Published online Oct 18, 2017. doi: 10.5312/wjo.v8.i10.798
Peer-review started: March 28, 2017
First decision: April 17, 2017
Revised: July 19, 2017
Accepted: August 2, 2017
Article in press: August 3, 2017
Published online: October 18, 2017
Processing time: 205 Days and 11.8 Hours
To clarify the effectiveness of scaffold-based therapy for osteochondral lesions of the talus (OLT).
A systematic search of MEDLINE and EMBASE databases was performed during August 2016 and updated in January 2017. Included studies were evaluated with regard to the level of evidence (LOE) and quality of evidence (QOE) using the Modified Coleman Methodology Score. Variable reporting outcome data, clinical outcomes, and the percentage of patients who returned to sport at previous level were also evaluated.
Twenty-eight studies for a total of 897 ankles were included; 96% were either LOE III or IV. Studies were designated as either of poor or fair quality. There were 30 treatment groups reporting six different scaffold repair techniques: 13 matrix-induced autologous chondrocyte transplantation (MACT), nine bone marrow derived cell transplantation (BMDCT), four autologous matrix-induced chondrogeneis (AMIC), and four studies of other techniques. The categories of general demographics (93%) and patient-reported outcome data (85%) were well reported. Study design (73%), imaging data (73%), clinical variables (49%), and patient history (30%) were also included. The weighted mean American Orthopaedic Foot and Ankle Society (AOFAS) score at final follow-up was: 86.7 in MACT, 88.2 in BMDCT, and 82.3 in AMIC. Eight studies reported that a weighted mean of 68.3% of patients returned to a previous level of sport activity.
Scaffold-based therapy for OLT may produce favorable clinical outcomes, but low LOE, poor QOE, and variability of the data have confounded the effectiveness of this treatment.
Core tip: This systematic review demonstrated that scaffold-based therapy for lesions of the talus (OLT) may produce favorable clinical outcomes. However, 96% of included studies were classified into the category of poor level of evidence and no papers were of good methodological quality. Therefore, careful attention should be paid when evaluating scaffold-based therapy for OLT. In addition, large variability and underreporting of clinical data between studies made it difficult to reliably compare the results. Further well-designed studies are necessary to determine the effectiveness of scaffold-based therapy for OLT, especially when compared to the available traditional treatments.
- Citation: Shimozono Y, Yasui Y, Ross AW, Miyamoto W, Kennedy JG. Scaffolds based therapy for osteochondral lesions of the talus: A systematic review. World J Orthop 2017; 8(10): 798-808
- URL: https://www.wjgnet.com/2218-5836/full/v8/i10/798.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i10.798
Numerous surgical treatment strategies for osteochondral lesions of the talus (OLT) have been proposed, but a universally ideal treatment has yet to be established[1,2]. The operative treatment for OLT can be divided into two broad categories: Reparative and replacement procedures. Reparative procedures aim to regenerate tissue with biomechanical properties similar to normal hyaline cartilage. Bone marrow stimulation (BMS) is the most common reparative procedure, which stimulates mesenchymal stem cell proliferation and promotes fibrous cartilage repair tissue at the defect site. However, the fibrous cartilage repair tissue has different biological and mechanical properties compared to native hyaline cartilage and is likely to degenerate over time[3]. Autologous chondrocyte implantation (ACI) is another reparative procedure that attempts to regenerate damaged cartilage with more hyaline-like repair tissue, but this procedure has the disadvantage of the need for a two-staged intervention, which increases both cost and the potential for morbidity[4].
Recently, tissue-engineering approaches using various types of bioavailable scaffolds has emerged with greater potential for cellular differentiation and maturation. The templates are typically seeded with elements selected to improve the quality of reparative cartilage and include stem cells and growth factors. Matrix-induced autologous chondrocyte transplantation (MACT) is a second-generation ACI technique, which uses a type I/III bilayer collagen membrane seeded with cultured autologous chondrocytes. However, MACT also requires a two stage procedure[5,6]. Autologous matrix-induced chondrogenesis (AMIC) is a one-step scaffold-based therapy that combines bone marrow stimulation (BMS) with the use of a porcine collagen I/III matrix scaffold[7]. Bone marrow-derived cell transplantation (BMDCT) is also a one-step procedure and is a combination of concentrated bone marrow aspirate and scaffold material[8].
Scaffold-based therapy for OLT offers alternative reparative procedures and is quickly becoming more popular as data supporting clinical efficacy increases[9]. However, no consensus has been reached regarding the effectiveness of scaffold-based therapy on OLT to date.
The purpose of the current systematic review was to clarify the effectiveness of scaffold-based therapy for OLT based on available clinical evidence.
Two independent reviewers performed a systematic review of the databases PubMed/MEDLINE and EMBASE in January 2017 based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[10].
The combination of search terms were: (cartilage OR cartilage injury OR cartilage damage OR cartilage repair OR cartilage defect OR osteochondral lesion OR osteochondral dissecans OR osteochondral defect OR osteochondral injury OR osteochondral fracture OR osteochondritis dissecans) AND (ankle OR talus OR tibia OR talocrural joint) AND (scaffold OR scaffold-based repair OR matrix-assisted chondrocyte implantation OR cartilage regeneration OR osteochondral repair). The reference list of all articles and relevant studies were also scanned for additional articles potentially not identified through our electronic search alone.
The inclusion and exclusion criteria are shown in the Table 1. No time limit was given to publication date.
| Inclusion criteria | |
| Therapeutic clinical studies evaluating the effect of scaffolds for ankle cartilage repair | |
| All patients included had > 6-mo follow-up | |
| Published in a peer-reviewed journal | |
| Published in English | |
| Full-text version available | |
| Exclusion criteria | |
| Review articles | |
| Case reports | |
| Technique articles | |
| Cadaveric studies | |
| Animal studies | |
| In vivo studies |
The titles and abstracts were reviewed by applying the aforementioned criteria, and the full text of potentially relevant studies was then selected. Scaffold-based therapy for OLT was defined as operative treatment using any scaffolds for OLT.
Differences between reviewers were discussed until agreement was achieved, and the senior author was consulted in the event of persistent disagreement.
Two independent investigators reviewed each study and the LOE was determined using previously published criteria[11].
Two independent investigators evaluated the methodological quality of evidence (QOE) of the included studies using the Modified Coleman Methodology Score (MCMS) (Table 2)[12,13]. Instances of discrepancy were resolved by consensus and if any disagreement persisted, a senior author was consulted and a consensus was reached. Excellent studies were considered those that scored 85 to 100 points; good studies scored 70 to 84 points; fair studies scored 55 to 69 points, and poor studies scored less than 55 points[14].
| Score | ||
| Part A: Only 1 score to be given for each section | ||
| Number of study patients | ||
| > 60 | 10 | |
| 41-60 | 7 | |
| 20-40 | 4 | |
| < 20, not stated | 0 | |
| Mean follow-up (mo) | ||
| > 24 | 5 | |
| 12-24 | 2 | |
| < 12, not stated or unclear | 0 | |
| Number of different surgical procedures included in each reported outcome | ||
| 1 | 10 | |
| > 1, but > 90% of patients undergoing the 1 procedure | 7 | |
| Not stated, unclear, or < 90% of subjects undergoing the 1 procedure | 0 | |
| Type of study | ||
| Randomized controlled trial | 15 | |
| Prospective cohort study | 10 | |
| Retrospective cohort study | 0 | |
| Diagnostic certainty (MRI) | ||
| In all | 5 | |
| In > 80% | 3 | |
| In < 80% | 0 | |
| Description of surgical procedure given | ||
| Adequate (technique stated and necessary details of that type of procedure provided) | 5 | |
| Fair (technique only stated without elaboration) | 3 | |
| Inadequate, not stated, or unclear | 0 | |
| Description of postoperative rehabilitation | ||
| Well described (ROM, WB, and sport) | 10 | |
| Not adequately described (2 items between ROM, WB, and sport) | 5 | |
| Protocol not reported | 0 | |
| Part B: Scores may be given for each option in each of the 3 sections if applicable | ||
| Outcome criteria | ||
| Outcome measures clearly defined | 2 | |
| Timing of outcome assessment clearly stated (e.g., at best outcome after surgery or follow-up) | 2 | |
| Objective, subjective, and imaging criteria | 6 | |
| 2 items between objective, subjective, and imaging criteria | 4 | |
| Objective, subjective, or radiological criteria | 2 | |
| Procedure for assessing outcomes | ||
| Patients recruited (results not taken from surgeons' files) | 5 | |
| Investigator independent of surgeon | 4 | |
| Written assessment | 3 | |
| Completion of assessment by patinets themselves with minimal investigator assistance | 3 | |
| Description of patient selection process | ||
| Selection criteria reported and unbiased | 5 | |
| Recruitment rate reported | ||
| > 80% | 5 | |
| < 80% | 3 | |
| Eligible patients not included in study satisfactorily accounted for or 100% recruitment | 5 | |
Two reviewers independently extracted data from each study and assessed variable reporting of outcome data using parameters of previously published criteria[15]. In addition, clinical outcomes and the percentage of patients who returned to sport at previous level were evaluated.
All statistical analysis was performed using a commercially available statistical software package (SAS 9.3; SAS Institute, Inc., Cary, NC, United States). Descriptive statistics were calculated for each study and parameters analyzed. For each variable, the number and percentage of studies that reported the variable was calculated. Variables were reported as weighted average ± weighted standard deviation where applicable.
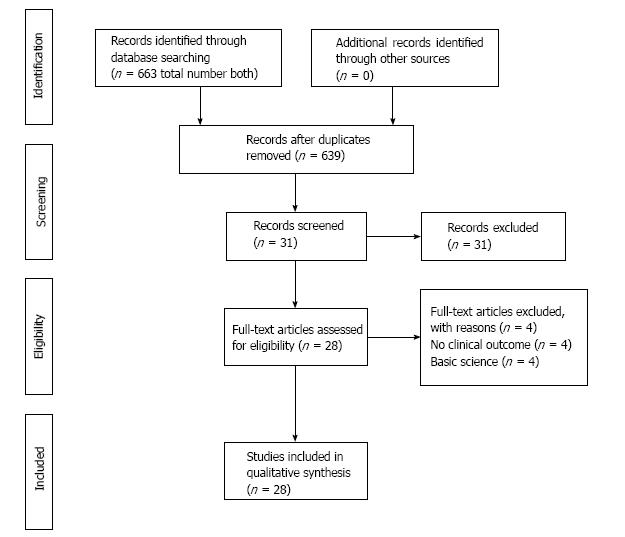
After full text review, 28 clinical studies for a total of 897 ankles were identified for inclusion in the current study (Figure 1)[4-8,16-38]. The weighted mean follow-up was 37.7 (range 6-87) mo, with only three studies reporting a follow-up time of greater than five years[20,22,24].
Of the 28 clinical studies, there were 30 treatment groups, including six different scaffold-based therapies: 13 MACT[5,6,16-26], nine BMDCT[4,8,26-31], four AMIC[7,32-34], two cartilage extracellular matrix[35,36], one autologous collagen-induced chondrogenesis (ACIC)[37], and one cell free scaffold therapy[38]. All included studies of scaffold-based therapy were summarized in Table 3. Patient demographics and clinical characteristics of each procedure are shown in Table 4.
| Procedure | Product | Scientific publication | Type of study | LOE | No. of patients | Lesion size (cm2) | Follow-up (mo) | Results |
| Two-step | ||||||||
| MACT | MACI | Schneider et al[6], 2009 | Case series | IV | 20 | 2.3 | 21 | Significant improvement in functional score |
| Pain improved in 70% of patients | ||||||||
| Giza et al[5], 2010 | Case series | IV | 10 | 1.3 | 24 | Significant clinical improvement at 1 yr and maintained at 2 yr | ||
| Aurich et al[16], 2011 | Case series | IV | 18 | - | 25 | Significant improvement in all clinical scores 64% were excellent or good Age and symptoms duration were correlated with results | ||
| Dixon et al[17], 2011 | Case series | IV | 25 | 1.3 | 44 | 72% improved symptoms 78% patients over 40 yr reported restricted recreational activity | ||
| Lee et al[18], 2013 | Case series | IV | 38 | 1.9 | 24 | Functional outcomes improved significantly at 2 yr 68% were excellent or good outcome 75% ICRS grade I or II in 2nd look arthroscopy at 1 yr | ||
| Johnson et al[19], 2013 | Case series | IV | 18 | 1.9 | 82 | Functional outcomes improved at final follow-up | ||
| Giannini et al[20], 2014 | Case series | IV | 46 | 1.6 | 87 | Significant clinical improvement at 1 yr and maintained at 3 yr; 3 failures | ||
| Hyalograft C | Giannini et al[21], 2008 | Case series | IV | 46 | 1.6 | 36 | Significant clinical improvement at 1 yr and 3 yr Results correlated with age and previous surgery Hyaline-like cartilage regeneration in histological evaluation | |
| Battaglia et al[22], 2011 | Case series | IV | 20 | 2.7 | 60 | Significant clinical improvement T2 mapping MRI showed 69% of lesion are covered with repair tissue | ||
| Nehrer et al[23], 2011 | Case series | IV | 13 | - | 47 | Significant clinical improvement in all cases | ||
| Domayer et al[24], 2012 | Comparative study | III | 18 | 1.2 | 65 | Significant clinical improvement but no significant difference compared to MFX group No difference between MFX and MACT on T2 maps | ||
| Apprich et al[25], 2012 | Case series | IV | 10 | 1.2 | 48 | Significant clinical improvement No differences in functional outcome and MOCART score between MFX and MACT | ||
| Two-step | ||||||||
| BMDCT | Spontostan Powder HYAFF-11 | Giannini et al[8], 2009 | Case series | IV | 48 (25 HA membrane, 23 collagen powder) | 2.1 | 29 | Significant clinical improvement at 1 yr maintained at 2 yr Similar results with two scaffolds Correlation between clinical outcome and lesion size |
| Spontostan Powder HYAFF-11 | Giannini et al[26], 2010 | Comparative study | III | 25 BMDCT 46 two-step MACI | 2.2 1.6 | 39 57 | Significant clinical improvement at 1 yr and further improvement at 3 yr 76% complete intergration with surrounding cartilage on MRI Hyaline-like cartilage tissue on histological evaluation | |
| HYAFF-11 | Battaglia et al[27], 2011 | Case series | IV | 20 | 1.5 | 24 | 85% excellent or good clinical results at 2 yr 78% of lesion are covered with repair tissue comparable to hyaline cartilage | |
| Spontostan Powder HYAFF-11 | Giannini et al[28], 2013 | Case series | IV | 49 | 2.1 | 29 | Significant clinical improvement at 1 yr with subsequent significant decrease at 2 and 3 yr 78% of repaired tissue similar to hyaline cartilage on T2 maps | |
| Spongostan Powder | Buda et al[29], 2014 | Case series | IV | 64 | 5.3 | 53 | Clinical results peaked at 2 yr, declining gradually at follow-up of 6 yr | |
| Biopad | Cadossi et al[30], 2014 | Comparative study | III | 15 BMDCT 15 BMDCT with PEMF | 2 1.9 | 12 12 | Significant clinical improvement in both groups | |
| HYAFF-11 | Buda et al[4], 2015 | Case series | IV | 40 | 1.8 | 48 | Significant clinical improvement Higher presence of hyaline-like cartilage in BMDCT than ACI on MRI T2 mapping | |
| HYAFF-11 Spongostan Powder Biopad | Vannini et al[31], 2017 | Case series | IV | 140 | 2 | 26 | Significant clinical improvement at 2 yr maintained at 4 yr Return to sports at preinjury level; 32.1% at 12 mo, 72.8% at 48 mo | |
| AMIC | Unclear | Wiewiorski et al[7], 2013 | Case series | IV | 23 | - | 23 | Significant clinical improvement |
| Chondro-Gide | Valderrabano et al[32], 2013 | Case series | IV | 26 | - | 31 | Significant clinical improvement Normal signal intensity of repair tissue was seen in 15% on MRI | |
| Chondro-Gide | Kubosch et al[33], 2016 | Case series | IV | 17 | 2.4 | 39 | Significant clinical improvement MOCART score correlated with AOFAS score | |
| Chondro-Gide | Wiewiorski et al[34], 2016 | Case series | IV | 60 | - | 47 | Calcaneal osteotomy was performed in 63% of patients Low rate for return to sports; postoperative sports activity levels remain stable when compared with preoperative levels | |
| Cartilage ECM | BioCartilage | Desai S[35], 2016 | Case series | IV | 9 | 1.3 | 12 | 78% excellent, 22% good clinical outcomes |
| Clanton et al[36], 2014 | Case series | IV | 7 | - | 8 | Significant clinical improvement | ||
| ACIC | Cartifill | Volpi P et al[37], 2014 | Case series | IV | 5 | 3.1 | 6 | Significant clinical improvement at 6 mo |
| Cell-free scaffold | MaioRegen® | Christensen et al[38], 2015 | Case series | IV | 4 | - | 30 | No clinical scores improvement No improvement in MOCART score and 3 patients had 0%-10% bone formation in defect at 1 yr on CT |
| Procedure | |||||||
| Total | MACT | BMDCT | AMIC | Cartilage ECM | ACIC | Cell-free scaffold | |
| Treatment groups, n | 30 | 13 | 9 | 4 | 2 | 1 | 1 |
| Ankles, n | 897 | 330 | 416 | 126 | 16 | 5 | 4 |
| Sex, male/female/unknown, n | 501/322/72 | 174/111/45 | 238/153/22 | 79/47/0 | 7/9/0 | 3/2/0 | - |
| Age, yr, weighted mean (range) | 30.9 (19-61) | 30.1 | 30.2 | 34.9 | 42.7 | 25.6 | - |
| Duration of symptoms, mo, weighted mean (range) | 34.3 (6-216) | 34.5 | 36.5 | 23 | - | - | - |
| Lesion size, mm2, weighted mean (range) | 215 (116-527) | 171 | 248 | 240 | 130 | - | - |
| Follow-up, mo, weighted mean (range) | 37.7 (6-87) | 45.8 | 32.7 | 38.2 | 10.4 | 6 | 30 |
There was one (3.6%) study of LOE II[30], three studies (10.7%) of LOE III[4,24,26], and 24 studies (85.7%) of LOE IV[5-8,16-23,25,27-29,31-38] (Table 5) according to established criteria[11]. No study of LOE I was reported. The further data of LOE in each procedure group was shown in Table 5.
| Total Studies | Procedure groups | |||||||
| MACT | BMDCT | AMIC | Cartilage ECM | ACIC | Cell-free scaffold | |||
| Level of evidence | ||||||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2 | 1 (3.6) | 0 | 2 (22.2) | 0 | 0 | 0 | 0 | |
| 3 | 3 (10.7) | 2 (15.4) | 2 (22.2) | 0 | 0 | 0 | 0 | |
| 4 | 24 (85.7) | 11 (84.6) | 5 (55.6) | 4 (100) | 2 (100) | 1 (100) | 1 (100) | |
| Quality of evidence | ||||||||
| Excellent (MCMS ≥ 85) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Good (MCMS 70-84) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Fair (MCMS 55-69) | 7 (25.0) | 3 (23.1) | 4 (44.4) | 0 | 0 | 0 | 0 | |
| Poor (MCMS < 55) | 21 (75.0) | 10 (76.9) | 5 (55.6) | 4 (100) | 2 (100) | 1 (100) | 1 (100) | |
The weighted mean MCMS of the overall population of studies was 49.3 ± 10.0 out of a possible 100 points. There were seven studies (25%) of fair quality [8,18,20,21,30,31,38] and the remainder (75%) were of poor quality[4-7,16,17,19,22-29,32-37] (Table 5). Further QOE data is shown in Table 5.
The defined data that were reported in the studies included in this review are listed and the each data according to procedure group is shown in Table 6. General demographic information including age and gender were reported in 93% of the studies. While the study design, imaging data, and patient-reported outcomes were well-reported variables with 73%, 73% and 85% respectively, patient history was the least reported variable of all with 30% of the data being reported. Clinical variables were reported in only 49% of studies.
| Procedure | ||||||||
| Total | MACT | BMDCT | AMIC | Cartilage ECM | ACIC | Cell-free scaffold | ||
| Procedure groups, n | 30 | 13 | 9 | 4 | 2 | 1 | 1 | |
| Demographic information | 93 | 92 | 94 | 100 | 100 | 100 | 0 | |
| Sex | 90 | 85 | 89 | 100 | 100 | 100 | 0 | |
| Mean age + range | 97 | 100 | 100 | 100 | 100 | 100 | 0 | |
| Patient history | 30 | 35 | 31 | 44 | 13 | 0 | 0 | |
| Body mass index | 33 | 31 | 33 | 50 | 50 | 0 | 0 | |
| Mean duration of symptoms | 23 | 38 | 22 | 25 | 0 | 0 | 0 | |
| Previous traumatic experience(s) | 33 | 38 | 44 | 50 | 0 | 0 | 0 | |
| Activities of daily living/athletic participation | 30 | 31 | 22 | 50 | 0 | 0 | 0 | |
| Study design | 73 | 71 | 78 | 72 | 56 | 63 | 38 | |
| Type of study | 50 | 23 | 56 | 25 | 0 | 0 | 100 | |
| Number of patients | 97 | 100 | 100 | 100 | 100 | 100 | 0 | |
| Percentage of patients in follow-up | 97 | 100 | 100 | 100 | 100 | 100 | 0 | |
| Consecutive patients | 23 | 23 | 22 | 50 | 0 | 0 | 0 | |
| Follow-up time + range/standard deviation | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Method of lesion size measurement | 43 | 54 | 44 | 50 | 0 | 0 | 0 | |
| Lesion classification system utilized | 77 | 77 | 100 | 50 | 50 | 100 | 0 | |
| Surgical approach used to access lesion | 97 | 92 | 100 | 100 | 100 | 100 | 100 | |
| Clinical variables | 49 | 53 | 50 | 58 | 33 | 33 | 33 | |
| Lesion size | 93 | 100 | 100 | 75 | 50 | 100 | 100 | |
| Lesion location | 77 | 77 | 100 | 75 | 50 | 0 | 0 | |
| Presence of cyst | 13 | 23 | 0 | 25 | 50 | 0 | 0 | |
| Associated pathology | 13 | 23 | 0 | 25 | 0 | 0 | 0 | |
| Concomitant procedures | 20 | 15 | 22 | 50 | 0 | 0 | 0 | |
| Description of rehabilitation | 80 | 77 | 78 | 100 | 50 | 100 | 100 | |
| Imaging data | 73 | 81 | 83 | 75 | 50 | 0 | 100 | |
| Imaging used to identify lesion | 80 | 92 | 89 | 75 | 50 | 0 | 100 | |
| Imaging used at follow-up | 67 | 69 | 78 | 75 | 50 | 0 | 100 | |
| Patient-reported outcomes | 85 | 85 | 100 | 88 | 0 | 100 | 100 | |
| Pain, function, and activity scale, pre-operative | 80 | 77 | 100 | 75 | 0 | 100 | 100 | |
| Pain, function, and activity scale, at follow-up | 90 | 92 | 100 | 100 | 0 | 100 | 100 | |
Clinical outcomes were evaluated using a number of different scoring systems for scaffolds-based therapy for OLT (Table 7). The American Orthopaedic Foot and Ankle Society (AOFAS) score was the most frequently utilized in 25 studies of the included[4-8,16-18,20-34,37,38]. Of the 25 studies that used AOFAS, 22 studies investigated both pre- and post-operative scores[4-8,16,18,20-23,25-32,34,37,38].
| Score | Studies, total | Procedure group | |||||
| MACT | BMDCT | AMIC | Cartilage ECM | ACIC | Cell-free scaffold | ||
| AOFAS | 25 (89) | 12 (92) | 9 (100) | 4 (100) | 0 (0) | 1 (100) | 1 (100) |
| VAS | 7 (25) | 1 (8) | 2 (22) | 4 (100) | 0 (0) | 1 (100) | 0 (0) |
| Tegner activity score | 3 (11) | 1 (8) | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 1 (100) |
| SF-36 | 2 (7) | 1 (8) | 2 (22) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| FFI | 2 (7) | 1 (8) | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 0 (0) |
| FADI | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | 0 (0) | 0 (0) |
| HSS | 1 (4) | 1 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| LEAS | 1 (4) | 1 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| AHS | 1 (4) | 1 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| AAOS | 1 (4) | 1 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ARS | 1 (4) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 0 (0) |
| Halasi score | 1 (4) | 0 (0) | 1 (11) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Mazur ankle score | 1 (4) | 1 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Cincinnati score | 1 (4) | 1 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Twelve of 13 MACT groups reported pre and postoperative AOFAS scores and of the 310 patients who underwent MACT[5,6,16-18,20-26], the mean AOFAS score improved from 59.1 to 86.7 at a mean follow-up of 47.9 mo. Of the 416 patients from the nine BMDCT groups[4,8,26-31], the mean AOFAS score improved from 61.1 to 88.2 at a mean of 32.7 mo of follow-up. Of the 126 patients from the four AMIC groups[7,32-34], the mean AOFAS score improved from 50.7 to 82.3 at a mean follow-up of 38.2 mo. Of the two cartilage ECM studies included, one publication reported outcomes at less than one year follow-up[36], and the other one did not describe clinical outcomes[35]. There was only one publication reporting ACIC data but clinical evaluation was insufficient due to a follow-up of only six mo[37]. In the cell-free scaffold group, only one study was published, which showed no clinical improvement in AOFAS score at a mean 30 mo (from 48.7 to 52.7) follow-up. However, these results only included four studies[38].
In this systematic review, 12 procedure groups reported sequential clinical outcomes at two or more post-operative time points[4,5,8,18,20,21,26,28-31]. Four groups, which were all BMDCT studies, found temporal improvement in AOFAS scores over the first 2-3 years of post-operative follow-up with a mean decrease in AOFAS score of 87.1 reported at a mean 41.8 mo follow-up[4,28,29,31]. In contrast, eight groups, including four MACT and four BMDCT groups, demonstrated that there were no deteriorations during a weighted mean 38-mo follow-up[5,8,18,20,21,26,30].
Overall, eight studies (MACT: One study, AMIC: Two studies, BMDCT: five studies) reported that a mean 68.3% of patients receiving scaffold-based therapy with mean lesion size of 250 mm2 returned to previous sport activity at previous level[4,8,21,28,29,31,32,34]. Of the MACT procedures, Giannini et al[21] showed that 20 of 29 patients (69%) returned to sport at previous levels. In patients treated with AMIC procedures, Valderrabano et al[32] reported only nine of 20 patients (45%) returned to previous sport activity level, and Wiewiorski et al[34] also showed no significant difference when comparing preoperative and postoperative activity scores (ARS, Tegner). BMDCT was the most reported in five studies, and of these studies, 74.5% of patients were able to resume sports at preinjury level, with a range of 69% to 78%[4,8,28,29,31].
The results from this systematic review demonstrate that recommendations for scaffold-based therapy based solely on evidence is not yet conclusive. In the current evaluation, 96% of included studies in which scaffold-based therapy was performed for the treatment of OLT were classified into the category of poor LOE. In addition, of 28 included articles, no papers were of good or better methodological quality. According to the principles of evidence-based medicine[39], a high level of clinical evidence and good methodological quality are fundamentally warranted to treat patients because low LOE and QOE studies are more likely to show overestimated outcomes compared to higher LOE and QOE studies[40,41]. Careful attention therefore should be paid when evaluating outcomes following the studies of scaffold-based therapy for OLT.
The results from the current systematic review demonstrate large variability and underreporting of clinical data between studies reflecting and inability to compare the results across studies. These inconsistencies and general underreporting of data make it difficult to pool data, which furthermore makes it difficult to draw conclusions about effectiveness of the use of scaffold in the treatment for OLT. As Hannon et al[15] described, adequate reporting of data in the studies of the treatment for OLT should be required to perform high quality studies, and investigators should be encouraged to implement data collection both before and after surgery according to recommended list described by Hannon et al[15] in this review, the categories of imaging data were reported in 73% of included studies. Compared with reporting of outcome data on microfracture for OLT in the systematic review by Hannon et al[15], imaging data was reported in only 39% among the studies. However, this review showed a higher percentage of reporting of imaging data (73%). Nevertheless, only 67% of studies used MRI for patient follow-up evaluation, although MRI evaluation for scaffold-based treatment of OLT is crucial because the aim of the use of scaffolds and is generally believed to promote the subchondral bone and cartilage repair. In addition, the categories of clinical variables and patient history were reported only with 49% and 30% respectively. As these data including BMI, lesion location, presence of cyst, associated pathology, and concomitant procedures can have significant effect on patient outcome, what is alarming is that appropriate information is not enough taken in the current studies.
Lesion size has been widely accepted as the most commonly used predictor of clinical outcomes after BMS for OLT[42,43]. Choi et al[42] demonstrated that BMS should be indicated for lesions less than 150 mm2 and lesions greater than this value resulted in poor outcomes. More recently, Ramponi et al[13] suggested that BMS could be best reserved for lesion size of less than 107.4 mm2 rather than 150 mm2. In the current review, however, the mean lesion size treated with scaffolds was 215 mm2, which is much larger than traditional indication size for BMS or the most current new indication size of 107 mm2[13]. This suggests that the use of scaffolds may further improve the potential of reparative techniques. However, further well-designed studies are necessary to determine the effectiveness of scaffold-based therapy on OLT because of low LOE and QOE and the large variability in the data.
Despite of high frequency of OLT in the athletic population, little is reported regarding return to sport following surgical treatment of OLT in this population. In the current review, weighted mean 68.3% of patients receiving scaffold therapy with weighted mean 250 mm2 of lesion size returned to previous sport activity at previous level in eight studies. There are no studies investigating the effectiveness of BMS alone for athletic populations who have large lesion as described above, but Choi et al[42] reported clinical failure rate in patients with lesion area ≥ 150 mm2 was 80%. Furthermore, Chuckpaiwong et al[43] reported a 97% of failure rate in 32 patients with a lesion area ≥ 150 mm2. This suggests that the use of scaffolds may provide better outcomes than BMS alone for larger lesions but high quality studies are warranted. On the other hand, in replacement procedures, including autologous osteochondral transplantation, which is generally indicated for larger lesions, several studies reported that more than 90% of patients returned to play sport at previous levels[44,45]. Although there is inconsistency in indications for the treatment strategy, the rate of return to sport following scaffold-based therapy appears to be relatively lower than AOT procedures. The highest rate of return to sport after scaffold-based therapy was only 78.0% in athletes treated with BMDCT[31]. However, there was variability of sport type, postoperative rehabilitation protocol, and time to return to sport, which makes it difficult to assess these results appropriately.
Our review found that there were 12 different scoring systems used to assess clinical outcomes, with AOFAS score being the most commonly used (89%). However, there remains no validated scoring system for the clinical follow-up for the treatment of OLT[13]. Moreover, four BMDCT groups have shown that clinical outcomes deteriorate after peaking at 2-3 year postoperatively[4,28,29,31], whereas four MACT and four BMDCT groups have no deterioration during follow-up[5,8,18,20,21,26,30]. A potential reason for these lags in clinical outcome data may be the invalid clinical evaluation methods after OLT surgery in addition to the use of the different kinds of scaffolds. A novel validated scoring system for the clinical follow-up of the treatment for OLT are currently warranted.
The appropriate treatment for OLT is still controversial. While the ideal procedure would regenerate a tissue with biomechanical properties similar to normal hyaline cartilage, reparative techniques can offer the replacement of the articular cartilage with a hyaline-like repair tissue. Scaffolds have been introduced to improve the requirements of the cartilage regeneration process, as ACI, the first generation approach for cartilage treatment, has evident biological and surgical limitations[46]. In fact, the use of scaffolds has overcome the drawbacks and simplified the procedure. However, any available substitute materials have not yet matched the properties of the normal cartilage, and there is no consensus about the superior effectiveness of these procedures over the other procedures, including replacement procedures. While the scaffold-based treatment has shown promising clinical results in numerous studies of case series, the current systematic review showed low LOE and poor methodological quality of the use of scaffolds for OLT. Further long-term comparative studies are warranted to investigate the potential of a bioengineered approach compared to other treatments. Furthermore, the definitive indications for this technique, including lesion size and character of the lesion, still remains controversial[13].
This systematic review has several inherent limitations and/or potential biases. The criterion was limited to MEDLINE, EMBASE and Cochrane Library Database articles published exclusively in English. The variables may not be all inclusive of data in each study, but they should be a representative summary of the most commonly used data. Another inherent concern was the overlapping of cohorts or subgroups of several cohorts studies in longitudinal follow-up studies. Finally, the data extraction was not performed blindly, but was performed by two independent reviewers and later confirmed by the lead author.
In conclusion, this systematic review demonstrated that the scaffold-based therapy for the treatment of OLT may produce favorable clinical outcomes, but low level of evidence, poor quality of evidence, and the variability of the data have confounded the effectiveness of scaffold-based therapy for OLT. Further, well-designed studies, are necessary to determine the effectiveness of the use of scaffold for the treatment of OLT, especially when compared to available traditional treatments.
Recently scaffold-based therapy for osteochondral lesions of the talus (OLT) has become more popular as an alternative reparative procedure. However, no consensus has been reached regarding the effectiveness of scaffold-based therapy in the treatment of OLT to date. In this study, the effectiveness of scaffold-based therapy was systematically reviewed based on available clinical evidence.
Scaffolds have been introduced to improve the requirements of the cartilage regeneration process, as autologous chondrocyte implantation (ACI), the first generation approach for cartilage treatment, has evident biological and surgical limitations. Recently, the use of scaffolds has overcome the drawbacks and simplified the procedure.
The scaffold-based treatment has shown promising clinical results in numerous studies of case series and the use of scaffolds may further improve the potential of reparative techniques. Retrieved manuscripts were reviewed by the authors, and the data were extracted.
This systematic review suggests that the scaffold-based therapy for the treatment of OLT may produce favorable clinical outcomes, but low level of evidence, poor quality of evidence, and the variability of the data have confounded the effectiveness of scaffold-based therapy for OLT.
Matrix-induced autologous chondrocyte transplantation is a second-generation ACI technique, which uses a type I/III bilayer collagen membrane seeded with cultured autologous chondrocytes. Autologous matrix-induced chondrogenesis is a one-step scaffold-based therapy that combines bone marrow stimulation with the use of a porcine collagen I/III matrix scaffold. Bone marrow-derived cell transplantation is also a one-step procedure and is a combination of concentrated bone marrow aspirate and scaffold material.
The paper adequately concludes what is already suspected in that variable quality, small studies with limited outcome data serve to confuse the authors’ knowledge. It’s a useful review.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lee SH, Malik H, Vulcano E S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Wodicka R, Ferkel E, Ferkel R. Osteochondral Lesions of the Ankle. Foot Ankle Int. 2016;37:1023-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18:238-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 441] [Cited by in RCA: 409] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 3. | Hannon CP, Smyth NA, Murawski CD, Savage-Elliott I, Deyer TW, Calder JD, Kennedy JG. Osteochondral lesions of the talus: aspects of current management. Bone Joint J. 2014;96-B:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Buda R, Vannini F, Castagnini F, Cavallo M, Ruffilli A, Ramponi L, Pagliazzi G, Giannini S. Regenerative treatment in osteochondral lesions of the talus: autologous chondrocyte implantation versus one-step bone marrow derived cells transplantation. Int Orthop. 2015;39:893-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Giza E, Sullivan M, Ocel D, Lundeen G, Mitchell ME, Veris L, Walton J. Matrix-induced autologous chondrocyte implantation of talus articular defects. Foot Ankle Int. 2010;31:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Schneider TE, Karaikudi S. Matrix-Induced Autologous Chondrocyte Implantation (MACI) grafting for osteochondral lesions of the talus. Foot Ankle Int. 2009;30:810-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Wiewiorski M, Miska M, Kretzschmar M, Studler U, Bieri O, Valderrabano V. Delayed gadolinium-enhanced MRI of cartilage of the ankle joint: results after autologous matrix-induced chondrogenesis (AMIC)-aided reconstruction of osteochondral lesions of the talus. Clin Radiol. 2013;68:1031-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res. 2009;467:3307-3320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 211] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 9. | Vannini F, Filardo G, Kon E, Roffi A, Marcacci M, Giannini S. Scaffolds for cartilage repair of the ankle joint: The impact on surgical practice. Foot Ankle Surg. 2013;19:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65-W94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3566] [Cited by in RCA: 4306] [Article Influence: 269.1] [Reference Citation Analysis (0)] |
| 11. | Marx RG, Wilson SM, Swiontkowski MF. Updating the assignment of levels of evidence. J Bone Joint Surg Am. 2015;97:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 230] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 12. | Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 765] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 13. | Ramponi L, Yasui Y, Murawski CD, Ferkel RD, DiGiovanni CW, Kerkhoffs GMMJ, Calder JDF, Takao M, Vannini F, Choi WJ. Lesion Size Is a Predictor of Clinical Outcomes After Bone Marrow Stimulation for Osteochondral Lesions of the Talus: A Systematic Review. Am J Sports Med. 2017;45:1698-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 201] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 14. | Jakobsen RB, Engebretsen L, Slauterbeck JR. An analysis of the quality of cartilage repair studies. J Bone Joint Surg Am. 2005;87:2232-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Hannon CP, Murawski CD, Fansa AM, Smyth NA, Do H, Kennedy JG. Microfracture for osteochondral lesions of the talus: a systematic review of reporting of outcome data. Am J Sports Med. 2013;41:689-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Aurich M, Bedi HS, Smith PJ, Rolauffs B, Mückley T, Clayton J, Blackney M. Arthroscopic treatment of osteochondral lesions of the ankle with matrix-associated chondrocyte implantation: early clinical and magnetic resonance imaging results. Am J Sports Med. 2011;39:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Dixon S, Harvey L, Baddour E, Janes G, Hardisty G. Functional outcome of matrix-associated autologous chondrocyte implantation in the ankle. Foot Ankle Int. 2011;32:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Lee KT, Kim JS, Young KW, Lee YK, Park YU, Kim YH, Cho HK. The use of fibrin matrix-mixed gel-type autologous chondrocyte implantation in the treatment for osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2013;21:1251-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Johnson B, Lever C, Roberts S, Richardson J, McCarthy H, Harrison P, Laing P, Makwana N. Cell cultured chondrocyte implantation and scaffold techniques for osteochondral talar lesions. Foot Ankle Clin. 2013;18:135-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Giannini S, Buda R, Ruffilli A, Cavallo M, Pagliazzi G, Bulzamini MC, Desando G, Luciani D, Vannini F. Arthroscopic autologous chondrocyte implantation in the ankle joint. Knee Surg Sports Traumatol Arthrosc. 2014;22:1311-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Giannini S, Buda R, Vannini F, Di Caprio F, Grigolo B. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: surgical technique and results. Am J Sports Med. 2008;36:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Battaglia M, Vannini F, Buda R, Cavallo M, Ruffilli A, Monti C, Galletti S, Giannini S. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: mid-term T2-mapping MRI evaluation. Knee Surg Sports Traumatol Arthrosc. 2011;19:1376-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Nehrer S, Domayer SE, Hirschfeld C, Stelzeneder D, Trattnig S, Dorotka R. Matrix-Associated and Autologous Chondrocyte Transplantation in the Ankle: Clinical and MRI Follow-up after 2 to 11 Years. Cartilage. 2011;2:81-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Domayer SE, Apprich S, Stelzeneder D, Hirschfeld C, Sokolowski M, Kronnerwetter C, Chiari C, Windhager R, Trattnig S. Cartilage repair of the ankle: first results of T2 mapping at 7.0 T after microfracture and matrix associated autologous cartilage transplantation. Osteoarthritis Cartilage. 2012;20:829-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Apprich S, Trattnig S, Welsch GH, Noebauer-Huhmann IM, Sokolowski M, Hirschfeld C, Stelzeneder D, Domayer S. Assessment of articular cartilage repair tissue after matrix-associated autologous chondrocyte transplantation or the microfracture technique in the ankle joint using diffusion-weighted imaging at 3 Tesla. Osteoarthritis Cartilage. 2012;20:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Giannini S, Buda R, Cavallo M, Ruffilli A, Cenacchi A, Cavallo C, Vannini F. Cartilage repair evolution in post-traumatic osteochondral lesions of the talus: from open field autologous chondrocyte to bone-marrow-derived cells transplantation. Injury. 2010;41:1196-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Battaglia M, Rimondi E, Monti C, Guaraldi F, Sant’Andrea A, Buda R, Cavallo M, Giannini S, Vannini F. Validity of T2 mapping in characterization of the regeneration tissue by bone marrow derived cell transplantation in osteochondral lesions of the ankle. Eur J Radiol. 2011;80:e132-e139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Giannini S, Buda R, Battaglia M, Cavallo M, Ruffilli A, Ramponi L, Pagliazzi G, Vannini F. One-step repair in talar osteochondral lesions: 4-year clinical results and t2-mapping capability in outcome prediction. Am J Sports Med. 2013;41:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 29. | Buda R, Vannini F, Cavallo M, Baldassarri M, Natali S, Castagnini F, Giannini S. One-step bone marrow-derived cell transplantation in talarosteochondral lesions: mid-term results. Joints. 2014;1:102-107. [PubMed] |
| 30. | Cadossi M, Buda RE, Ramponi L, Sambri A, Natali S, Giannini S. Bone marrow-derived cells and biophysical stimulation for talar osteochondral lesions: a randomized controlled study. Foot Ankle Int. 2014;35:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Vannini F, Cavallo M, Ramponi L, Castagnini F, Massimi S, Giannini S, Buda RE. Return to Sports After Bone Marrow-Derived Cell Transplantation for Osteochondral Lesions of the Talus. Cartilage. 2017;8:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Valderrabano V, Miska M, Leumann A, Wiewiorski M. Reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med. 2013;41:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 33. | Kubosch EJ, Erdle B, Izadpanah K, Kubosch D, Uhl M, Südkamp NP, Niemeyer P. Clinical outcome and T2 assessment following autologous matrix-induced chondrogenesis in osteochondral lesions of the talus. Int Orthop. 2016;40:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Wiewiorski M, Werner L, Paul J, Anderson AE, Barg A, Valderrabano V. Sports Activity After Reconstruction of Osteochondral Lesions of the Talus With Autologous Spongiosa Grafts and Autologous Matrix-Induced Chondrogenesis. Am J Sports Med. 2016;44:2651-2658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Desai S. Surgical Treatment of a Tibial Osteochondral Defect With Debridement, Marrow Stimulation, and Micronized Allograft Cartilage Matrix: Report of an All-Arthroscopic Technique. J Foot Ankle Surg. 2016;55:279-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Clanton TO, Jhonson NS, Matheny LM. Use of cartilage extracellular matrix and bone marrow aspirate concentrate in treatment of osteochondral lesions of the talus. Tech Foot and Ankle. 2014;13:212220. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Volpi P, Bait C, Quaglia A, Redaelli A, Prospero E, Cervellin M, Stanco D, de Girolamo L. Autologous collagen-induced chondrogenesis technique (ACIC) for the treatment of chondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2014;22:1320-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Christensen BB, Foldager CB, Jensen J, Jensen NC, Lind M. Poor osteochondral repair by a biomimetic collagen scaffold: 1- to 3-year clinical and radiological follow-up. Knee Surg Sports Traumatol Arthrosc. 2016;24:2380-2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 39. | Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8991] [Cited by in RCA: 7464] [Article Influence: 257.4] [Reference Citation Analysis (0)] |
| 40. | Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, Pham B, Klassen TP. Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses. Health Technol Assess. 1999;3:i-iv, 1-98. [PubMed] |
| 41. | Moher D, Jadad AR, Klassen TP. Guides for reading and interpreting systematic reviews: III. How did the authors synthesize the data and make their conclusions? Arch Pediatr Adolesc Med. 1998;152:915-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med. 2009;37:1974-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 320] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 43. | Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy. 2008;24:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 341] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 44. | Fraser EJ, Harris MC, Prado MP, Kennedy JG. Autologous osteochondral transplantation for osteochondral lesions of the talus in an athletic population. Knee Surg Sports Traumatol Arthrosc. 2016;24:1272-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 45. | Kennedy JG, Murawski CD. The Treatment of Osteochondral Lesions of the Talus with Autologous Osteochondral Transplantation and Bone Marrow Aspirate Concentrate: Surgical Technique. Cartilage. 2011;2:327-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 46. | Filardo G, Kon E, Roffi A, Di Martino A, Marcacci M. Scaffold-based repair for cartilage healing: a systematic review and technical note. Arthroscopy. 2013;29:174-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |