Published online Oct 18, 2017. doi: 10.5312/wjo.v8.i10.790
Peer-review started: May 12, 2017
First decision: July 10, 2017
Revised: July 20, 2017
Accepted: August 2, 2017
Article in press: August 2, 2017
Published online: October 18, 2017
Processing time: 158 Days and 23.5 Hours
To evaluate the joint geometry and the clinical outcome of stemless, anatomical shoulder arthroplasty with the TESS system.
Twenty-one shoulders with a mean follow-up 18 of months were included. On scaled digital radiographs the premorbid center of rotation (CoR) was assessed and compared to the CoR of the prosthesis by using the MediCAD® software. Additionally, the pre- and post-operative geometry of the CoR was assessed in relation to the glenoid, the acromion as well as to the proximal humerus. Radiological changes, such as radiolucencies, were also assessed. Clinical outcome was assessed with the Constant and DASH score.
Both, the Constant and DASH scores improved significantly from 11% to 75% and from 70 to 30 points, P < 0.01 respectively. There were no significant differences regarding age, etiology, cemented or metal-backed glenoids, etc. (P > 0.05). The pre- and postoperative humeral offset, the lateral glenohumeral offset, the height of the CoR, the acromiohumeral distance as well as neck-shaft angle showed no significant changes (P > 0.05). The mean deviation of the CoR of the prosthesis from the anatomic center was 1.0 ± 2.8 mm. Three cases showed a medial deviation of more than 3 mm. These deviations of 5.1, 5.7 and 7.6 mm and were caused by an inaccurate humeral neck cut. These 3 patients showed a relatively poor outcome scoring.
TESS arthroplasty allows an anatomical joint reconstruction with a very good outcome. Outliers described in this study sensitize the surgeon for an accurate humeral neck cut.
Core tip: By using bony landmarks that are not altered by osteoarthritic changes, the premorbid center of rotation (CoR) was assessed in comparison to the postoperative one after TESS arthroplasty. Furthermore, joint geometry changes were assessed in relation to the glenoid, the acromion and the proximal humerus. Our data demonstrate a precise restoration of the joint and a very good clinical outcome. This study also describes outliers with a clinically relevant medialized CoR. Being caused by a slightly inaccurate humeral neck cut, this study might sensitize us that this osteotomy is a crucial step to ensure a good clinical outcome.
- Citation: von Engelhardt LV, Manzke M, Breil-Wirth A, Filler TJ, Jerosch J. Restoration of the joint geometry and outcome after stemless TESS shoulder arthroplasty. World J Orthop 2017; 8(10): 790-797
- URL: https://www.wjgnet.com/2218-5836/full/v8/i10/790.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i10.790
The traditional stemmed design of anatomical total shoulder arthroplasties is based on the principles of total hip replacement. Similarly to hip arthroplasty, stem-related complications, such as a bone loss secondary to stress shielding, humeral fractures, etc., are not so infrequent[1-4]. A further difficulty of a stemmed shoulder arthroplasty is that the restoration of the individual anatomy with its offset and center of rotation (CoR) is not always reached even with newer modular designs[5,6]. Another aspect is the revision surgery, where severe difficulties may arise during and after stem revision. A recent report describes complications such as a canal perforation, bone destructions and humerus fractures in around 50% of the cases[7]. Thus, avoiding stem-related complications, improved options to gain an anatomic reconstruction of the proximal humerus and preserving the bone stock for easier revisions are practical reasons why stemless designs have been introduced as an alternative to traditional designs. However, both patients and surgeons have high expectations regarding activity levels and return to sports following shoulder replacement surgery[8]. In regard to these data, the ongoing development of shoulder arthroplasty is a logical consequence.
The Total Evolutive Shoulder System (TESS, Biomet-Zimmer, Warsaw, IN, United States) uses different sizes of an impaction-implanted 6-armed corolla for a peripheral metaphyseal anchoring close to the cortical bone. This method of fixation is different to those with a much more central anchoring within the metaphysis, e.g., the threaded central cage of the Arthrex Eclipse (Arthrex, Karlsfeld, Germany) or the Simpliciti system with a nucleus and 3 fins for central impaction (Wright Medical, formerly Tornier, Montbonnot, France)[9]. The principle of a peripheral metaphyseal anchoring might influence the reconstruction of the individual anatomy of the proximal humerus. The purpose of this study was to evaluate the restoration of the joint geometry as well as the clinical and radiographic outcome of the TESS system for anatomical shoulder arthroplasty.
This study has been approved by the Ethical Committee of the University of Duesseldorf (Study No. 4426). All patients were operated at the Department of Orthopedics, Trauma Surgery and Sports Medicine of the Johanna-Etienne Hospital Neuss. Patients included in this study had an anatomical shoulder arthroplasty with the TESS system (TESS, Biomet-Zimmer, Warsaw, IN, United States). Pre-operative planning of the prosthesis components was performed in all cases on scaled anteroposterior digital radiographs using the MediCAD® software. After a deltopectoral approach, the elevation of the subscapularis tendon and the dislocation of the humeral head, the rotator cuff insertions, the humeral head and the anatomical neck were visualized. The cutting guide was held parallel to the anatomical neck and the inclination, retroversion and the height of the cut were adjusted by using these landmarks. After the saw cut, the size of the corolla broach was measured using the humeral sizing templates. Then the glenoid was prepared. A cemented all-polyethylene component or a metal-backed glenoid which allows a conversion to a reversed version were available. After broaching and impaction of the corolla into the metaphysis, different trial heads with a diameter of 41, 43, 45, 48, 50 and 52 mm with or without an offset were available. The subscapularis tendon was reattached to its origin by using transosseus Ethibond sutures. A biceps tenodesis was performed in patients with slender overarms. Physiotherapy with restricted external rotation was started directly after the operation. Besides the exclusion of patients with rotator cuff tears or a defect arthropathy, there were no further exclusion criteria for the implantation of an anatomical TESS prosthesis. All patients received a non-stemmed version. The decision whether to use a stemmed or non-stemmed design was made intraoperatively depending on the metaphyseal bone quality. One patient with a humeral head necrosis had an incorrect positioning of the humeral component leading to an extensively elevated humeral offset. In this patient, a revision to a stemmed version was performed immediately. This case was considered a surgical failure. Another patient suffered a fall with a traumatic rotator cuff tear before the follow-up appointment. Both patients were excluded from this study. Finally, 21 shoulders in 19 patients (m/f = 10/9) with anatomic TESS shoulder prostheses were evaluated regarding their clinical and radiological outcome. The mean follow-up was 18 ± 9 mo. The average age at surgery was 66 years (range 32-79 years). In 10 cases, the dominant side was involved. 15 shoulders received a total arthroplasty with a metal-backed glenoid, four a cemented PE glenoid and three received a hemiarthroplasty. Indications were an osteoarthritis (n = 19) and a humeral head necrosis (n = 2). One necrosis was caused by a thalassaemia and one was posttraumatic after plate fixation of a proximal humeral fracture.
As recommended by Booker et al[10], the clinical outcome of the patients was assessed with the combination of two outcome scoring tools. The Disabilities of the Arm, Shoulder, Hand (DASH) score was used as a patient self-assessment measurement tool and the constant score (CS) as a clinically-based outcome measuring.
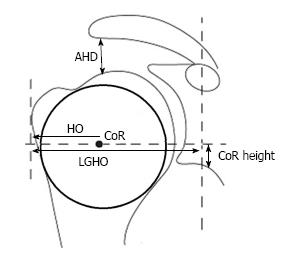
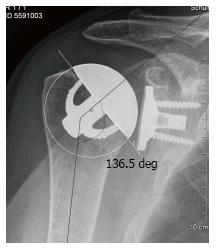
A standardized anterior-posterior- and an axillary view were performed preoperatively and at the follow-up appointment. Preoperative X-rays were scaled by using a 25 mm diameter ball marker. Postoperative X-rays were scaled using the size of the glenosphere. Measurements were performed with the MediCAD® software. The premorbid CoR was assessed with the best-fit circle method generated with three bony landmarks which are not altered by the osteoarthritic articular surface (Figures 1 and 2): The lateral cortex of the greater tuberosity, the medial calcar at the inflection point where the calcar meets the articular surface, and the medial edge of the greater tuberosity at the medial supraspinatus insertion[11]. This way, the deviation of the CoR of the implanted humeral head can be assessed in comparison to the native anatomic one. As described by Alolabio et al[12], a deviation of more than 3 mm was considered as being clinically significant. A medial deviation compared to the premorbid CoR was defined as an overstuffing (Figure 3), whereas a lateral deviation was defined as an understuffing (Figure 4). To further assess the geometry of the pre- and postoperative CoR in relation to the glenoid, the acromion as well as to the proximal humerus, further parameters were measured as described by Thomas et al[13] (Figure 1). Because some preoperative X-rays showed a poor positioning quality, four cases had to be excluded from the assessment of these pre- to postoperative geometry changes. The following differences of the pre- and postoperative values were calculated: The acromiohumeral distance (AHD) is defined as the shortest distance between the humerus and the acromion, the humeral offset (HO) as the distance between the CoR and the lateral border of the greater tuberosity, the lateral glenohumeral offset (LGHO) as the distance between the basis of the coracoid and the lateral border of the greater tuberosity and the height of the CoR regarding to the inferior border of the glenoid (CoR height)[13]. Pre- to post-operative neck shaft angles, defined as the medial angle between the shaft axis and a perpendicular line to the anatomic neck, were also measured (Figure 2).
Statistical analysis were performed with SPSS Statistics software 22.0 (SPSS Inc., Chicago, Illinois, United States). The Wilcoxon test was used for the comparison between the pre- and postoperative data of the clinical scores, the Mann-Whitney-U test to compare the clinical scores between two different groups of the population and the Kruskal-Walis test to compare the clinical scores between several groups. The parametric Students t-test was used to compare pre- and post-operative geometrical measurements. The level of significance was set to alpha < 0.05. A statistical review was conducted by a biomedical statistician.
The relative CS and DASH score improved significantly from a median of 11% ± 19% to 75% ± 26% and from 70 ± 22 points to 30 ± 19 points, P < 0.01, respectively. In the symptoms section of the DASH score, the patients improved from 24 ± 7 points to 12 ± 5 points (P < 0.01). In the function section, the score improved from 82 ± 19 points to 53 ± 18 points. There were no significant differences regarding age, sex, etiology groups, side of surgery, cemented or metal back glenoids treatment with a hemi- or total arthroplasty (P > 0.05).
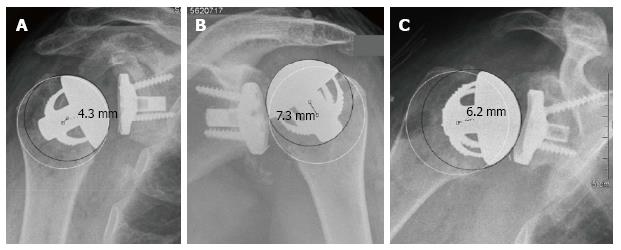
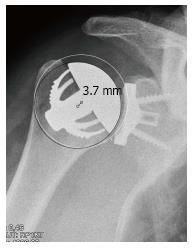
With the best-fit circle method, the mean deviation of the CoR of the prosthesis from the anatomic CoR was 1.0 ± 2.8 mm. Of the 21 cases, four (19%) exhibited a deviation of more than 3 mm. Three cases (14%) showed an overstuffing with a medial deviation of 4.9, 6.2 and 7.6 mm. These patients showed a relatively poor outcome with a CS of 39, 41 and 51 points. The reasons for these deviations were a too high resection level (Figure 3A and C) and an inaccurate inclination of the humeral neck cut (Figure 3B). In the fourth case, the CoR of the humeral component was 3.7 mm lateral to the anatomical CoR. This deviation with an understuffing was caused by a slight undersizing of the humeral head (Figure 4). This patient showed a relatively high postoperative CS of 77 points.
The geometry of the CoR in relation to the glenoid, the acromion as well as to the proximal humerus, described by the AHD, HO, LGHO, the CoR height and the neck-shaft angle, showed only slight differences between the pre- and post-operative measurements. By using the Students t-test all these minor changes of the geometry presented in Table 1 were not significant (P > 0.05).
| Mean | Median | SD | Minimum | Maximum | |
| Pre-OP neck shaft angle | 135.4° | 135.3° | 3.0° | 131.6° | 139.7° |
| Post-OP neck shaft angle | 136.6° | 133.8° | 9.4° | 119.3° | 158.9° |
| Pre-OP AHD (mm) | 6 | 6.5 | 3.1 | 2 | 13 |
| Post-OP AHD (mm) | 9.6 | 7 | 6.8 | 2 | 25 |
| Pre-OP HO (mm) | 25.3 | 25 | 2.8 | 22 | 32 |
| Post-OP HO (mm) | 25.2 | 25.5 | 4.2 | 18 | 36 |
| Pre-OP LGHO (mm) | 63.9 | 63 | 6 | 52 | 74 |
| Post-OP LGHO (mm) | 60.9 | 62.5 | 6.5 | 49 | 74 |
| Pre-OP CoR height (mm) | 17.2 | 17 | 6.3 | 8 | 28 |
| Post-OP CoR height (mm) | 17.7 | 17.5 | 6.5 | 5 | 29 |
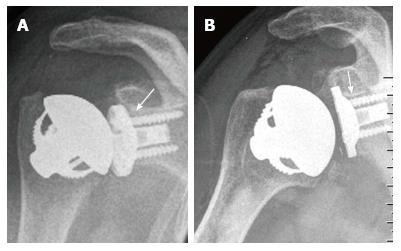
Small radiolucent lines were seen in two of 15 cases with a metal-backed glenoid (13%) (Figure 5). In both cases it was above the superior part of the upper screw and behind the superior third of the baseplate. Both radiolucencies measured a maximal thickness of 2 mm. Further signs of a loosening were not noticed. At the last follow-up, both patients were pain-free and showed a CS of 75 and 52 points. At the cemented all-polyethylene glenoid radiolucent lines and/or osteolyses were not noticed. Radiolucent lines were also not detected around the 21 humeral components.
We observed three (14%) complications. One patient with a posttraumatic humeral head necrosis developed a frozen shoulder which was treated with an arthroscopic capsular release. The CS at the last follow-up was 48 points. One patient showed a partial brachial plexus lesion. He underwent an intensive rehabilitation. At the follow-up appointment, he recovered partially but still showed a CS of only 15 points. One of the patients with an overstuffed positioning of the humeral component (Figure 3B) suffered a cuff failure three months after the last follow-up appointment nine months postoperatively. This patient showed a CS of only 51 points. A revision to a reversed prosthesis was performed.
In this study, we had to document three complications which lead to an overall complication rate of 14%. Looking closer, one partial brachial plexus lesion was treated with an intensive rehabilitation, one shoulder arthrofibrosis was treated with an arthroscopic capsular release and one cuff failure needed a revision to a reversed prosthesis. In recent review articles, the overall complication rate lies between 4.2% and 15.2%[14,15]. In the literature, an arthrofibrosis after shoulder arthroplasty is rarely documented[15,16], whereas a rotator cuff failure is reported with incidences between 1.3% and 14%[17-19] and a plexus lesion with incidences up to 15%[20,21]. Taken together, our complication rate is high and lies in the upper range compared to the literature. In our opinion, these results are poor and interfere with the outcome scorings. This should be highlighted at the beginning of this discussion.
The humeral head varies individually in its retroversion, inclination as well as its medial and posterior offset[22,23]. Therefore, first and second generation stemmed arthroplasties did not meet the requirements to reach an exact restoration of the anatomy[22]. Even if newer modular stemmed designs have improved the adaptation to the individual anatomy, an exact anatomic match is not always achieved[5,6]. In a finite element analysis, Büchler and Farron[24] demonstrated the importance of an anatomically reconstructed humeral head to avoid an eccentric glenoid loading. In a study on patients with dissatisfaction after shoulder arthroplasty, main findings were substantially malpositioned components with or without loosened glenoids, stiffness and instabilities[25]. These clinical and biomechanical studies demonstrate the importance of an exact reconstruction of the joint geometry to achieve a good clinical outcome.
The impacted corolla of the TESS prosthesis provides a peripheral metaphyseal anchoring[9]. This relatively stable fixation close to the cortical bone might explain why findings indicating a loosening were not noticed. This is in accordance to previous studies where no radiolucent lines were noticed around the corolla of the TESS implant[26,27]. On the other hand, the peripheral metaphyseal anchoring with different sizes might influence the reconstruction of the joint geometry. Our hypothesis was that the stemless TESS system provides a reliable reconstruction of the individual anatomy with a good clinical outcome. In our series, the relative CS and DASH scores improved significantly with results that are in a similar range to previous reports on the anatomic TESS prosthesis[26-28]. Youderian et al[11] demonstrated that the premorbid CoR can be accurately predicted by a circle fitted from preserved nonarticular bony landmarks. We used this best-fit circle to measure the deviation of the center of the prosthesis to the premorbid CoR. Previous studies demonstrated that a malpositioning of 3 to 4 mm can affect the clinical outcome[5,12,29-31]. According to Alolabi et al[12] and Kadum et al[31], we defined a deviation of 3 mm as clinically relevant. In our series, 81% showed no deviation or a deviation of less than 3 mm. Another study also used the best-fit circle method to assess the restoration of the CoR with different anatomical prosthesis types. This study demonstrated no deviation or a deviation of less than 3 mm with lower rates lying between 34.9% and 68.8%. The mean deviation between the premorbid CoR and the center of the prosthesis measured between 2.5 and 3.8 mm which is two to four times higher compared to our study[12]. However, even if our results are relatively good, we have to notice that we were not able to demonstrate a 100% rate of an exact restoration of the CoR. Thus, four patients (19%) showed a deviation of more than 3 mm. We hypothesized that a significant deviation might lead to a relatively poor clinical outcome. One patient showed an understuffing with a lateral deviation of the implant CoR which was caused by a relatively small humeral component. Showing a relatively high CS of 77 points, this deviation did not lead to a poor clinical outcome. Three patients showed an overstuffing caused by an inaccurate resection level for the humeral neck cut. With 51, 39 and 41 points, these patients showed a relatively poor CS. Because the inaccurate humeral neck cut lead to a clinically relevant overstuffing, these cases have to be characterized as avoidable failures during surgery. Besides a poor clinical outcome, one of these three patients suffered a cuff failure after the last follow-up, requiring a revision to a reversed arthroplasty. Showing an incidence of 11%, a recent systematic review suggests that these cuff tears following total shoulder arthroplasty may be more common than previously thought[19]. Maybe these data should sensitize the surgeon to be aware of an exact identification of anatomical landmarks for a correct humeral neck cut. Besides a digital scaled preoperative planning, the use of the best-fit circle method might support the surgeon’s ability to find the right resection level and to choose the correct head size. In some cases, osteophytes as bony landmarks might be helpful to mark the correct resection level and angle during surgery. In some cases, an intraoperative fluoroscopy, where the best-fit circle method can be used again, might provide an increased security to achieve an exact humeral head position and size. Especially in cases with advanced deformities or in cases where the achievement of an optimal soft tissue balancing of the implant is not completely satisfactory, such additional intraoperative X-rays might be helpful.
The pre- and post-operative AHD, HO, LGHO and the CoR height were measured as described by Thomas et al[13]. Table 1 depicts that these measurements, including the pre- and postoperative neck shaft angles, showed only minimal changes. Thus, the geometry of the CoR in relation to the glenoid, the proximal humerus and the acromion does not seem to be altered. Regarding these data, the TESS system allows a reliable restoration of the individual joint geometry. This might explain the relatively good clinical outcome of the TESS prosthesis described in our series as well as in previous studies with follow-up times ranging from 6 to 45 mo[26-28,32-34].
At the cemented all-polyethylene glenoid components, radiolucent lines were not noticed. This is similar to previous studies on pegged designs, where radiolucent lines were not detected[35,36]. At the metal-backed glenoids, small radiolucent lines were seen in two cases (2/15, 13%) behind the superior third of the baseplate and above the upper screw (Figure 5). Further signs of a loosening were not noticed. In previous studies with newer metal-backed glenoids, radiolucencies were also noticed in 7%[37], 10%[38,39] and 23%[40] of the cases. The TESS system has a central convex section in both the polyethylene and the metal-backed component. Compared to those with a flat-backed glenoid, this design showed lower distraction forces in biomechanical testings[41] as well as a lower presence and progression of radiolucencies[40]. Moreover, the metal-backed glenoid baseplate of the TESS system has a double coating with porous titanium and hydroxyapatite. Besides the design chracteristics, these material features have also been shown not to be as critical as those with older flat shaped, uncoated metal-backed glenoid components[40,42,43]. These features of the TESS system might explain why radiolucencies were noticed in our series in only two cases of metal-backed components and in none of the cases with a polyethylene glenoid component.
We acknowledge that our study has several limitations. There was no randomized control group treated with a conventional stemmed prosthesis to compare our results. Further limitations of this study are the short mean follow-up of 18 mo and the small number of 21 shoulders being evaluated. The study presented here was a necessary first step in exploring our first experiences with the TESS system, which seems to provide reasonable advantages. Therefore, the outcome scorings and the assessment of complications should be regarded cautiously. Larger studies with longer follow-up intervals are needed to assess the sustainability of the clinical outcome as well as long-term changes of the joint geometry. For this reason we recently applied for ethical approval of a long term study on the TESS prosthesis.
In conclusion, the stemless shoulder arthroplasty using the TESS system allows a reliable reconstruction of the individual anatomy with an excellent clinical outcome. On the other hand, we noticed three cases with a slight but clinically relevant overstuffed reconstruction of the CoR caused by an inaccurate humeral neck cut. This should increase our awareness. An optimized bone cut is a crucial step to ensure a good clinical outcome during surgery.
An exact reconstruction of the individual anatomy of the shoulder joint is vital to reach a good clinical outcome of anatomical shoulder replacement. The restoration of the joint geometry as well as the clinical and radiographic outcome of stemless, peripheral metaphyseal anchored shoulder arthroplasty by using the TESS system is evaluated.
Besides modular prosthesis designs, current developments in shoulder arthroplasty include smaller, bone sparing components. Research is needed to interpret advantages, pitfalls and the clinical efficacy.
Stemless TESS shoulder arthroplasty allows an exact reconstruction of the premorbid center of rotation (CoR). Additional parameters, such as the relation between the pre- and postoperative CoR and the glenoid, the acromion as well as the proximal humerus are reconstructed. Thus, the data demonstrate a precise restoration of the joint geometry with a good clinical outcome. A loosening of the metaphyseal anchoring was not detected. Similarly to previous studies, this article also demonstrates that even a slightly inaccurate humeral neck cut can cause a clinically relevant medialized CoR.
Surgical precision work and a highly modular prosthesis system with variable sizes is needed to ensure a good clinical outcome. The use of the best-fit circle method during the preoperative planning might be helpful to find the right resection level and to choose the correct head size. During surgery, an exact identification of anatomical landmarks is vital to find the correct level and angle for the humeral neck cut. In some cases, intraoperative fluoroscopy might be an additional support.
TESS: Total Evolutive Shoulder System; DASH score: Disabilities of the Arm, Shoulder and Hand score; CS: Constant score; CoR: Center of rotation; AHD: Acromiohumeral distance; HO: Humeral offset; LGHO: Lateral glenohumeral offset.
It is a well-written manuscript with information useful to the readers of the journal.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chen YK, Iban MAR, Li JM S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Nagels J, Stokdijk M, Rozing PM. Stress shielding and bone resorption in shoulder arthroplasty. J Shoulder Elbow Surg. 2003;12:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 348] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 2. | Raiss P, Edwards TB, Deutsch A, Shah A, Bruckner T, Loew M, Boileau P, Walch G. Radiographic changes around humeral components in shoulder arthroplasty. J Bone Joint Surg Am. 2014;96:e54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 3. | Rockwood CA Jr, Wirth MA. Observation on retrieved Hylamer glenoids in shoulder arthroplasty: problems associated with sterilization by gamma irradiation in air. J Shoulder Elbow Surg. 2002;11:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am. 2004;86-A:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Pearl ML, Kurutz S. Geometric analysis of commonly used prosthetic systems for proximal humeral replacement. J Bone Joint Surg Am. 1999;81:660-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Antoni M, Barthoulot M, Kempf JF, Clavert P. Revisions of total shoulder arthroplasty: Clinical results and complications of various modalities. Orthop Traumatol Surg Res. 2016;102:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Johnson CC, Johnson DJ, Liu JN, Dines JS, Dines DM, Gulotta LV, Garcia GH. Return to sports after shoulder arthroplasty. World J Orthop. 2016;7:519-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Churchill RS, Athwal GS. Stemless shoulder arthroplasty-current results and designs. Curr Rev Musculoskelet Med. 2016;9:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Booker S, Alfahad N, Scott M, Gooding B, Wallace WA. Use of scoring systems for assessing and reporting the outcome results from shoulder surgery and arthroplasty. World J Orthop. 2015;6:244-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Youderian AR, Ricchetti ET, Drews M, Iannotti JP. Determination of humeral head size in anatomic shoulder replacement for glenohumeral osteoarthritis. J Shoulder Elbow Surg. 2014;23:955-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Alolabi B, Youderian AR, Napolitano L, Szerlip BW, Evans PJ, Nowinski RJ, Ricchetti ET, Iannotti JP. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1740-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Thomas SR, Sforza G, Levy O, Copeland SA. Geometrical analysis of Copeland surface replacement shoulder arthroplasty in relation to normal anatomy. J Shoulder Elbow Surg. 2005;14:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Bohsali KI, Bois AJ, Wirth MA. Complications of Shoulder Arthroplasty. J Bone Joint Surg Am. 2017;99:256-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 222] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 15. | Roberson TA, Bentley JC, Griscom JT, Kissenberth MJ, Tolan SJ, Hawkins RJ, Tokish JM. Outcomes of total shoulder arthroplasty in patients younger than 65 years: a systematic review. J Shoulder Elbow Surg. 2017;26:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Harmer L, Throckmorton T, Sperling JW. Total shoulder arthroplasty: are the humeral components getting shorter? Curr Rev Musculoskelet Med. 2016;9:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 311] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 18. | Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88:2279-2292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 335] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 19. | Levy DM, Abrams GD, Harris JD, Bach BR Jr, Nicholson GP, Romeo AA. Rotator cuff tears after total shoulder arthroplasty in primary osteoarthritis: A systematic review. Int J Shoulder Surg. 2016;10:78-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Lynch NM, Cofield RH, Silbert PL, Hermann RC. Neurologic complications after total shoulder arthroplasty. J Shoulder Elbow Surg. 1996;5:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 131] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Pape G, Raiss P, Aldinger PR, Loew M. [Comparison of short-term results after CUP prosthesis with cemented glenoid components and total shoulder arthroplasty: a matched-pair analysis]. Z Orthop Unfall. 2010;148:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Roberts SN, Foley AP, Swallow HM, Wallace WA, Coughlan DP. The geometry of the humeral head and the design of prostheses. J Bone Joint Surg Br. 1991;73:647-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Boileau P, Walch G. The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br. 1997;79:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Büchler P, Farron A. Benefits of an anatomical reconstruction of the humeral head during shoulder arthroplasty: a finite element analysis. Clin Biomech (Bristol, Avon). 2004;19:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Hasan SS, Leith JM, Campbell B, Kapil R, Smith KL, Matsen FA 3rd. Characteristics of unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2002;11:431-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 221] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 26. | Berth A, Pap G. Stemless shoulder prosthesis versus conventional anatomic shoulder prosthesis in patients with osteoarthritis: a comparison of the functional outcome after a minimum of two years follow-up. J Orthop Traumatol. 2013;14:31-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Huguet D, DeClercq G, Rio B, Teissier J, Zipoli B; TESS Group. Results of a new stemless shoulder prosthesis: radiologic proof of maintained fixation and stability after a minimum of three years’follow-up. J Shoulder Elbow Surg. 2010;19:847-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Williams GR Jr, Wong KL, Pepe MD, Tan V, Silverberg D, Ramsey ML, Karduna A, Iannotti JP. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Harryman DT, Sidles JA, Harris SL, Lippitt SB, Matsen FA 3rd. The effect of articular conformity and the size of the humeral head component on laxity and motion after glenohumeral arthroplasty. A study in cadavera. J Bone Joint Surg Am. 1995;77:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 137] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Kadum B, Hassany H, Wadsten M, Sayed-Noor A, Sjödén G. Geometrical analysis of stemless shoulder arthroplasty: a radiological study of seventy TESS total shoulder prostheses. Int Orthop. 2016;40:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Kadum B, Mukka S, Englund E, Sayed-Noor A, Sjödén G. Clinical and radiological outcome of the Total Evolutive Shoulder System (TESS®) reverse shoulder arthroplasty: a prospective comparative non-randomised study. Int Orthop. 2014;38:1001-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Castricini R, De Benedetto M, Pirani P, Panfoli N, Pace N. Shoulder hemiarthroplasty for fractures of the proximal humerus. Musculoskelet Surg. 2011;95 Suppl 1:S49-S54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Hawi N, Tauber M, Messina MJ, Habermeyer P, Martetschläger F. Anatomic stemless shoulder arthroplasty and related outcomes: a systematic review. BMC Musculoskelet Disord. 2016;17:376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Everding NG, Levy JC, Formaini NT, Blum S, Gil CC, Verde K. Observation of initial postoperative radiolucent lines using a modern pegged-glenoid design. Int J Shoulder Surg. 2016;10:67-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Edwards TB, Labriola JE, Stanley RJ, O’Connor DP, Elkousy HA, Gartsman GM. Radiographic comparison of pegged and keeled glenoid components using modern cementing techniques: a prospective randomized study. J Shoulder Elbow Surg. 2010;19:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Panti JP, Tan S, Kuo W, Fung S, Walker K, Duff J. Clinical and radiologic outcomes of the second-generation Trabecular Metal™ glenoid for total shoulder replacements after 2-6 years follow-up. Arch Orthop Trauma Surg. 2016;136:1637-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Uschok S, Magosch P, Moe M, Lichtenberg S, Habermeyer P. Is the stemless humeral head replacement clinically and radiographically a secure equivalent to standard stem humeral head replacement in the long-term follow-up? A prospective randomized trial. J Shoulder Elbow Surg. 2017;26:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 39. | Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22:535-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Castagna A, Randelli M, Garofalo R, Maradei L, Giardella A, Borroni M. Mid-term results of a metal-backed glenoid component in total shoulder replacement. J Bone Joint Surg Br. 2010;92:1410-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Anglin C, Wyss UP, Pichora DR. Mechanical testing of shoulder prostheses and recommendations for glenoid design. J Shoulder Elbow Surg. 2000;9:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Boileau P, Avidor C, Krishnan SG, Walch G, Kempf JF, Molé D. Cemented polyethylene versus uncemented metal-backed glenoid components in total shoulder arthroplasty: a prospective, double-blind, randomized study. J Shoulder Elbow Surg. 2002;11:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 209] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 43. | Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90:2180-2188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |