Peer-review started: June 20, 2016
First decision: July 29, 2016
Revised: August 29, 2016
Accepted: October 25, 2016
Article in press: October 27, 2016
Published online: January 18, 2017
Processing time: 211 Days and 19.6 Hours
To present the incidence of heterotopic ossification after the use of recombinant human bone morphogenetic protein-7 (rhBMP-7) for the treatment of nonunions.
Bone morphogenetic proteins (BMPs) promote bone formation by auto-induction. Recombinant human BMP-7 in combination with bone grafts was used in 84 patients for the treatment of long bone nonunions. All patients were evaluated radiographicaly for the development of heterotopic ossification during the standard assessment for the nonunion healing. In all patients (80.9%) with radiographic signs of heterotopic ossification, a CT scan was performed. Nonunion site palpation and ROM evaluation of the adjacent joints were also carried out. Factors related to the patient (age, gender), the nonunion (location, size, chronicity, number of previous procedures, infection, surrounding tissues condition) and the surgical procedure (graft and fixation type, amount of rhBMP-7) were correlated with the development of heterotopic ossification and statistical analysis with Pearsons χ2 test was performed.
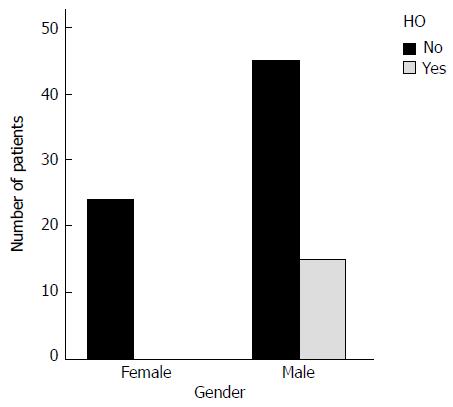
Eighty point nine percent of the nonunions treated with rhBMP-7, healed with no need for further procedures. Heterotopic bone formation occurred in 15 of 84 patients (17.8%) and it was apparent in the routine radiological evaluation of the nonunion site, in a mean time of 5.5 mo after the rhBMP-7 application (range 3-12). The heterotopic ossification was located at the femur in 8 cases, at the tibia in 6, and at the humerus in οne patient. In 4 patients a palpable mass was present and only in one patient, with a para-articular knee nonunion treated with rhBMP-7, the size of heterotopic ossification affected the knee range of motion. All the patients with heterotopic ossification were male. Statistical analysis proved that patient’s gender was the only important factor for the development of heterotopic ossification (P = 0.007).
Heterotopic ossification after the use of rhBMP-7 in nonunions was common but it did not compromise the final clinical outcome in most cases, and affected only male patients.
Core tip: Bone morphogenetic proteins are identified as factors promoting osteogenesis. In this study an attempt was made to estimate the rate of heterotopic bone formation in patients with long bone nonunions treated with recombinant human bone morphogenetic protein-7 (rhBMP-7), and to identify predisposing factors, related to the patient, the nonunion characteristics, and the surgical procedure. Eighteen percent of the patients developed heterotopic ossification on the radiographs, without functional limitations. All patients that developed heterotopic ossification were male. This rate of heterotopic ossification after rhBMP-7 use for the treatment of long bone nonunions is higher than the rates reported in literature.
- Citation: Papanagiotou M, Dailiana ZH, Karachalios T, Varitimidis S, Hantes M, Dimakopoulos G, Vlychou M, Malizos KN. Heterotopic ossification after the use of recombinant human bone morphogenetic protein-7. World J Orthop 2017; 8(1): 36-41
- URL: https://www.wjgnet.com/2218-5836/full/v8/i1/36.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i1.36
Heterotopic ossification is commonly complicating orthopaedic procedures and trauma but its highest incidence occurs after brain injuries. The hip, the elbow, and the knee are the most commonly affected joints after muscle damage, intramuscular hematoma and brain trauma[1-7]. Depending on the site and the location of the heterotopic bone, it may interfere with muscle and tendon function and limit the range of joint motion[3,4]. In experimental models, heterotopic bone is induced after implantation of bone marrow cells in muscle or in the peritoneal cavity[7] and allo-transplatation of demineralized bone matrix[8]. The later is linked to the activity of Bone Morphogenetic Proteins (BMPs) as factors promoting osteogenesis by auto-induction in extra-skeletal sites, as described by Urist et al[8].
Since their identification BMPs have been isolated and experimentally applied in several preclinical models. Recombinant (rh) forms are available for two of the BMPs (rhBMP-2 and rhBMP-7), and have been licensed by the american food and drug administration for clinical use in tibia nonunions (rhBMP-7)[9-11], and acute tibia fractures (rhBMP-2)[12,13]. Although the formation of heterotopic bone after the rhBMPs application in experimental animal models is well known[14], only a few reports confirm these findings in the clinical practice.
The aim of our study is to describe the development of heterotopic ossification in a series of 84 patients with long bone nonunions treated with bone graft and rhBMP-7, and to identify risk factors related to the patient, the nonunion and the surgical procedure.
Eighty-four patients (60 men and 24 women), with long bone nonunions treated with the combination of bone grafts and rhBMP-7 (Osigraft, Stryker Pharmaceutical) between 2004 and 2008[15] were evaluated for the development of heterotopic ossification. Nonunions were located in the upper (13) and lower (71) extremity and all patients had undergone at least one previous failed procedure for the treatment of the nonunion. The product used consisted of 3.3 mg of lyophilized rhBMP-7 combined with 6.7 mg of type I bovine collagen. The standard surgical procedure consisted of debridement of the nonunion site till normal viable bone margins. RhBMP-7 was applied mixed with bone grafts[15]. After rhBMP-7 implantation, irrigation was avoided, so as to prevent product leakage at the surrounding tissues.
Ηeterotopic ossification was diagnosed as a delayed postoperative complication, during the standard postoperative radiographic evaluation for the assessment of healing of the nonunion. The efficacy of rhBMP-7 on the treatment of long bone nonunions, in this series of patients, has already been published in a previous study[15]. In all patients with apparent signs of heterotopic bone formation on the radiographs, a quantitative computed tomography (Q-CT) and 3D-CT reconstruction (employing Osirix software) were obtained to confirm the diagnosis. The patients with heterotopic ossification were additionally evaluated clinically by palpation and examination of the range of motion of the adjacent joints.
Factors related to the patient (age, gender), the nonunion (location, size, chronicity, number of previous failed procedures, presence of infection, and condition of the surrounding soft tissues) and the type of the index surgical procedure (type of graft and amount of rhBMP-7 used), were also analyzed and correlated with the presence of heterotopic ossification.
Statistical analysis was performed with the Pearson χ2 test and with a logistic regression model under Firth’s correction (Stata version 10).
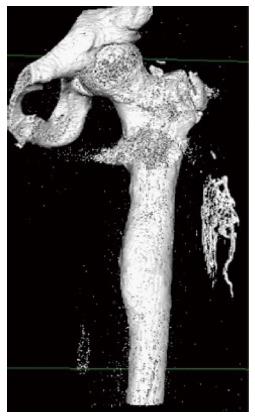
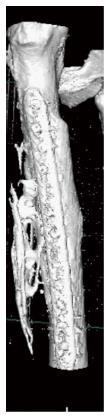
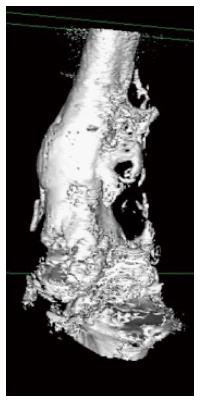
Heterotopic bone formation was diagnosed as a delayed complication within the first postoperative year in 15 patients (17.8%) (Figures 1-3). All patients were male (Figure 4) and the mean time to heterotopic ossification radiographic appearance was 5.5 mo (3 to 12 mo) after the index procedure. The heterotopic ossification was located at the lower extremity in 14 cases, 6 of them at the tibia and in 8 at the femur. In one case heterotopic ossification developed in a patient with a humeral nonunion. The ectopic bone was palpable in 4 patients, but only one had a limitation of the range of motion after the treatment of a para-articular distal femoral nonunion. This patient developed a significant restriction of flexion and extension of the knee joint (Figure 3).
The results were presented with the use of means and standard deviations or counts and percentages, where appropriate. The effect of categorical variables on the main outcome was examined with the use of the χ2 test, while the effect of scale variables with the use of the t test for independent samples or the Mann-Whitney test where normality did not hold, after implementation of the Shapiro Wilk test. The variables were then used in a logistic regression model under Firth’s correction. Significance was in all cases set at 0.05. The analysis was carried out with the use of the software Stata v.10.0.
Statistical analysis with the use of χ2 test, proved that patient’s gender was the only factor significantly correlating with the development of heterotopic ossification with statistical significance (P = 0.007) (Table 1 and Figure 4), while no other parameter had any effect on the development of this complication. The logistic regression model which was used, under Firth’s correction, did not show any parameter significantly affecting heterotopic bone formation, probably due to the small number of patients who developed this complication (Table 1).
| Parameter | No. of patients | HO | HO rate (%) | P value (χ2) | P value (logistic regression firth’s correction) | |
| Gender | Male | 60 | 15 | 25 | ||
| Female | 24 | 0 | 0.0 | 0.007 | 0.081 | |
| ≤ 30 | 20 | 6 | 30.0 | |||
| Age | 31-55 | 39 | 8 | 20.5 | 0.067 | 0.205 |
| ≥ 56 | 25 | 1 | 4.0 | |||
| Extremity | Upper | 13 | 1 | 7.7 | ||
| Lower | 71 | 14 | 19.7 | 0.298 | 0.733 | |
| Defect size | Critical | 42 | 8 | 19.0 | ||
| Non critical | 42 | 7 | 16.0 | 0.776 | 0.997 | |
| Chronicity | > 1 yr | 33 | 4 | 12.1 | ||
| < 1 yr | 51 | 11 | 20.3 | 0.229 | 0.731 | |
| Infection | Yes | 30 | 4 | 13.3 | ||
| No | 54 | 11 | 20.3 | 0.420 | 0.451 | |
| Soft tissue defects | Yes | 9 | 3 | 33.3 | ||
| No | 75 | 12 | 16.0 | 0.200 | 0.287 | |
| Previous procedures | 1 | 56 | 8 | 14.3 | ||
| 2-3 | 28 | 7 | 25.0 | 0.227 | 0.315 | |
| Graft type | Autograft | 67 | 13 | 19.4 | ||
| Allograft | 9 | 0 | 0 | 0.463 | 0.663 | |
| No graft | 8 | 2 | 25.0 | |||
| Amount of rhBMP-7 | 1 vial | 75 | 14 | 18.6 | ||
| 2 vials | 9 | 1 | 11.1 | 0.576 | 0.629 | |
The incidence of heterotopic bone formation in this series is relatively high (17.8%), but in the majority of cases (14 of 15) it did not compromise the functional outcome of the limb. This high incidence may be attributed to the preexisting muscle trauma and extensive excision of the scar at the site of rhBMP-7 application, as these patients had undergone several operations prior to the index procedure. It has been demonstrated that repeated blunt trauma in the extremities, may induce degenerative changes in the muscle predisposing to ectopic bone formation[3,7,16].
The development of heterotopic ossification has been studied in several experimental models, however the exact pathophysiology of this process has not been completely elucidated. According to the rhBMP-7 commercial product’s safety database, the rate of undesirable effects (erythema, tenderness swelling and ectopic ossification) ranges from 1% to 10%[17].
Male gender significantly influenced the appearance of heterotopic bone. In a study evaluating the osteogenic capacity of mice skeletal muscle-derived stem cells (MDSCs), the male MDSCs revealed significantly greater ALP activity and expression of osteogenic genes when stimulated with rhBMP-4 in vitro. In addition, the implantation of these cells into intramuscular pockets in the mice led to more bone formation in the male mice compared to the female regardless of the implanted cells gender[18]. In a recent study in mice with cranial defect treated with MDSCs after transduction with a retrovirus encoding BMP-4, male mice demonstrated more rapid bone formation and larger volumes of ectopic bone than female[19]. These findings suggest a specific effect of the gender in the heterotopic ossification development.
The potential of mesenchymal progenitor cells to differentiate into osteoprogenitor cells in muscles, has been shown in animal models[20-22]. In a resent in vitro study human skeletal muscle-derived progenitor cells have been isolated and characterized for their osteogenic properties indicating a potential effect on heterotopic bone formatio[23]. These cells were identified as PDGFRα+ cells, able to differentiate into osteoprogenitor cells under the stimulation of bone morphogenetic proteins in mice[21]. The role of BMPs in the heterotopic bone formation has also been elucidated in the fibrodysplasia ossificans progressiva, where BMPs were found to promote muscle mesenchymal stem cells differentiation in preosteoblasts and osteoblasts[24].
Since 2001, when the use of rhBMP-7 was approved by the American Food and Drug Administration and the European Medicines Agency (European Marketing Authorization Number: EU/1/01/179/001) for the treatment of tibia nonunions, its use was extended to skeletal nonunions and spinal fusions with successful outcomes presented in several series[25-27]. Although rhBMP-7 has been widely used in the clinical practice, only few cases of heterotopic bone formation were reported in the literature after 2006. A case of myositis ossificans in a 49-year old woman, who underwent L4-L5 decompression and fusion with the use of rhBMP-7 was presented by Bennet et al[28]. The first case of heterotopic ossification in a long bone (distal humerus nonunion) after the use of rhBMP-7 was reported by Wysocki et al[29]. The patient gradually developed elbow stiffness from an extensive heterotopic ossification in the triceps muscle. Another small series of four patients that developed heterotopic ossification after the use of rhBMP-7 (3 patients) and rhBMP-2 (one patient) for the treatment of acute fractures or nonunions of the humerus was presented by Axelrad et al[30]. All patients had painful restriction of motion and underwent surgical excision of the heterotopic bone with good postoperative outcome. Heterotopic ossification after the use of rhBMP-7 for the treatment of femoral head osteonecrosis was also reported. In this case, it series the majority of patients developed heterotopic ossification at the lateral surface of the femur around the entry point of core decompression and fibula insertion, which, however, did not affect the range of motion[31].
The main limitation of this study is the lack of a control group of patients who did not received rhBMP-7. As all patients had several previous unsuccessful surgical procedures, the treating physicians decided to use all available means to treat the nonunions and considered unethical the deprivation of rhBMP-7 in the patients of a control group.
In conclusion, heterotopic ossification following the use of rhBMP-7 for the treatment of long bone nonunions was a relatively common complication. Also, a positive correlation between the male gender and the development of this side effect was found. Careful observation of postoperative radiographs may increase the reported heterotopic ossification rates in the literature as the majority of cases lack clinical relevance.
Dr. Konstantinos Kokkinis: Chairman at the Department of Computed Tomography KAT Hospital Athens, Greece.
Bone morphogenetic proteins (BMPs) have been identified as factors promoting osteogenesis by auto-induction in extra-skeletal sites. In the recent years they have been used in the treatment of long bone nonunions in combination with bone grafts. In this study, the rate of heterotopic ossification occurring after the use of commercially available recombinant human bone morphogenetic protein-7 (rhBMP-7) for the treatment of long bone nonunions was estimated. In addition, the correlation between the development of heterotopic ossification and factors related to the patient (age, gender), the nonunion (location, size, chronicity, previous procedures, infection, surrounding tissues condition), and the surgical procedure (graft type, amount of rhBMP-7) was evaluated.
BMPs and especially rhBMP-2 and rhBMP-7 have been used in several clinical studies but there are only few reported data related to the complications related to their use.
In this study, heterotopic ossification was a relatively common complication of the use of rhBMP-7, with higher rates than the ones reported in literature. In addition, a significant correlation between patient’s gender and the development of this complication was found.
This study suggests that heterotopic ossification is a relatively common complication after the use of rhBMP-7 for the treatment on nonunions, related significantly with patient’s gender. Although not clinically significant in the majority of cases, this complication should be acknowledged to the patients, especially the male, before rhBMP-7 use.
rhBMP-7: Recombinant human bone morphogenetic protein-7; HO: Heterotopic ossification.
The rate of heterotopic ossification after the use of rhBMP-7 was higher in the present series than the rates reported in the literature and a significant correlation between male gender and the development of this complication was found. The lack of any clinical relevance of this complication in the vast majority of cases may be the reason of the low reported rates up to now. Thus, careful evaluation of postoperative radiographs may increase the reported rates.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Emara KM, Frenkel B, Zhao JW S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Rath E, Sherman H, Sampson TG, Ben Tov T, Maman E, Amar E. The incidence of heterotopic ossification in hip arthroscopy. Arthroscopy. 2013;29:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Neal B, Gray H, MacMahon S, Dunn L. Incidence of heterotopic bone formation after major hip surgery. ANZ J Surg. 2002;72:808-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Dailiana ZH, Gunneson EE, Urbaniak JR. Heterotopic ossification after treatment of femoral head osteonecrosis with free vascularized fibular graft. J Arthroplasty. 2003;18:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Solomon L, Warwick DJ, Nayagam S. Apley’ system of orthopaedic and fractures, 8th edition. |
| 5. | Keschner MT, Paksima N. The stiff elbow. Bull NYU Hosp Jt Dis. 2007;65:24-28. [PubMed] |
| 6. | Ilahi OA, Strausser DW, Gabel GT. Post-traumatic heterotopic ossification about the elbow. Orthopedics. 1998;21:265-268. [PubMed] |
| 7. | Ekelund A, Brosjö O, Nilsson OS. Experimental induction of heterotopic bone. Clin Orthop Relat Res. 1991;2:102-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Urist MR. Bone: formation by autoinduction. Science. 1965;150:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4079] [Cited by in RCA: 3691] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 9. | Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A Suppl 1:S151-S158. [PubMed] |
| 10. | Ristiniemi J, Flinkkilä T, Hyvönen P, Lakovaara M, Pakarinen H, Jalovaara P. RhBMP-7 accelerates the healing in distal tibial fractures treated by external fixation. J Bone Joint Surg Br. 2007;89:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Kanakaris NK, Calori GM, Verdonk R, Burssens P, De Biase P, Capanna R, Vangosa LB, Cherubino P, Baldo F, Ristiniemi J. Application of BMP-7 to tibial non-unions: a 3-year multicenter experience. Injury. 2008;39 Suppl 2:S83-S90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Börner MG. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A:2123-2134. [PubMed] |
| 13. | Aro HT, Govender S, Patel AD, Hernigou P, Perera de Gregorio A, Popescu GI, Golden JD, Christensen J, Valentin A. Recombinant human bone morphogenetic protein-2: a randomized trial in open tibial fractures treated with reamed nail fixation. J Bone Joint Surg Am. 2011;93:801-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 14. | Love DA, Lietman SA. The effect of osteogenic protein-1 dosing regimen on ectopic bone formation. Clin Orthop Relat Res. 2004;264-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Papanagiotou M, Dailiana ZH, Karachalios T, Varitimidis S, Vlychou M, Hantes M, Malizos KN. RhBMP-7 for the treatment of nonunion of fractures of long bones. Bone Joint J. 2015;97-B:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Mavrogenis AF, Soucacos PN, Papagelopoulos PJ. Heterotopic ossification revisited. Orthopedics. 2011;34:177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | European Medicines Agency. Osigraft summary of product Characteristics. Available from: http//www.ema.europa.eu last updated 15/05/2014. |
| 18. | Zaccalini PS, Urist MR. Traumatic periosteal proliferations in rabbits. the enigma of experimental myositis ossificans traumatica. J Trauma. 1964;4:344-357. [PubMed] |
| 19. | Corsi KA, Pollett JB, Phillippi JA, Usas A, Li G, Huard J. Osteogenic potential of postnatal skeletal muscle-derived stem cells is influenced by donor sex. J Bone Miner Res. 2007;22:1592-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Meszaros LB, Usas A, Cooper GM, Huard J. Effect of host sex and sex hormones on muscle-derived stem cell-mediated bone formation and defect healing. Tissue Eng Part A. 2012;18:1751-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 966] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 22. | Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124:3654-3664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 490] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 23. | Oishi T, Uezumi A, Kanaji A, Yamamoto N, Yamaguchi A, Yamada H, Tsuchida K. Osteogenic differentiation capacity of human skeletal muscle-derived progenitor cells. PLoS One. 2013;8:e56641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Billings PC, Fiori JL, Bentwood JL, O’Connell MP, Jiao X, Nussbaum B, Caron RJ, Shore EM, Kaplan FS. Dysregulated BMP signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (FOP). J Bone Miner Res. 2008;23:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Dimitriou R, Dahabreh Z, Katsoulis E, Matthews SJ, Branfoot T, Giannoudis PV. Application of recombinant BMP-7 on persistent upper and lower limb non-unions. Injury. 2005;36 Suppl 4:S51-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Bong MR, Capla EL, Egol KA, Sorkin AT, Distefano M, Buckle R, Chandler RW, Koval KJ. Osteogenic protein-1 (bone morphogenic protein-7) combined with various adjuncts in the treatment of humeral diaphyseal nonunions. Bull Hosp Jt Dis. 2005;63:20-23. [PubMed] |
| 27. | Leach J, Bittar RG. BMP-7 (OP-1) safety in anterior cervical fusion surgery. J Clin Neurosci. 2009;16:1417-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Bennett M, Reynolds AS, Dickerman RD. Recent article by Shields et al titled “adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion”. Spine (Phila Pa 1976). 2006;31:2029-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Wysocki RW, Cohen MS. Ectopic ossification of the triceps muscle after application of bone morphogenetic protein-7 to the distal humerus for recalcitrant nonunion: a case report. J Hand Surg Am. 2007;32:647-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Axelrad TW, Steen B, Lowenberg DW, Creevy WR, Einhorn TA. Heterotopic ossification after the use of commercially available recombinant human bone morphogenetic proteins in four patients. J Bone Joint Surg Br. 2008;90:1617-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Papanagiotou M, Malizos KN, Vlychou M, Dailiana ZH. Autologous (non-vascularised) fibular grafting with recombinant bone morphogenetic protein-7 for the treatment of femoral head osteonecrosis: preliminary report. Bone Joint J. 2014;96-B:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |