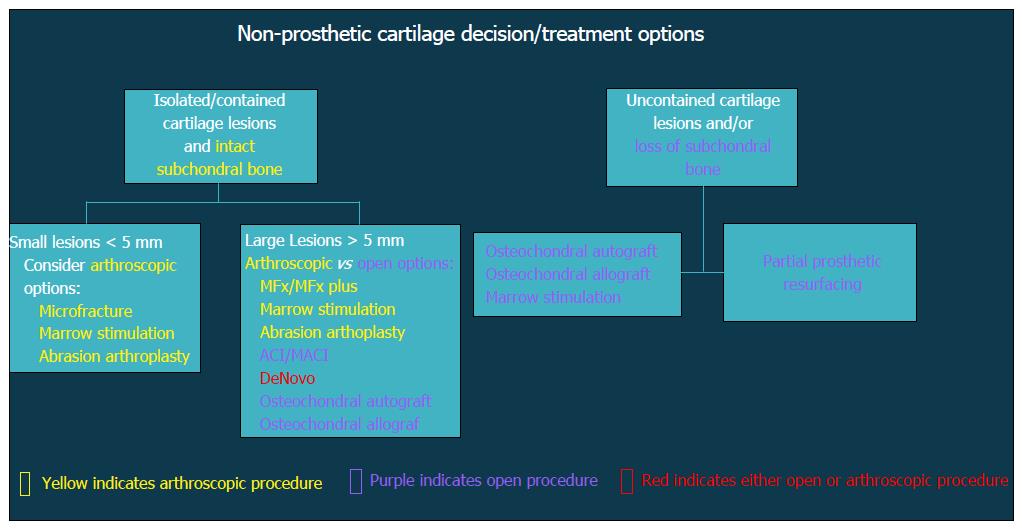
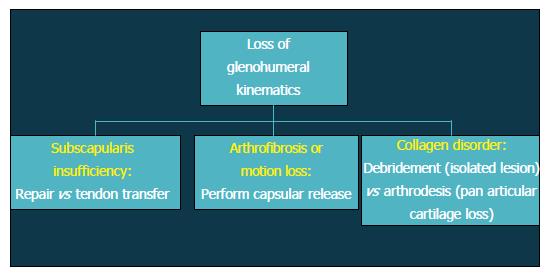
Patients with altered glenohumeral kinematics must be identified and addressed alone or in conjunction with an additional surgical procedure (Figure 2). When post-instability arthropathy has developed due to over-tensioning of either the anterior or posterior capsule, an arthroscopic capsular release may be considered restore normal kinematics should be performed in these patients. Furthermore, loss of appropriate rotator cuff function, particularly the subscapularis, can result in shoulder dysfunction and must also be addressed accordingly. Finally, patients with a collagen disorder (e.g., Ehlers Danlos) represent a particularly difficult population to manage. Given that many of these patients will have had several prior surgeries, reconstructive options will be limited in the setting of post-instability arthropathy, including continuing conservative care, arthroscopic debridement, or potentially, arthrodesis.
Figure 2 Flow-chart demonstrating decision algorithm for non-prosthetic cartilage treatment options.
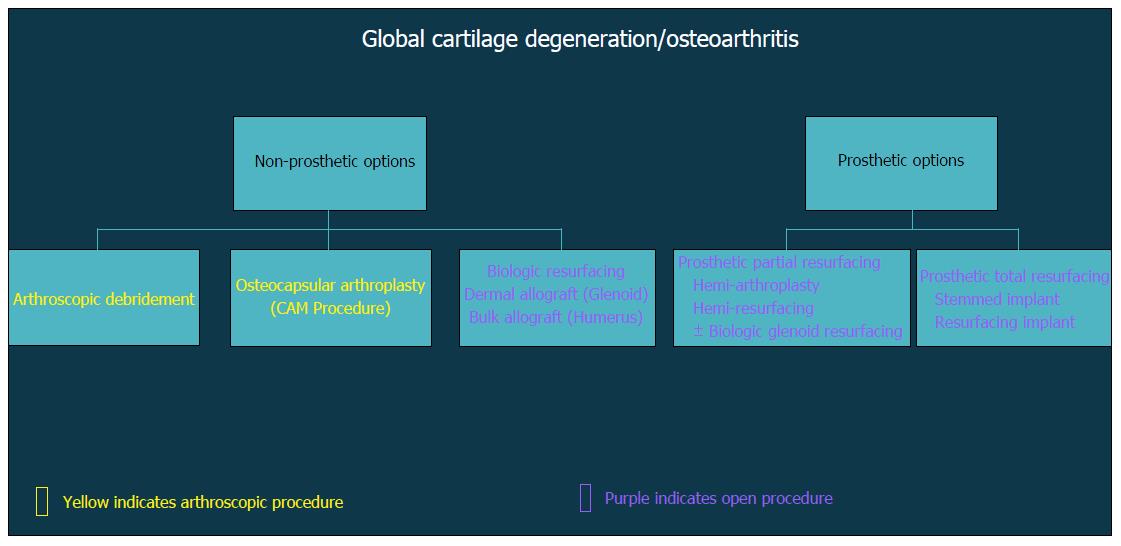
In general, surgical strategies for post-instability arthropathy are contingent on the degree and severity of the associated articular cartilage lesions. Variable arthroscopic and open procedures may be indicated for localized disease and/or focal defects of the glenohumeral joint (Figure 3). However, when the articular involvement is more advanced and secondary arthritis has developed (Figure 4), arthroplasty options may be preferentially considered in the absence of marked rotator cuff disease, significant neurologic deficits, and/or structural glenohumeral bone loss with residual instability.
Figure 3 Flow-chart depicting treatment options after loss of glenohumeral kinematics.
Figure 4 Flow-chart demonstrating decision algorithm for non-prosthetic vs prosthetic treatment options that include arthroscopic and open procedures.
Non-arthroplasty options
Glenohumeral debridement: Arthroscopic debridement of the glenohumeral joint (Figure 5) has been reported as having variable, if not modest success among several case series[71-73]. While most of these studies do not detail the precise surgical interventions, simple removal of symptomatic loose bodies or foreign bodies (e.g., prominent suture material), debridement of hypertrophic synovitis, and/or capsular release may have a positive short-term effect on most patients[74].
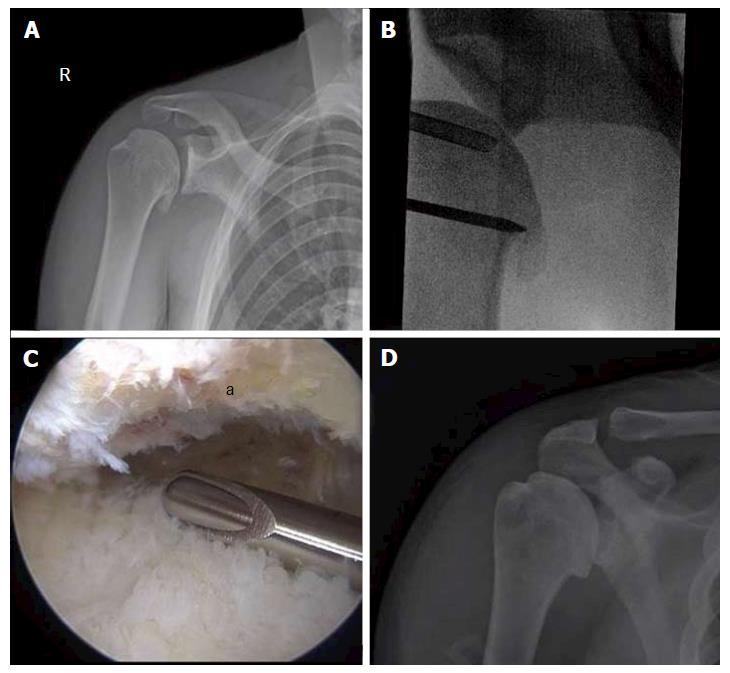
Figure 5 Comprehensive arthroscopy management of glenohumeral arthropathy.
A: Images from a 37-year-old male with instability arthropathy demonstrating preoperative anteroposterior radiograph with large inferior humeral head osteophyte and loss of glenohumeral joint space; B: Intra-operative fluoroscopy localization of extent of inferior humeral head osteophyte; C: Intra-operative arthroscopic image viewing from posterior portal, demonstrating inferior humeral neck (a) status post debridement of osteophyte, the arthroscopic shaver is on the inferior capsule; D: Post-operative anteroposterior radiograph demonstrating debridement of osteophyte and biceps tenodesis with a biocomposite screw.
Adjunctive procedures: The addition of adjunctive procedures has not been extensively evaluated, although treatment of biceps-labral complex, rotator cuff, acromioclavicular, or subacromial pathology may also yield partial, symptomatic relief. Skelley et al[75] revealed worse surgical results when arthroscopic glenohumeral debridement and capsular release was performed strictly without any additional procedures. As a result, the authors suggest that isolated arthroscopic debridement and capsular release when done without any other concomitant procedures may not provide lasting enough benefit to justify its use.
More recently, the Comprehensive Arthroscopic Management (CAM) procedure was described by Millet et al[76] for younger, active patients early glenohumeral arthritis. The CAM procedure differs from previous arthroscopic debridement in that it also features an extensive capsular release, humeral osteoplasty with inferior osteophyte removal, axillary neurolysis, and a biceps tenodesis, when appropriate. Retrospective, short term follow-up of 30 shoulders in 29 patients with an average age of 52 years (range 33 to 68 years) revealed 85% survivorship at 2 years with statistically significant improvement in pain and functional scores. Worse preoperative functional scores and joint space measurements less than 2 mm were more likely experience clinical failure and require subsequent shoulder arthroplasty. By contrast, patients with worse pre-operative motion experienced greater postoperative satisfaction, suggesting that the CAM procedure may be ideally suited for those patients affected by with motion loss due to impinging osteophytes or capsular tightness. In a separate analysis, the authors further evaluated the CAM procedure with a Markov decision model and discovered that arthroscopic management was the preferred strategy for patients younger than 47 years, whereas TSA was optimal for patients over 66 years and both treatment strategies may be considered between 47-66 years[77].
Chondral restoration: Isolated glenohumeral chondral lesions without global or diffuse cartilage involvement may be considered for a restorative procedure. Identification of symptomatic cartilage lesions can be a challenge, as coexistent pathology may be frequently be present and difficult to independently distinguish. As previously mentioned, diagnostic injections may help identify focal areas of pathology masquerading as glenohumeral disease[78].
Microfracture or other marrow stimulation techniques have been employed with clinical success in the knee, although only three small case series have been evaluated its role in the glenohumeral joint[79-81]. The merits of this procedure include that it can be performed arthroscopically without the surgical morbidity associated with an open approach. However, the utility of isolated microfracture is probably limited, and its durability of this procedure is in question due to failure rates approaching 20%[80]. Additionally, some authors have expressed concerns about the potentially harmful effects that of subchondral bone particularly in the glenoid may be harmful[82]. Newer modifications incorporating marrow stimulation with orthobiologics are also emerging, but these techniques have not yet been described in the shoulder[83].
Osteochondral autograft transfer (OATS) or osteochondral allograft transplantation have demonstrated narrow indications in the shoulder, mostly for humeral-based lesions (Figure 6), although the literature is notably limited. Due to the concave geometry of the glenoid vault, centrally located glenoid lesions may be able to accommodate osteochondral transfer, although remains technically challenging[84]. Only one known series has evaluated the surgical outcomes of 8 patients with OATS from the lateral femoral condyle for Outbridge grade IV lesions of the shoulder (7 humeral, 1 glenoid). All patients experienced improvement in both function and pain postoperatively, but two patients experienced mild, persistent shoulder limitations and one patient with donor site knee pain requiring arthroscopic debridement[85]. In a systematic review of 35 patients with osteochondral allografts of the humeral head at mean 57 mo follow-up, Saltzman et al[86] showed significant improvements in range of motion and American Shoulder and Elbow Society scores. Of note, however, 8.7% demonstrated graft necrosis, 26.7% of patients underwent reoperation, and 35.7% developed secondary arthritic changes. The authors also acknowledged that the majority of grafts were derived from frozen allografts, thereby limiting chondrocyte viability vis-à-vis fresh ostechondral allografts.
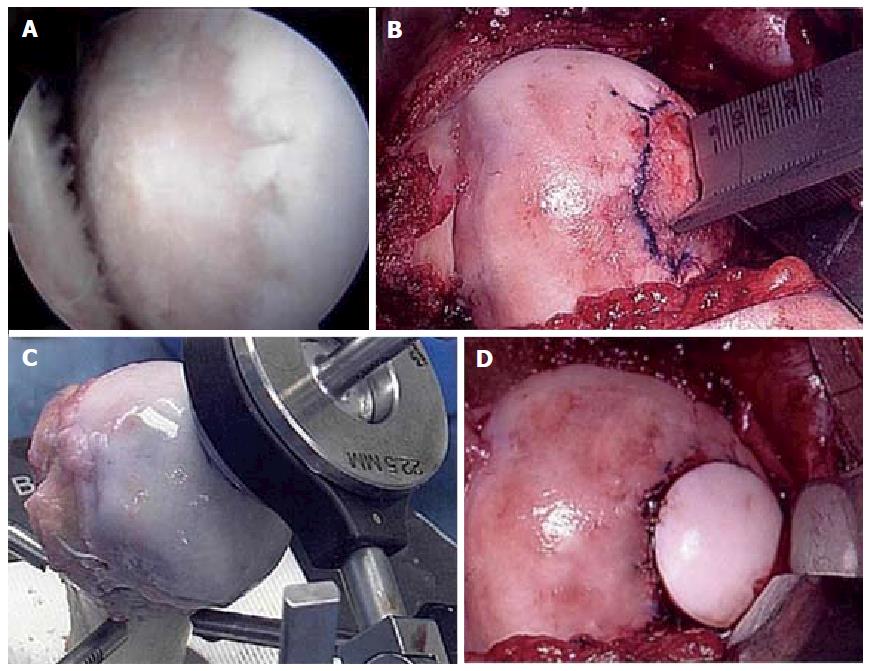
Figure 6 Fresh osteochondral allograft transplantation.
A: Intra-operative arthroscopic image of central humeral articular lesion while viewing from a posterior portal in a 39-year-old patient; B: After an open approach, preparation of the central lesion; C: Harvesting a corresponding osteochondral plug from a size-matched, fresh allograft humerus; D: Status post insertion of the osteochondral plug into the defect.
Arthroplasty options
Arthroplasty options exist for post-instability arthropathy that incorporates a prosthesis of the humeral head, the glenoid, or both. Survivorship as a concept for surgical treatment is the period of time free from revision surgery. Another way to describe survivorship is durability of outcome or durability of patient satisfaction. Recent literature has highlighted the discrepancy of implant survival with patient satisfaction in shoulder arthroplasty in patients under the age of 50 years[87].
Partial resurfacing/biologic resurfacing/hemiarthroplasty: Partial humeral head resurfacing with a prosthesis has been suggested as an option for patients with isolated or unipolar humeral disease. Concomitant pathology as well as prior surgical procedures have been found to decrease outcomes and could be considered a contraindication to this procedure[88]. Soft-tissue interpositional arthroplasty procedures involve humeral head arthroplasty and then securing soft-tissue to cover the arthritic glenoid in an effort to improve upon the outcomes of humeral hemiarthroplasty (HA) alone. Unfortunately, multiple authors have reported unacceptably poor outcomes following this procedure[89-91]. Another option that can be performed with humeral HA is the “ream-and-run” technique popularized by Gilmer et al[92]. This technique provides concentric glenoid reaming with an over-sized reamer with a goal of creating a smooth concavity for articulation. This has been shown by the author to be a viable alternative to TSA in the young patient[93,94], although published series from other institutions are limited[95]. In the previous studies, the ream-and-run technique utilized in the arthritic shoulder in patients 55 years old or less led to a significant improvement in the Simple Shoulder Test as well as minimal medial glenoid erosion.
HA has been promulgated as an option which theoretically would allow patients to pursue more aggressive/demanding activities and avoid the risk of glenoid component loosening. However, concerns for the durability of pain relief is a concern with glenoid wear over time[96].
Total shoulder arthroplasty: Total shoulder arthroplasty (TSA) outperforms HA both functionally and in terms of implant survivorship both in the short term and long-term follow-up[97-100]. Despite initial concerns over the possibility of glenoid loosening, recent studies have shown that patients have a significantly increased return to sports after TSA compared to HA[101,102]. Large meta-analysis of pooled data has also demonstrated that TSA provides greater pain improvement and increased range of motion compared to HA in young patients with glenohumeral arthritis[103]. Certainly, the young patient with arthritis is very difficult to treat with any arthroplasty modality, and outcomes in this population are worse than standard arthroplasty patients[87,104]. Concern over a future revision procedure can influence the surgeon’s choice of the initial arthroplasty procedure, as surgeons worry about medializing glenoid wear with a HA or aseptic glenoid loosening with a TSA. There remains a higher risk of revision surgery in the population of patients under 60 receiving a HA compared to a TSA[104].
TSA is by no means an operation without complications and adverse effects in this young patient population. In our own experience of treating a young, active military population with instability arthropathy, we have found a high rate of complications to include component failure, neurologic injury, adhesive capsulitis and venous thrombosis. In our series of 26 TSAs in a predominantly male cohort with a mean age of 45.8 years (range, 35-54 years), we experienced 9 patients with 12 complications (46.2%) leading to a 23.1% reoperation rate at an average of 3.5 years follow-up. Nine patients (37.5%) were unable to continue their high-demand activities and underwent a medical discharge for persistent shoulder disability[105].
CONCLUSION
So-called instability (or dislocation) arthropathy may develop in high-risk patients with a history of recurrent glenohumeral instability, both with and without surgical stabilization. The incidence and rates of arthritic progression may vary widely, with radiographic changes present in up to two out of three patients after primary Bankart repair. However, the presence of secondary arthrosis does not predict poor patient-reported function. When oral medication, periarticular injections, and physical therapy have failed, surgical options will depend on patient-specific factors, anticipated upper extremity demands, size and extent of articular involvement, and other anatomic factors. A variety of arthroscopic and open non-arthroplasty procedures are available as temporizing measures, and data regarding the efficacy of chondral restoration options are currently limited. Total shoulder arthroplasty remains the most reliable option for the treatment of post-instability arthropathy, although the clinical outcomes, wear characteristics, and implant survivorship remains a concern among active, young patients. Further investigations are warranted to evaluate the comparative efficacy of management options in this challenging, young patient demographic with early arthritis.