Published online Dec 18, 2017. doi: 10.5312/wjo.v8.i12.853
Peer-review started: June 19, 2017
First decision: August 4, 2017
Revised: September 23, 2017
Accepted: October 17, 2017
Article in press: October 17, 2017
Published online: December 18, 2017
Processing time: 187 Days and 1.3 Hours
Cartilage disorders, including focal cartilage lesions, are among the most common clinical problems in orthopedic practice. Left untreated, large focal lesions may result in progression to osteoarthritis, with tremendous impact on the quality of life of affected individuals. Current management strategies have shown only a modest degree of success, while several upcoming interventions signify better outcomes in the future. Among these, stem cell therapies have been suggested as a promising new era for cartilage disorders. Certain characteristics of the stem cells, such as their potential to differentiate but also to support healing made them a fruitful candidate for lesions in cartilage, a tissue with poor healing capacity. The aim of this editorial is to provide an update on the recent advancements in the field of stem cell therapy for the management of focal cartilage defects. Our goal is to present recent basic science advances and to present the potential of the use of stem cells in novel clinical interventions towards enhancement of the treatment armamentarium for cartilage lesions. Furthermore, we highlight some thoughts for the future of cartilage regeneration and repair and to explore future perspectives for the next steps in the field.
Core tip: An increasing interest in stem cell application for cartilage defect repair is recently expressed, as a consequence of advancements demonstrating the critical function of mesenchymal stem cells as a potential alternative cell source for cartilage repair, as well as of recent clinical data exhibiting the effectiveness of these management strategies. Future research will determine the role of combining stem cells, primary chondrocytes, and signaling molecules towards cartilage regeneration.
- Citation: Paschos NK, Sennett ML. Update on mesenchymal stem cell therapies for cartilage disorders. World J Orthop 2017; 8(12): 853-860
- URL: https://www.wjgnet.com/2218-5836/full/v8/i12/853.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i12.853
Cartilage lesions are among the most often recognized pathologies in young adults undergoing arthroscopy. Approximately 60% of the patients treated arthroscopically for any reason had at least one chondral lesion at their knee[1]. Due to the relatively young age of these patients (mean age ranged from 37 to 43 years old) and because the majority of lesions has been graded as II or III, it was suggested that impending osteoarthritis would be inevitable without treatment in these patients[1].
Effective management of these lesions can be extremely challenging, thus, creating a burden for both patients and physicians. With conservative treatment being unsuccessful, several surgical interventions have been proposed for focal cartilage lesions, including microfracture, autologous chondrocyte implantation [with either periosteum (ACI) or matrix-assisted (MACI)], osteochondral autograft or allograft transplantation, and particulated autologous or allogeneic articular cartilage[2-4]. Despite the plethora of available techniques, the effectiveness of these in terms of preventing or delaying the development of osteoarthritis is questionable.
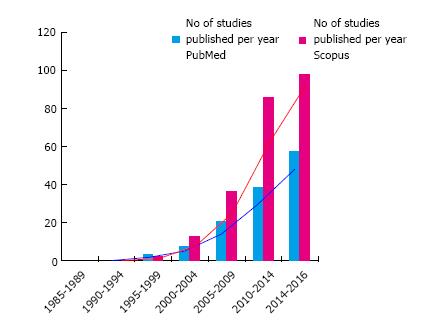
In the armamentarium against cartilage defects, stem cell-based interventions have gradually taken a more prominent role. As shown in Figure 1, there is an exponential growth in the number of published studies that deal with stem cell use in cartilage disorders (Figure 1). Mesenchymal stem cells (MSCs) are multipotent cells that have been isolated from a variety of tissue types, including bone marrow, synovium, and adipose tissue[5-7]. MSCs have been observed to undergo differentiation down osteogenic, chondrogenic, myogenic, and tenogenic lineages, making them of great importance to the orthopedic and tissue engineering communities.
The regenerative potential of marrow-derived elements was determined in the 1960’s, long before the discovery of MSCs, with the observation that superficial chondral defects exhibit limited healing potential, while defects penetrating the subchondral plate result in fibrocartilaginous ingrowth[8]. This observation ultimately led to the development of marrow stimulating techniques for chondral repair such as microfracture[9]. Penetrating the subchondral bone allows bleeding, and formation of a clot containing MSCs and various other bone marrow elements to form. While this technique has been shown to produce improved patient-reported outcomes at short time-points, the repair tissue is fibrocartilaginous in nature, and exhibits poor mechanical properties when compared to native cartilage. At longer time-points, this tissue may become fibrillated and require further management.
The premise behind the use of MSCs for cartilage repair was supported on two characteristics of the stem cells. These multipotent cells, under the appropriate environmental conditions, could differentiate into chondrocytes and repair the chondral defect[10]. Differentiation of both adipose-derived and bone marrow MSCs towards chondrocytes can be enhanced with the use of growth factors[11,12]. Thus, the ideal perspective would be to promote MSC differentiation towards chondrocytes and to utilize the healing response with new chondrocyte-like cells.
Another equally important ability has been revealed for MSCs, i.e., their capacity to actively interact with primary cells and extracellular matrix via continuous feedback mechanisms[13]. As a consequence, stem cells could act as advocators of the existing chondrocytes via their anti-inflammatory and immunomodulatory effect. In addition, this interaction can formulate an appropriate response towards differentiation, proliferation or secretion of supportive molecules that allows stem cells to be actively engaged in the cartilage healing response[14].
MSC transplantation is a technique that offers several potential benefits over other cartilage repair methods. MSCs may be isolated from adipose tissue, which is much less invasive than the harvest of chondrocytes for ACI. MSCs are also phenotypically stable during cell culture expansion, while chondrocytes undergo dedifferentiation. Furthermore, MSCs may be isolated in greater quantities than chondrocytes, allowing for the possibility of single-step procedures, which could decrease economic burden for the patient. Currently, clinical studies involving MSC transplantation are limited.
The aim of this editorial is to summarize the recent work in basic science and clinical interventions in an attempt to provide an update on the recent advances in stem cell treatment options for cartilage disorders. Furthermore, some thoughts for the future of cartilage regeneration and repair via stem cell application will be discussed. Due to the plethora of studies in the literature, the main focus of this study would be on clinical interventions for focal cartilage defects.
A better understanding of the pathogenetic phenomena that initiate the process of cartilage degeneration is of paramount importance, as this would allow the formulation of therapeutic approaches that aim to prevent osteoarthritis at its infancy. TGF-beta1 is proven to be a key factor for cartilage homeostasis, and its function is utilized in tissue engineering for chondrocyte proliferation and enhancement of functional properties[15]. Recently, an interesting aspect of the role of transforming growth factor-beta (TGF-beta) in osteoarthritis has been proposed. Specifically, it was found that mechanical load increases in ACL-deficient knees result in recruitment of additional osteoclasts at the subchondral bone. This leads to activation of TGF-beta1 that assists in recruitment of MSCs that causes atypical subchondral bone formation, a potential initial step in the osteoarthritic pathogenetic cascade[16].
Cell to cell and cell to extracellular matrix interactions are important in the mechanical environment of the joint for suitable response to injury or degeneration. Stem cells appear to play a major role in these interactions with numerous applications in both therapeutic strategies but also in the design of tissue-engineered scaffolds. A recent study demonstrated that interactions of N-cadherin in MSCs could modify the perception of the mechanical properties of the microenvironment, and thus, generate corresponding response of stem cells towards proliferation and differentiation[17]. Furthermore, mechanical stimuli including tension stimulation can result in significant improvement of the functional properties of tissue-engineered human cartilage that could be used for replacing chondral defects[18].
The initial reports for the use of autologous bone marrow-derived MSCs for focal cartilage defects used marrow obtained from the iliac crest and, via a subsequent surgery using an open approach, the stem cells were injected into the defect with a periosteal cover sutured on top of the lesion. In the first study, two patients with patellar cartilage defects underwent treatment with MSCs. Significant improvement in pain and functional outcome was maintained for at least 4 years after surgery[19]. In a case report involving a patient with a defect of the medial femoral condyle, autologous transplantation of bone marrow derived MSCs was also associated with improved outcome. Second-look arthroscopy revealed full coverage of the lesion 7 mo after surgery with hyaline-like cartilage as well as significant improvement in clinical symptoms. However, MRI showed irregularities of the repair tissue[20]. The favorable outcome of autologous bone-marrow derived MSCs for cartilage defects at the patellofemoral joint was confirmed by another report of three cases demonstrating clinical improvement in kissing lesions which are usually more challenging to treat[21].
In recent years, as arthroscopic techniques have evolved, all-inside arthroscopic techniques for cartilage repair using MSCs developed with favorable outcome. In a case report, after microfracture, a collagen membrane that was immersed in bone marrow derived MSCs were secured with fibrin glue. MRI imaging at 12 mo demonstrated filling of the cartilage defect, while the patient remained asymptomatic until at least 24 mo[22]. In addition, single stage arthroscopic techniques were also described. In a prospective study, 30 patients with cartilage defects at the knee joint were treated using a combination of microfracture and subsequent application of a mixture gel containing bone marrow aspirated cells, hyaluronic acid (HA), and fibrin. A significant improvement was recorded with an improvement in Lysholm score from 50.8 to 80.1 and subjective IKDC from 39 to 83 preoperatively and postoperatively, respectively[23]. An analogous technique where bone marrow-derived cells were mixed with collagen powder or HA membrane and platelet gel was used for osteochondral defects of the talus[24]. In these patients, a significant improvement in the AOFAS score was recorded with an increase from 64.4 to 91.4, preoperatively to postoperatively, respectively[24]. Furthermore, using bone marrow cells aspirated from the iliac crest, isolated via centrifugation and embedded in a HA membrane, 20 patients with chondral defects at their knee experienced significant improvement in pain and function[25]. Both MRI and histological analysis confirmed the presence of regenerated cartilaginous tissue[25]. Nine patients with focal cartilage defects were treated with microfracture supplemented with coverage of a polyglycolic acid/hyaluronan matrix membrane with autologous bone marrow concentrate cells. A significant improvement in IKDC, Lysholm and Tegner scores was reported at 22 mo follow up[26]. The recent advancements in the field have allowed for the successful combination of autologous stem cells with therapeutic interventions already employed in cartilage repair.
When evaluating studies that compared outcomes with or without stem cell augmentation, promising findings are reported. The first attempt to compare clinical outcome between patients treated with autologous chondrocyte implantation and patients treated with autologous bone marrow-derived MSCs, showed that bone marrow MSCs could be equally effective as chondrocytes for focal cartilage lesions. Indeed, both groups demonstrated significant improvement postoperatively with no statistical difference between the groups in Lysholm IKDC and Tegner activity scores[27]. In a comparative study for patellofemoral chondral lesions, 18 patients were treated with bone marrow cells embedded in a biodegradable HA-based scaffold while 19 patients were treated with matrix-induced autologous chondrocyte implantation (MACI). At 2 year follow up, both groups showed a significant improvement in clinical outcome and pain scores, but bone marrow aspirate concentrate treated patients had significantly better subjective IKDC score[28]. In another study that combined therapeutic approaches, two different techniques using marrow derived MSCs were compared. One combined microfracture with subsequent injection of MSCs with HA. In the other group, MSCs were seeded in a periosteal patch that was sutured over the cartilage defect. Both groups showed improvement postoperatively with a trend towards better outcome for the MSC/HA group[29]. Combination of intra-articular injections of autologous bone marrow-derived MSCs and HA 3 wk after microfracture and medial opening-wedge high tibial osteotomy in 28 patients resulted in approximately 7.6 added improvement for IKDC and Lysholm scores compared to HA injections alone. In this randomized controlled trial an improvement in Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score at 1 year was also seen[30]. Further improvement in the current armamentarium against cartilage defects has leads to technical adaptations that explore the potential use of autologous MSCs instead of primary chondrocytes. The above findings suggest that the use of MSCs result in analogous - if not better - outcomes.
Several carriers have also been successfully used recently for autologous MSCs. In a case series, platelet rich fibrin glue was successfully used as a cell carrier for autologous bone marrow derived MSCs that were subsequently sutured with a periosteal flap. All five patients experienced improvement in clinical scores, while second look arthroscopy performed in two patients demonstrated good integration and healing of the repair area[31]. A type I collagen scaffold was used as a carrier for autologous MSCs in two patients with periosteal graft sutured on top. Both KOOS and IKDC score improved significantly postoperatively at 30 mo follow up[32]. In a pilot clinical study application of a poly-ethylene glycol diacrylate (PEGDA) hydrogel biomaterial after microfracture in 15 patients was compared with the outcome of microfracture alone in three patients. It was suggested that pain was improved in the biomaterial group, however, additional data are necessary before making safe conclusions[33].
Finally, a recent randomized trial used allogeneic MSCs for patients with osteoarthritis. It was suggested that allogeneic bone marrow MScs resulted in improvement in pain, quality of life and cartilage quality as evaluated by MRI compared to HA[34]. Based on these promising data, use of allogeneic bone marrow MSCs may be a viable solution for focal cartilage lesions in some patients.
Since their identification as a potential source of cartilage matrix molecules, adipose derived MSCs (ADSCs) have become a valuable source in several models for cartilage regeneration[7,35]. A primary advantage of ADSCs is their abundance and the relative ease of obtaining cells from the patient[14,36]. Another advantage of ADSCs is that they have been demonstrated to have a prominent chondro-inductive effect. As shown in an in vitro co-culture model, the combination of articular chondrocytes with ADSCs resulted in a two-fold increase in GAG content and increased collagen II gene expression[37].
Expression of pro-chondrogenic genes in ADSCs via adenovirus-mediated gene transfer of TGF beta2 could lead to ectopic neocartilage formation, while recently a combined expression of IGF-1 and FGF-2 in ADSCs demonstrated a synergistic effect towards enhanced chondrogenic differentiation[38,39]. Towards this direction, poly lactic-co-glycolic acid) (PLGA) nanoparticles that deliver a specific plasmid of bone morphogenetic protein 4 (BMP-4) into rabbit ADSCs significantly enhanced chondrogenesis and appear to benefit cartilage repair in vivo[40].
Concerns about the degree of stemness of ADSCs have been raised since ADSCs demonstrate an inferior chondrogenic potential when compared to BMSCs[41]. Indeed, ADSCs may need specific conditions to chondro-differentiate and this process may require a prior step of pre-differentiation[42]. However, the immunosuppressive effect and the chondroprotective role of ADSCs should not be underestimated. In a mice model of osteoarthritis injection of ADSCs resulted in inhibition of cartilage destruction and synovial thickening[43]. Similarly, in a rabbit model, intra-articular injections of ADSCs delayed the progression of osteoarthritis and meniscus damage via inhibition of metalloproteinase and TNFa expression[44]. In one study performed in a rat OA model, fluorescein-labeled ADSCs were injected into an arthritic knee joint and were detectable via non-invasive bioluminescence imaging for up to 10 wk post-injection. Histological analysis confirmed that injected cells were present and proliferating in synovial, meniscal, and articular cartilage tissues. Furthermore, ADSC-treated rats showed a significantly increased O’Driscoll histological score, suggesting a chondro-protective or regenerative effect from these cells[45]. Additional research is needed to better understand the protective or reparative mechanism of ADSCs. Recently, a similar role in chondroprotection has been demonstrated for synovial derived MSCs[46].
Interest in ADSCs increased considerably after a case series reported that patients that received intra-articular injection of ADSCs had reduced pain and improved knee function[47,48]. In humans, ADSCs have been used in intra-articular injections since 2011. To date, eleven studies have been recorded. ADSCs have been isolated either from the abdominal area, buttocks, or infrapatellar fat pad. In all studies, ADSC injection showed improved clinical outcome in terms of reduced pain and improvement in functional scores. Data obtained from MRI at 6 mo showed promising data suggesting cartilage regeneration[47]. Also, macroscopic appearance from second-look arthroscopy showed hyaline-like cartilage with smooth surface[47]. Finally, histology in a specimen obtained also confirmed characteristics of hyaline cartilage. Unfortunately, there are some disadvantages in these clinical studies. First, most are case reports without a control group. Another limitation is the fact that in most cases an additional therapeutic intervention was simultaneously performed[49]. High-level of evidence studies are necessary in order to confirm the promising results of the clinical cases described.
In a randomized single blinded study evaluating only 14 patients synovial mesenchymal cells were used in a matrix collagen membrane and were compared with matrix autologous chondrocyte implantation[50]. Functional outcomes were similar between the two groups, while mesenchymal cells were reported to have better outcome in certain outcomes, such as KOOS score[50].
Synovial MSCs, cultured in autologous human serum, arthroscopically implanted in 10 patients with single cartilage defect showed promising results[51]. Specifically, synovial cells were harvested and were cultured in autologous human serum[51]. At 3-year follow up, improved MRI features and Lysholm score were reported, however no benefit was seen in Tegner activity level[51].
Another type of stem cells used in clinical studies is the autologous peripheral blood progenitor cells (PBPCs). Specifically, one week after arthroscopic drilling of a cartilage defect, 8 mL of PBPCs were injected at the knee together with 2 mL of HA[52]. Articular cartilage biopsies in five patients showed the presence of hyaline cartilage[52]. Clinical outcomes from the same group have been reported in a randomized study that compared the outcomes in patients that underwent subchondral drilling and subsequent HA injections with and without PBPCs[53]. It was shown that the presence of PBPCs did not result in better clinical outcome (IKDC scores of 74.8 vs 71.1 for PBPC and no PBPC group, respectively). However, PBPC group exhibited better histologic and MRI scores compared to control[53].
Other types of stem cells have been proposed as potential candidates for cartilage repair treatment. In a recent animal model, weekly injections of embryonic MSC-derived exosomes demonstrated restoration of osteochondral defects and presence of hyaline cartilage[54]. Chondrocytes have also been demonstrated to adopt stem cell-like characteristics when cultured under specific biochemical and mechanical conditions[55]. In this study, dedifferentiated chondrocytes were found to be highly proliferative and chondrogenic, making them an attractive source for cartilage repair. Additional research should identify additional progenitor cell populations that may be used in cartilage repair and should focus in further characterizing their properties.
Autologous MSCs have demonstrated potential in the repair of cartilage defects, and that lead to an increased interest has been expressed for the use of stem cells in cartilage lesions (Figure 1). However, an analysis of these studies in terms of level of evidence shows that less than 10 studies are randomized and approximately 15 studies have a control group. Additional randomized controlled clinical studies are necessary to determine whether isolated MSCs or ADSCs offer any benefits over traditional marrow stimulating techniques such as microfracture. Furthermore, long-term follow-up with MRI or second-look arthroscopy will be critical for determining the durability of repair tissue generated by MSC implantation, particularly before utilizing these techniques in younger, active patients.
The use of MSCs is not without clinical limitations or disadvantages. Cost of stem cell preparation represents an unknown factor that needs to be included in the equation of their application. Detailed cost effectiveness studies are needed to clarify the potential benefit for their use in comparison to the current strategies for cartilage repair. Moreover, the use of autologous MSCs has a certain amount of donor morbidity. Iliac crest marrow aspiration has been associated with chronic pain, dysesthesia, potential wound drainage and scaring[56]. These complications are relatively minor and occur rarely, but it is important to establish a better understanding of the potential problems that are associated with stem cell use, as these techniques become more popular. For adipose and synovial derived MSCs donor morbidity is significantly less, but future studies should focus on potential withdraws for their use as well. Finally, the use of allogeneic MSCs in co-culture systems could offer a potential solution, but again, additional research should determine whether the potential risk of immune response related complications outweighs their benefits[14].
From a basic science perspective, the mechanisms underlying chondrogenic differentiation of MSCs and subsequent matrix production require elucidation. As it stands, the quality of repair tissue generated by marrow stimulation is known to be mechanically inferior to native articular cartilage. It may be the case that disruption to the subchondral plate contributes to this phenomenon, and implantation of autologous MSC or ADSC could circumvent this issue.
The success of autologous MSC implantation is dependent on determining their benefit over simpler techniques such as microfracture. Studies will also be necessary to determine which delivery method is most appropriate. An appealing aspect of MSC implantation is that it has the potential to be performed in a single surgical procedure. However, MSCs can also be isolated at an earlier time point and pre-differentiated prior to implantation. Studies comparing the efficacy of the multitude of cell delivery techniques are necessary to determine a standard of care.
A lot of interest has been focusing in the filed of lubricin expression from MSCs[57,58]. This potential may indicate that tissue engineering of cartilage from stem cell sources could ensure the presence of lubricin in the superficial cartilage[59].
Finally, effective application of stem cells appears to be linked with the presence of primary cells, and the interactions between primary chondrocytes and MSCs may worth to be examined further. This is an attractive field that may attract a lot of interest since recent work have shown that endogenous stem cells may have the capacity for cartilage repair and regeneration[55,60]. Additional research is also necessary to incorporate the advances in the field of tissue engineering and explore the potential of capitalizing the benefit from combining successful approaches from basic science to clinical practice.
Over the recent years, a growing number of studies arise in the field of MSCs use in cartilage repair, building a progressively stronger foundation for their clinical applications. With the assistance of basic science, a better understanding of their exact role in cartilage physiology is established. New studies initiate to demonstrate the critical role of MSCs in the initial steps of cartilage degeneration, opening a new horizon full of possible alternative methods to address the complicated problem of cartilage repair and regeneration. Clinical work has shared valuable data that confirm their effectiveness as an alternative cell source for cartilage repair. More importantly, recent clinical findings revealed the advantages and disadvantages of each type of MSCs signifying the importance of combining chondrocytes with different types of stem cells. The effective combination of different treatment approaches showed that careful selection of the treatment plan should be based on the characteristics of the patient.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cui Q, Musumeci G, Sakkas LI, Vynios D S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Paschos NK, Lim N, Hu JC, Athanasiou KA. Functional properties of native and tissue-engineered cartilage toward understanding the pathogenesis of chondral lesions at the knee: A bovine cadaveric study. J Orthop Res. 2017;35:2452-2464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Ambra LF, de Girolamo L, Mosier B, Gomoll AH. Review: Interventions for Cartilage Disease: Current State-of-the-Art and Emerging Technologies. Arthritis Rheumatol. 2017;69:1363-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 876] [Article Influence: 87.6] [Reference Citation Analysis (0)] |
| 4. | Paschos NK. Recent advances and future directions in the management of knee osteoarthritis: Can biological joint reconstruction replace joint arthroplasty and when? World J Orthop. 2015;6:655-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15195] [Article Influence: 584.4] [Reference Citation Analysis (0)] |
| 7. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5012] [Article Influence: 217.9] [Reference Citation Analysis (0)] |
| 8. | Campbell CJ. The healing of cartilage defects. Clin Orthop Relat Res. 1969;64:45-63. [PubMed] |
| 9. | Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;S362-S369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 823] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 10. | Caplan AI, Elyaderani M, Mochizuki Y, Wakitani S, Goldberg VM. Principles of cartilage repair and regeneration. Clin Orthop Relat Res. 1997;254-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 253] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 637] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 12. | Guilak F, Estes BT, Diekman BO, Moutos FT, Gimble JM. 2010 Nicolas Andry Award: Multipotent adult stem cells from adipose tissue for musculoskeletal tissue engineering. Clin Orthop Relat Res. 2010;468:2530-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2126] [Cited by in RCA: 2224] [Article Influence: 117.1] [Reference Citation Analysis (0)] |
| 14. | Paschos NK, Brown WE, Eswaramoorthy R, Hu JC, Athanasiou KA. Advances in tissue engineering through stem cell-based co-culture. J Tissue Eng Regen Med. 2015;9:488-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 15. | Kwon H, Paschos NK, Hu JC, Athanasiou K. Articular cartilage tissue engineering: the role of signaling molecules. Cell Mol Life Sci. 2016;73:1173-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, Askin FB, Frassica FJ, Chang W, Yao J. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 598] [Cited by in RCA: 760] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 17. | Cosgrove BD, Mui KL, Driscoll TP, Caliari SR, Mehta KD, Assoian RK, Burdick JA, Mauck RL. N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat Mater. 2016;15:1297-1306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 252] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 18. | Lee JK, Huwe LW, Paschos N, Aryaei A, Gegg CA, Hu JC, Athanasiou KA. Tension stimulation drives tissue formation in scaffold-free systems. Nat Mater. 2017;16:864-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004;13:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 266] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 20. | Kuroda R, Ishida K, Matsumoto T, Akisue T, Fujioka H, Mizuno K, Ohgushi H, Wakitani S, Kurosaka M. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 276] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 21. | Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 246] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 22. | Gigante A, Cecconi S, Calcagno S, Busilacchi A, Enea D. Arthroscopic knee cartilage repair with covered microfracture and bone marrow concentrate. Arthrosc Tech. 2012;1:e175-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Shetty AA, Kim SJ, Shetty V, Stelzeneder D, Shetty N, Bilagi Praveen, Lee H-J. Autologous bone-marrow mesenchymal cell induced chondrogenesis: Single-stage arthroscopic cartilage repair. Tissue Eng Regen M. 2014;11:247-253. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res. 2009;467:3307-3320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 211] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 25. | Buda R, Vannini F, Cavallo M, Grigolo B, Cenacchi A, Giannini S. Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. J Bone Joint Surg Am. 2010;92 Suppl 2:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 26. | Enea D, Cecconi S, Calcagno S, Busilacchi A, Manzotti S, Kaps C, Gigante A. Single-stage cartilage repair in the knee with microfracture covered with a resorbable polymer-based matrix and autologous bone marrow concentrate. Knee. 2013;20:562-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38:1110-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 394] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 28. | Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-Induced Autologous Chondrocyte Implantation versus Multipotent Stem Cells for the Treatment of Large Patellofemoral Chondral Lesions: A Nonrandomized Prospective Trial. Cartilage. 2015;6:82-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 29. | Lee KB, Wang VT, Chan YH, Hui JH. A novel, minimally-invasive technique of cartilage repair in the human knee using arthroscopic microfracture and injections of mesenchymal stem cells and hyaluronic acid--a prospective comparative study on safety and short-term efficacy. Ann Acad Med Singapore. 2012;41:511-517. [PubMed] |
| 30. | Wong KL, Lee KB, Tai BC, Law P, Lee EH, Hui JH. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy. 2013;29:2020-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 278] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 31. | Haleem AM, Singergy AA, Sabry D, Atta HM, Rashed LA, Chu CR, El Shewy MT, Azzam A, Abdel Aziz MT. The Clinical Use of Human Culture-Expanded Autologous Bone Marrow Mesenchymal Stem Cells Transplanted on Platelet-Rich Fibrin Glue in the Treatment of Articular Cartilage Defects: A Pilot Study and Preliminary Results. Cartilage. 2010;1:253-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 32. | Kasemkijwattana C, Hongeng S, Kesprayura S, Rungsinaporn V, Chaipinyo K, Chansiri K. Autologous bone marrow mesenchymal stem cells implantation for cartilage defects: two cases report. J Med Assoc Thai. 2011;94:395-400. [PubMed] |
| 33. | Sharma B, Fermanian S, Gibson M, Unterman S, Herzka DA, Cascio B, Coburn J, Hui AY, Marcus N, Gold GE. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci Transl Med. 2013;5:167ra6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 232] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 34. | Vega A, Martín-Ferrero MA, Del Canto F, Alberca M, García V, Munar A, Orozco L, Soler R, Fuertes JJ, Huguet M. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation. 2015;99:1681-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 448] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 35. | Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;290:763-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 706] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 36. | Schäffler A, Büchler C. Concise review: adipose tissue-derived stromal cells--basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 768] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 37. | Acharya C, Adesida A, Zajac P, Mumme M, Riesle J, Martin I, Barbero A. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J Cell Physiol. 2012;227:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 38. | Jin Xb, Sun Ys, Zhang K, Wang J, Shi Tp, Ju Xd, Lou Sq. Ectopic neocartilage formation from predifferentiated human adipose derived stem cells induced by adenoviral-mediated transfer of hTGF beta2. Biomaterials. 2007;28:2994-3003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Garza-Veloz I, Romero-Diaz VJ, Martinez-Fierro ML, Marino-Martinez IA, Gonzalez-Rodriguez M, Martinez-Rodriguez HG, Espinoza-Juarez MA, Bernal-Garza DA, Ortiz-Lopez R, Rojas-Martinez A. Analyses of chondrogenic induction of adipose mesenchymal stem cells by combined co-stimulation mediated by adenoviral gene transfer. Arthritis Res Ther. 2013;15:R80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Shi J, Zhang X, Zhu J, Pi Y, Hu X, Zhou C, Ao Y. Nanoparticle delivery of the bone morphogenetic protein 4 gene to adipose-derived stem cells promotes articular cartilage repair in vitro and in vivo. Arthroscopy. 2013;29:2001-2011.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage. 2005;13:845-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 627] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 42. | Ruetze M, Richter W. Adipose-derived stromal cells for osteoarticular repair: trophic function versus stem cell activity. Expert Rev Mol Med. 2014;16:e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | ter Huurne M, Schelbergen R, Blattes R, Blom A, de Munter W, Grevers LC, Jeanson J, Noël D, Casteilla L, Jorgensen C. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012;64:3604-3613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 273] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 44. | Desando G, Cavallo C, Sartoni F, Martini L, Parrilli A, Veronesi F, Fini M, Giardino R, Facchini A, Grigolo B. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Res Ther. 2013;15:R22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 45. | Li M, Luo X, Lv X, Liu V, Zhao G, Zhang X, Cao W, Wang R, Wang W. In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model. Stem Cell Res Ther. 2016;7:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 46. | Mak J, Jablonski CL, Leonard CA, Dunn JF, Raharjo E, Matyas JR, Biernaskie J, Krawetz RJ. Intra-articular injection of synovial mesenchymal stem cells improves cartilage repair in a mouse injury model. Sci Rep. 2016;6:23076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 47. | Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32:1254-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 644] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 48. | Koh YG, Jo SB, Kwon OR, Suh DS, Lee SW, Park SH, Choi YJ. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 258] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 49. | Koh YG, Choi YJ, Kwon SK, Kim YS, Yeo JE. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2015;23:1308-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 50. | Akgun I, Unlu MC, Erdal OA, Ogut T, Erturk M, Ovali E, Kantarci F, Caliskan G, Akgun Y. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: a 2-year randomized study. Arch Orthop Trauma Surg. 2015;135:251-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 51. | Sekiya I, Muneta T, Horie M, Koga H. Arthroscopic Transplantation of Synovial Stem Cells Improves Clinical Outcomes in Knees With Cartilage Defects. Clin Orthop Relat Res. 2015;473:2316-2326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 52. | Saw KY, Anz A, Merican S, Tay YG, Ragavanaidu K, Jee CS, McGuire DA. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. Arthroscopy. 2011;27:493-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 53. | Saw KY, Anz A, Siew-Yoke Jee C, Merican S, Ching-Soong Ng R, Roohi SA, Ragavanaidu K. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29:684-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 54. | Zhang S, Chu WC, Lai RC, Lim SK, Hui JH, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24:2135-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 488] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 55. | Jiang Y, Cai Y, Zhang W, Yin Z, Hu C, Tong T, Lu P, Zhang S, Neculai D, Tuan RS. Human Cartilage-Derived Progenitor Cells From Committed Chondrocytes for Efficient Cartilage Repair and Regeneration. Stem Cells Transl Med. 2016;5:733-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 56. | Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002;84-A:454-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 664] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 57. | Musumeci G, Lo Furno D, Loreto C, Giuffrida R, Caggia S, Leonardi R, Cardile V. Mesenchymal stem cells from adipose tissue which have been differentiated into chondrocytes in three-dimensional culture express lubricin. Exp Biol Med (Maywood). 2011;236:1333-1341. [PubMed] [DOI] [Full Text] |
| 58. | Nakagawa Y, Muneta T, Otabe K, Ozeki N, Mizuno M, Udo M, Saito R, Yanagisawa K, Ichinose S, Koga H. Cartilage Derived from Bone Marrow Mesenchymal Stem Cells Expresses Lubricin In Vitro and In Vivo. PLoS One. 2016;11:e0148777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 59. | Musumeci G, Castrogiovanni P, Leonardi R, Trovato FM, Szychlinska MA, Di Giunta A, Loreto C, Castorina S. New perspectives for articular cartilage repair treatment through tissue engineering: A contemporary review. World J Orthop. 2014;5:80-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 110] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 60. | Embree MC, Chen M, Pylawka S, Kong D, Iwaoka GM, Kalajzic I, Yao H, Shi C, Sun D, Sheu TJ. Exploiting endogenous fibrocartilage stem cells to regenerate cartilage and repair joint injury. Nat Commun. 2016;7:13073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |