Published online Oct 18, 2017. doi: 10.5312/wjo.v8.i10.747
Peer-review started: February 7, 2017
First decision: July 10, 2017
Revised: August 2, 2017
Accepted: August 15, 2017
Article in press: August 16, 2017
Published online: October 18, 2017
Processing time: 264 Days and 6 Hours
Osteonecrosis (ON) is caused by inadequate blood supply leading to bone death, which results in the collapse of the architectural bony structure. Femoral head is the most common site involved in ON. Magnetic resonance imaging (MRI) is a commonly used imaging modality to detect early ON. When MRI is inconclusive, bone scan is helpful in detecting ON during early phase of the disease. As newer nuclear medicine equipment, like single photon emission computed tomography/computed tomography (CT) and positron emission tomography/CT, are emerging in medical science, we review the role of these imaging modalities in ON of femoral head.
Core tip: Early diagnosis and treatment remains the key to hip preservation in osteonecrosis (ON). Till date magnetic resonance imaging (MRI) is considered as the gold standard diagnostic modality for ON. However with the improvement in nuclear imaging technique, the disease can be diagnosed even at a very early stage. Available literature suggests that single photon emission computed tomography/computed tomography (CT) bone scan and 18F-fluoride photon emission computed tomography/CT have similar or better results in comparison to MRI in ON of the femoral head. They also provide both morphological and metabolic information in the disease part and hence can indicate whether the disease is active or healed.
- Citation: Agrawal K, Tripathy SK, Sen RK, Santhosh S, Bhattacharya A. Nuclear medicine imaging in osteonecrosis of hip: Old and current concepts. World J Orthop 2017; 8(10): 747-753
- URL: https://www.wjgnet.com/2218-5836/full/v8/i10/747.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i10.747
Osteonecrosis (ON) is caused by inadequate blood supply leading to bone death, which results in the collapse of the architectural bony structure. ON can be due to interruption of the vascular supply as a result of local trauma or non-traumatic systemic conditions[1]. In general, symptomatic patients present with pain and reduced range of motion. Initially, pain is due to increase in intramedullary pressure resulting from medullary bone marrow edema. However, clinical diagnosis is often difficult due to lack of specific symptoms during the initial period. Common sites for ON include the femoral head, humeral head, knee, femoral and tibial metadiaphysis, scaphoid, lunate and talus[2]. Femoral head is the most common site involved in ON[1]. In the femoral head, site of necrosis is immediately below the weight bearing articular surface of the bone, i.e., the anterolateral aspect of the femoral head. Early recognition of the disease is essential for better patient management.
The clinical findings and imaging studies are the primary modalities used to diagnose and stage ON. In radiology, the imaging modalities to detect ON are conventional radiographs, computed tomography (CT) and magnetic resonance imaging (MRI). In nuclear medicine imaging, the imaging modalities are planar bone scintigraphy, single photon emission computed tomography (SPECT) only bone scintigraphy, SPECT/CT bone scintigraphy and 18F-fluoride positron emission tomography/CT (PET/CT) bone scan. Different staging systems have been developed based on the severity of symptoms and imaging findings. Knowledge of degree of involvement in ON of femoral head helps in selecting the optimal treatment and also predicting the prognosis. In general, the most important feature of the staging system is the initial collapse of the cortex of femoral head. Before collapse, the necrotic lesion in the cortex can undergo repair and the damage may be reversible; after collapse, the damage is irreversible[1].
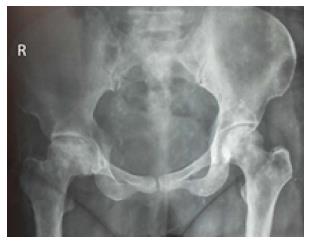
Being the least expensive and most widely available method, imaging evaluation of ON usually begins with conventional radiography. Ideally, both frontal and frog-leg lateral projections should be obtained. The typical radiographic appearance shows patchy areas of lucency with surrounding sclerosis[2]. The surrounding sclerotic margin correlates with the host bone response to wall off the areas of necrosis. X-rays may also show early articular collapse (Figure 1). However, radiography has low sensitivity during early stages.
In early femoral head ON, a CT scan may shows alteration of the normal “asterisk” that is formed due to condensation of the compressive and tensile trabeculae. Although, CT has lower sensitivity in detection of early changes than scintigraphy or MR imaging, it is helpful for detecting articular collapse location and extent in epiphyseal ON[3-5].
MRI is considered the most sensitive and specific imaging modality in detection of ON[6,7]. Generally, early fatty marrow necrosis is not associated with changes in signal intensity on MR studies. Death of cellular marrow components initiates tissue reaction, which results in appearance of a reactive interface between live and necrotic marrow areas. The hallmark of early avascular necrosis lesions is clear delineation of normal-appearing epiphyseal area with either a low-signal-intensity band on T1-weighted images or a rim of sclerosis on radiographs[2]. The rim of sclerosis is often crescentic or wedge shaped. The interface appears as a low-signal-intensity band with a sharp inner face and a blurred outer face on MRI[8]. A “double-line” sign on T2 weighted MRI has been demonstrated in 65%-85% of cases of early ON[8]. This double-line sign consists of an outer low signal intensity rim of sclerosis and a second inner high signal intensity representing the reparative granulation tissue of the reactive interface. However, some authors have attributed the outer low signal intensity rim to a potential chemical-shift artifact[9].
In a minority of patients, the area of ON may also show intrinsic characteristics such as hemorrhage (high signal intensity on T1- and T2-weighted), cystic changes (low signal intensity on T1-weighted and high signal intensity on T2-weighted images), and fibrous tissue (low signal intensity with all pulse sequences). Non-specific diffuse marrow signal abnormality may be seen in patients with sickle cell anemia and Gaucher’s disease. On a contrast enhanced MR study, typically, there is lack of enhancement of the necrosed area. A peripheral rim of enhancement corresponding to the zone of creeping substitution granulation tissue is generally seen[2]. In advanced disease, lesions show low signal intensity on T1 weighted and variable signal intensity on T2 weighted images. However, previous studies have demonstrated that signal changes on MR imaging may not be evident despite histologic evidence of ON[10-12]. It has been shown that signal changes caused by the death of marrow cells from ischemia on T1- and T2-weighted images may not occur until 5 d after interruption in the blood supply[10]. Hence, MRI may be false negative in the early phase of ON.
Technetium-99m methylene diphosphonate (99mTc-MDP) bone scintigraphy is one of the most commonly performed nuclear medicine studies. It is highly sensitive in detection of different benign and malignant bone pathologies[13]. The skeleton is made up of inorganic calcium hydroxyapatite crystals. The tracer uptake in a bone scan primarily identifies areas of osteoblastic activity. 99mTc-MDP binding occurs by chemisorption in the hydroxyapatite component of the osseous matrix. However, blood flow is the other most important factor influencing uptake of the radiotracer. As low as 5% change in bone turnover can be detected on bone imaging, whereas 40%-50% of mineral must be lost to detect lucency within the bone on radiographs and CT[14].
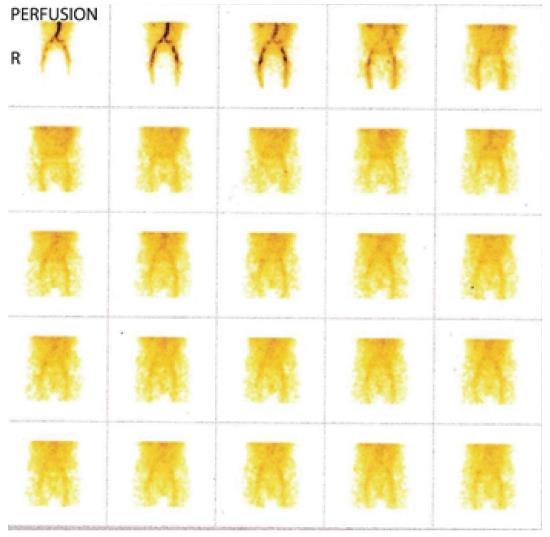
Three phase bone scan is usually performed in patients with suspected ON. In the three phase study, a bolus of 99mTc-MDP is injected intravenously with the concerned body parts under the gamma camera. The first phase of the study includes immediate dynamic images after radiotracer injection acquired for 60 s. The second phase or blood pool or soft tissue phase is acquired after approximately 5-10 min of radiotracer administration and delayed phase after 2-3 h.
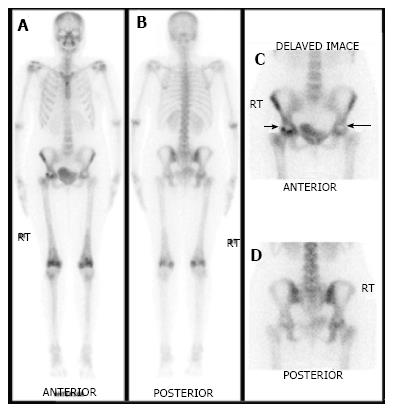
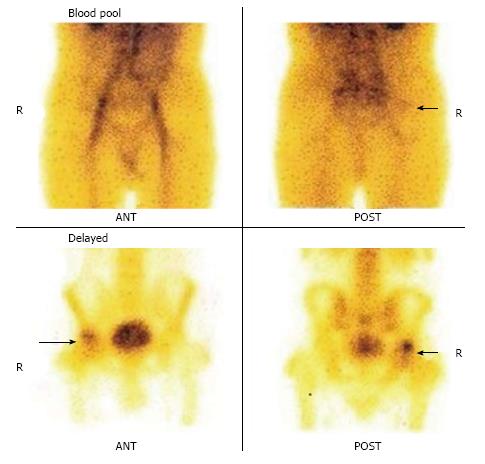
As ON is an evolving process, the appearance on bone scan depends on the stage of the disease. In the acute phase of ON, no radiotracer is delivered to the bone tissue. Therefore, initially for 7-10 d after the event, ON generally appears on bone imaging as a photopenic area (Figures 2 and 3). After 1-3 wk, increased radiotracer uptake is seen in a subchondral distribution due to osteoblastic activity at the reactive interface around the necrotic segment[15]. Imaging with pinhole collimator is useful as it increases the resolution. Overall sensitivity of bone scintigraphy for diagnosis of ON of femoral head is from 78% to 91%[16-18]. This variation in sensitivity is probably due to different etiology of ON of femoral head. For example, sensitivity is high in ON following femoral neck fracture due to sudden and nearly complete cut off of blood supply resulting in large, well defined cold lesion on bone scintigraphy. However, in chronic processes like steroid induced ON, typical cold lesion may not be identified and scintigraphy usually demonstrates increased tracer localization due to microcollapse and repair. Some studies have shown that bone scan is superior to conventional MRI in early detection of ON[19]. However, a planar bone scan has its own limitations of low specificity due to difficulty in distinguishing ON from fractures, transient osteoporosis or other conditions[1]. Although MRI is considered the diagnostic modality of choice in patients with femoral head ON, bone scan remains a valid alternative with fractured femoral neck with a metallic fixation device. Moreover, it is also helpful when involvement of multiple sites is suspected in patients with risk factors such as sickle cell disease.
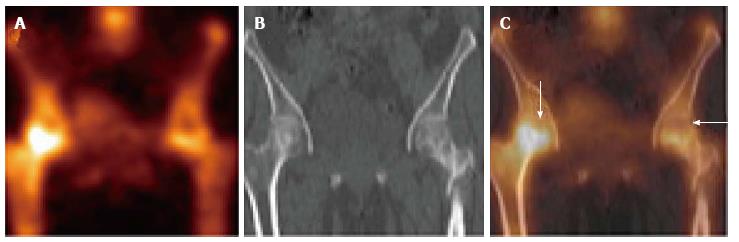
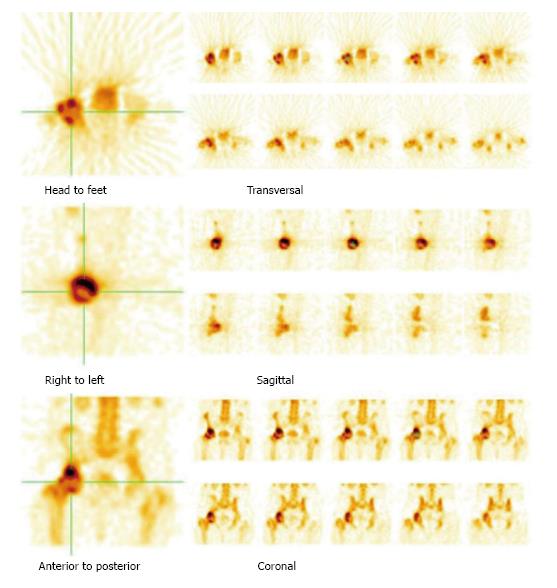
SPECT imaging is a nuclear medicine modality which produces cross-sectional images similar in presentation to CT and MRI in radiology. On planar bone scintigraphy, earliest and most evident finding of ON, i.e., photopenic region in the femoral head may be obscured by the superimposed acetabular and other surrounding bone activity[20]. This overlying increased activity could be due to osteoarthritis, fracture, inflammatory arthritis, etc., and results in false negative test results. As SPECT provides three-dimensional images, it is possible to separate the femoral head from other overlying bony structures (Figures 4-6). Siddiqui et al[21] demonstrated that both MRI and bone SPECT are complementary to each other in detecting subclinical avascular necrosis in asymptomatic renal allograft recipients. Ryu et al[20] showed that bone SPECT imaging is more sensitive than MRI in early detection of femoral head ON in renal transplant recipients. Their study revealed 100% sensitivity of SPECT in detection of ON of the femoral head, compared to 66% for MRI. Several other studies have shown that in ON of the femoral head, the sensitivity of MRI ranges from 85% to 100% and that of SPECT bone imaging ranges from 85% to 97%[22-24]. Hence, SPECT bone scan could be equally informative in patients with suspicion of ON of the femoral head.
Hybrid SPECT/CT provides both anatomical and metabolic information (Figures 2 and 3)[13]. CT component is helpful in localization and characterization of increased osteoblastic activity seen on planar or SPECT only images. CT scan added to SPECT can detect subtle collapse of the femoral head, which may not be easily visible on plain radiographs. In addition, morphological imaging may detect other underlying pain generators, which may explain the symptoms. Although SPECT-only bone scintigraphy has high sensitivity, its specificity is low. Luk et al[25] showed that SPECT/CT has similar sensitivity (100%) as SPECT bone scintigraphy, but better specificity compared to SPECT imaging alone (88% vs 82%) for the diagnosis of ON of the femoral head. In another study, SPECT/CT was found superior to planar and SPECT only bone scan for the diagnosis of ON. SPECT/CT demonstrated diagnostic accuracy of 95%, sensitivity of 98% and specificity of 87% compared to diagnostic accuracy of 67%, sensitivity of 75% and specificity of 40% for planar bone scan[26].
With wider use of PET/CT and reintroduction of the 18F-fluoride bone scan, its role in ON has been evaluated by researchers. 18F-fluoride is a positron-emitting radiotracer[13]. After diffusion through capillaries into bone extracellular fluid, 18F-ions exchange with hydroxyl groups in hydroxyapatite crystals to form fluorapatite[13]. 18F-fluoride PET has several advantages over 99mTc-MDP bone scan. 18F-fluoride has approximately 100% first-pass extraction in bone, allowing the estimation of bone blood flow. Further, its uptake in bone is two-fold higher than that of 99mTc-MDP. Moreover, it is not protein bound, which leads to faster blood clearance and better target-to-background ratio. In addition, PET/CT imaging has higher resolution compared to SPECT/CT leading to better appreciation of the characteristic photopenic region on PET/CT. Hence, 18F-fluoride bone scan has high sensitivity compared to 99mTc-MDP bone scan[13]. Quantification is another technical advantage of PET/CT imaging. Additional CT features theoretically results in high specificity of 18F-fluoride PET/CT in ON of femoral head.
Typically, a ring sign, i.e., circular pattern uptake surrounding a photopenic region is noted on 18F-fluoride PET/CT studies[27]. The central photopenia represents the necrotic area, whereas the surrounding uptake area probably reflects reactive bone formation around the necrotic area or microcollapse. A study by Gayana et al[28] revealed higher sensitivity and accuracy of 18F-fluoride PET/CT compared to MRI in ON of the femoral head. Recently, one group showed that metabolic information obtained from 18F-fluoride PET/CT is useful to predict femoral head collapse in ON[27]. A semi-quantitative parameter like SUVmax is used for quantifying bone metabolism. They concluded that SUVmax increases with stage progression and collapse of femoral head was observed within 12 mo after 18F-fluoride PET in patients with SUVmax more than 6.45. This finding could be very useful in predicting femoral head collapse in ON and may lead to early management change.
Imaging plays a crucial role in early diagnosis of ON of the femoral head. Although MRI is a commonly used modality in detecting ON, nuclear medicine imaging technology has improved tremendously in recent years. Although there is scarcity of literature, new imaging modalities like SPECT/CT bone scan and 18F-fluoride PET/CT have similar or better results in comparison to MRI in ON of the femoral head. In addition, they provide both morphological and metabolic information.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: Cui Q, Georgiev GPP, Liu JY, Lu KH S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32:94-124. [PubMed] |
| 2. | Murphey MD, Foreman KL, Klassen-Fischer MK, Fox MG, Chung EM, Kransdorf MJ. From the radiologic pathology archives imaging of osteonecrosis: radiologic-pathologic correlation. Radiographics. 2014;34:1003-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Hauzeur JP, Pasteels JL, Orloff S. Bilateral non-traumatic aseptic osteonecrosis in the femoral head. An experimental study of incidence. J Bone Joint Surg Am. 1987;69:1221-1225. [PubMed] |
| 4. | Stevens K, Tao C, Lee SU, Salem N, Vandevenne J, Cheng C, Neumann G, Valentin-Opran A, Lang P. Subchondral fractures in osteonecrosis of the femoral head: comparison of radiography, CT, and MR imaging. AJR Am J Roentgenol. 2003;180:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Yeh LR, Chen CK, Huang YL, Pan HB, Yang CF. Diagnostic performance of MR imaging in the assessment of subchondral fractures in avascular necrosis of the femoral head. Skeletal Radiol. 2009;38:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Glickstein MF, Burk DL Jr, Schiebler ML, Cohen EK, Dalinka MK, Steinberg ME, Kressel HY. Avascular necrosis versus other diseases of the hip: sensitivity of MR imaging. Radiology. 1988;169:213-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 70] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Kamata N, Oshitani N, Sogawa M, Yamagami H, Watanabe K, Fujiwara Y, Arakawa T. Usefulness of magnetic resonance imaging for detection of asymptomatic osteonecrosis of the femoral head in patients with inflammatory bowel disease on long-term corticosteroid treatment. Scand J Gastroenterol. 2008;43:308-313. [PubMed] |
| 8. | Vande Berg BE, Malghem JJ, Labaisse MA, Noel HM, Maldague BE. MR imaging of avascular necrosis and transient marrow edema of the femoral head. Radiographics. 1993;13:501-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 106] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Duda SH, Laniado M, Schick F, Claussen CD. The double-line sign of osteonecrosis: evaluation on chemical shift MR images. Eur J Radiol. 1993;16:233-238. [PubMed] |
| 10. | Brody AS, Strong M, Babikian G, Sweet DE, Seidel FG, Kuhn JP. John Caffey Award paper. Avascular necrosis: early MR imaging and histologic findings in a canine model. AJR Am J Roentgenol. 1991;157:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Genez BM, Wilson MR, Houk RW, Weiland FL, Unger HR Jr, Shields NN, Rugh KS. Early osteonecrosis of the femoral head: detection in high-risk patients with MR imaging. Radiology. 1988;168:521-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Koo KH, Jeong ST, Jones JP Jr. Borderline necrosis of the femoral head. Clin Orthop Relat Res. 1999;358:158-165. [PubMed] |
| 13. | Agrawal K, Marafi F, Gnanasegaran G, Van der Wall H, Fogelman I. Pitfalls and Limitations of Radionuclide Planar and Hybrid Bone Imaging. Semin Nucl Med. 2015;45:347-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Brenner AI, Koshy J, Morey J, Lin C, DiPoce J. The bone scan. Semin Nucl Med. 2012;42:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Fernandez-Ulloa M, Klostermeier TT, Lancaster KT. Orthopaedic nuclear medicine: the pelvis and hip. Semin Nucl Med. 1998;28:25-40. [PubMed] |
| 16. | Lucie RS, Fuller S, Burdick DC, Johnston RM. Early prediction of avascular necrosis of the femoral head following femoral neck fractures. Clin Orthop Relat Res. 1981;161:207-214. [PubMed] |
| 17. | Markisz JA, Knowles RJ, Altchek DW, Schneider R, Whalen JP, Cahill PT. Segmental patterns of avascular necrosis of the femoral heads: early detection with MR imaging. Radiology. 1987;162:717-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 75] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Beltran J, Herman LJ, Burk JM, Zuelzer WA, Clark RN, Lucas JG, Weiss LD, Yang A. Femoral head avascular necrosis: MR imaging with clinical-pathologic and radionuclide correlation. Radiology. 1988;166:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 94] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Koo KH, Kim R, Cho SH, Song HR, Lee G, Ko GH. Angiography, scintigraphy, intraosseous pressure, and histologic findings in high-risk osteonecrotic femoral heads with negative magnetic resonance images. Clin Orthop Relat Res. 1994;308:127-138. [PubMed] |
| 20. | Ryu JS, Kim JS, Moon DH, Kim SM, Shin MJ, Chang JS, Park SK, Han DJ, Lee HK. Bone SPECT is more sensitive than MRI in the detection of early osteonecrosis of the femoral head after renal transplantation. J Nucl Med. 2002;43:1006-1011. [PubMed] |
| 21. | Siddiqui AR, Kopecky KK, Wellman HN, Park HM, Braunstein EM, Brandt KD, Klatte EC, Capello WN, Leapman SB, Filo RS. Prospective study of magnetic resonance imaging and SPECT bone scans in renal allograft recipients: evidence for a self-limited subclinical abnormality of the hip. J Nucl Med. 1993;34:381-386. [PubMed] |
| 22. | Collier BD, Carrera GF, Johnson RP, Isitman AT, Hellman RS, Knobel J, Finger WA, Gonyo JE, Malloy PJ. Detection of femoral head avascular necrosis in adults by SPECT. J Nucl Med. 1985;26:979-987. [PubMed] |
| 23. | Kim KY, Lee SH, Moon DH, Nah HY. The diagnostic value of triple head single photon emission computed tomography (3H-SPECT) in avascular necrosis of the femoral head. Int Orthop. 1993;17:132-138. [PubMed] |
| 24. | Sarikaya I, Sarikaya A, Holder LE. The role of single photon emission computed tomography in bone imaging. Semin Nucl Med. 2001;31:3-16. [PubMed] |
| 25. | Luk WH, Au-Yeung AW, Yang MK. Diagnostic value of SPECT versus SPECT/CT in femoral avascular necrosis: preliminary results. Nucl Med Commun. 2010;31:958-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Agarwal KK, Mukherjee A, Sharma P, Bal C, Kumar R. Incremental value of 99mTc-MDP hybrid SPECT/CT over planar scintigraphy and SPECT in avascular necrosis of the femoral head. Nucl Med Commun. 2015;36:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Kubota S, Inaba Y, Kobayashi N, Tateishi U, Ike H, Inoue T, Saito T. Prediction of femoral head collapse in osteonecrosis using 18F-fluoride positron emission tomography. Nucl Med Commun. 2015;36:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Gayana S, Bhattacharya A, Sen RK, Singh P, Prakash M, Mittal BR. F-18 fluoride positron emission tomography/computed tomography in the diagnosis of avascular necrosis of the femoral head: Comparison with magnetic resonance imaging. Indian J Nucl Med. 2016;31:3-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |