Peer-review started: July 18, 2016
First decision: September 2, 2016
Revised: September 21, 2016
Accepted: December 1, 2016
Article in press: December 2, 2016
Published online: January 18, 2017
Processing time: 176 Days and 23.2 Hours
To analyze planning total hip arthroplasty (THA) with an additional anteroposterior hip view may increases the accuracy of preoperative planning in THA.
We conducted prospective digital planning in 100 consecutive patients: 50 of these procedures were planned using pelvic overview only (first group), and the other 50 procedures were planned using pelvic overview plus antero-posterior (a.p.) hip view (second group). The planning and the procedure of each patient were performed exclusively by the senior surgeon. Fifty procedures with retrospective analogues planning were used as the control group (group zero). After the procedure, the planning was compared with the eventually implanted components (cup and stem). For statistic analysis the χ2 test was used for nominal variables and the t test was used for a comparison of continuous variables.
Preoperative planning with an additional a.p. hip view (second group) significantly increased the exact component correlation when compared to pelvic overview only (first group) for both the acetabular cup and the femoral stem (76% cup and 66% stem vs 54% cup and 32% stem). When considering planning ± 1 size, the accuracy in the second group was 96% (48 of 50 patients) for the cup and 94% for the stem (47 of 50 patients). In the analogue control group (group zero), an exact correlation was observed in only 1/3 of the cases.
Digital THA planning performed by the operating surgeon and based on additional a.p. hip view significantly increases the correlation between preoperative planning and eventual implant sizes.
Core tip: Preoperative planning is an essential practice carried out prior to total hip arthroplasty (THA). However, the accuracy of digital preoperative planning in THA is variable and often lacks sufficient precision. Our prospective study analysed that preoperative planning with an additional antero-posterior hip view significantly increased the exact component correlation when compared to pelvic overview only for both the acetabular cup and the femoral.
- Citation: Stigler SK, Müller FJ, Pfaud S, Zellner M, Füchtmeier B. Digital templating in total hip arthroplasty: Additional anteroposterior hip view increases the accuracy. World J Orthop 2017; 8(1): 30-35
- URL: https://www.wjgnet.com/2218-5836/full/v8/i1/30.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i1.30
Preoperative planning for elective total hip arthroplasty (THA) is of paramount importance, irrespective of the level of difficulty. Not only does it prevent complications, but it also helps to optimise important geometric parameters such as leg length, centre of rotation, and femoro-acetabular offset adjustment by determining such components[1-4].
Previously, conventional X-ray images and measuring templates were used for this purpose. However, according to the literature, these practices resulted in low levels of correlation with the sizes of the eventually implanted devices in most cases[2]. With the increasing use of digital radiography, more and more digital planning software programmes are being offered, which, in theory, should deliver higher precision. However, it has been reported that there were only a few cases for which digital planning has resulted in more than low correlation between planning and implanted sizes[4-6].
Therefore, we conducted a comparative case-control study based on the null hypothesis that planning precision regarding the eventually implanted components can be increased with an additional antero-posterior (a.p.) hip view. This was based on the fact that the a.p. hip view with a central X-ray beam (directed to the proximal femur) reduces parallax shifts and rotational deviations[7].
Since 2014, we have exclusively performed preoperative THA planning in our hospital using digital software (MediCAD, HECTEC GmbH, Landshut, Germany). The digital planning has been performed using a 17-inch LCD screen with a resolution of at least 1.024 × 768 pixels.
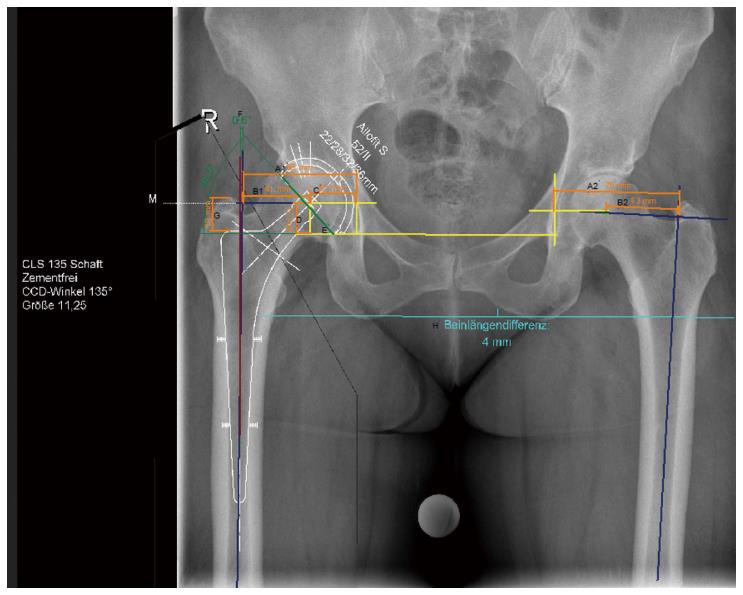
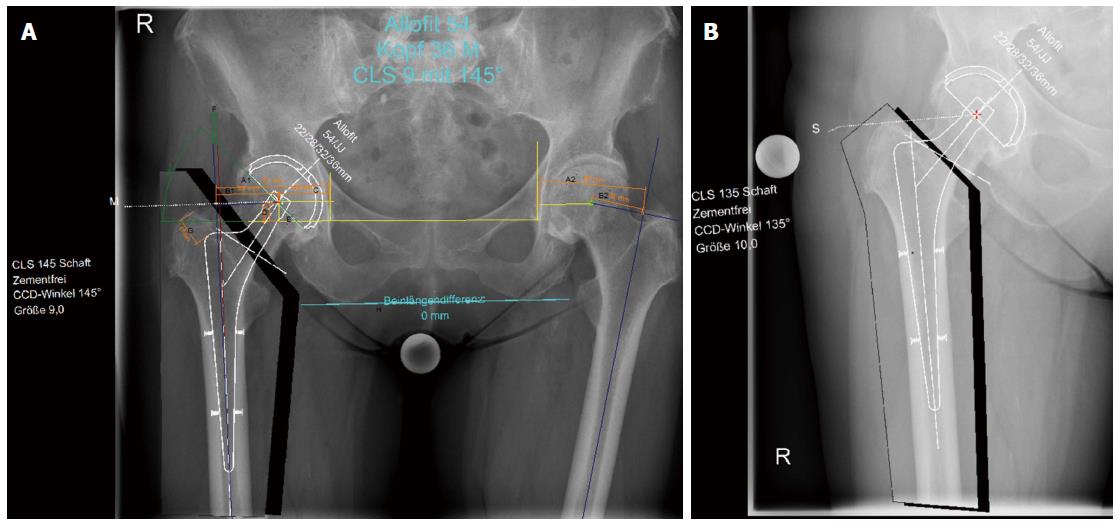
We used three groups for this comparative study: The first group included digital planning in 50 consecutive patients (who underwent surgery in 2015) using digital pelvic overview only (Figure 1). The second group also included digital planning in 50 consecutive patients (within the same year), but with an additional a.p. hip view for planning (Figure 2). All X-ray examinations (pelvic overview) were performed using a standardised technique with the patients in the supine position with a film-focus distance of 115 cm, a 10- to 15-degree internal rotation of the hip joint, and the central X-ray beam directed to the pubic symphysis.
A 25-mm external calibration marker (scaling sphere) was used for planning in both groups, and it was placed laterally from the hip joint requiring surgery or centred between the legs at the joint level at the height of the trochanter major. Moreover, surgeries in both groups were exclusively performed by the senior consultant surgeon who operated on the patients on the following day. Access to the hip was achieved exclusively in the lateral position using the minimally invasive technique according to Bertin and Röttinger[8]. The planning steps were performed according to the procedure described by Bono[3], Dastane et al[9], and Unnanuntana et al[10] (Figures 1 and 2).
Fifty consecutive patients with analogue planning (who underwent surgery the year before the digital software had become available) served as the control group (group zero). Here, the individual planning steps had been performed according to Eggli et al[11].
The indications for the 150 patients who received a cementless THA for both acetabular cup and femoral stem were primary osteoarthritis (n = 133), avascular femoral head necrosis (n = 11), and dysplasia (n = 6). The exclusion criteria were as follows: History of cemented or hybrid arthroplasty, additional osteotomies, and revision surgery for any reason.
Preoperative planning, surgical reports, and postoperative X-ray (within 6 wk) for the first and second group were performed prospectively, and group zero was evaluated retrospectively. The cup component was a Fitmore or an Allofit press-fit cup (Zimmer, Freiburg, Germany), and the stem was a CLS Spotorno (Zimmer), exclusively in all three of the groups. The study was approved by the local ethics committee.
SPSS version 23 (SPSS, Chicago, IL, United States) was used for statistical analysis. Descriptive analysis was performed by determination of values, averages, and standard deviations. Differences were compared using the χ2 test for nominal variables. The t-test was used for a comparison of continuous variables. A P value of < 0.05 was set as the significance threshold.
Among the 150 patients who underwent cementless THA, 63 were female, 87 were male, and the mean age was 63 years (30 to 83). No patient received bilateral THA. The descriptive data (sex, age, BMI, indications, and duration of surgery) within the three groups were not significantly different (P > 0.05).
For all 150 patients, no major postoperative complications, e.g., periprosthetic fracture, fracture of the trochanter tip, or hip dislocation - were documented.
The exact acetabular cup sizes within the three groups are shown in Table 1. The size increments within the two cups are 2 mm. Within the second group, including additional a.p. hip view, a total of 48 out of 50 (96%) were predicted within ± one size without a significant difference between both of the utilised components (Allofit or Fitmore). The results for exact size accuracy between the three groups were significantly different (P = 0.02).
| Preoperative planningvs implant used | Group zero n = 50 | First group n = 50 | Second group n = 50 |
| - 2 sizes smaller | - | - | - |
| - 1 size smaller | 6 (12) | 8 (16) | 5 (10) |
| Exact size | 17 (34) | 27 (54) | 38 (76) |
| + 1 size larger | 15 (30) | 12 (24) | 5 (10) |
| + 2 sizes larger | 9 (18) | 2 (4) | 2 (4) |
| + 3 sizes larger | 2 (4) | 1 (2) | - |
| + 4 sizes larger | 1 (2) | - | - |
The exact femoral stem sizes within the three groups are shown in Table 2. The increments of the femoral stems are between 1 and 1.25 mm. Within the second group that included an additional a.p. hip view, a total of 47 out of 50 (94%) were predicted within a one size deviation. The results for exact size accuracy between the first and the second group were significantly different (P < 0.001).
| Preoperative planning vs implant used | Group zero n = 50 | First group n = 50 | Second group n = 50 |
| - 2 sizes smaller | 1 (2) | 3 (6) | 3 (6) |
| - 1 size smaller | 11 (22) | 20 (40) | 9 (18) |
| Exact size | 16 (32) | 16 (32) | 33 (66) |
| + 1 size larger | 17 (34) | 10 (20) | 5 (10) |
| + 2 sizes larger | 5 (10) | 1 (2) | - |
A distance of ≤ 5 mm between the vertical and horizontal centre of rotation after implantation when compared to the scheduled position was found in a total of 48% (n = 24) of patients in group zero and in a total of 72% (n = 36) of patients in the digital group. The planning accuracy difference between both groups was significantly different (P = 0.014).
A femoral offset of ≤ 5 mm when compared to the scheduled position was found in a total of 68% (n = 34) in the analogue planning group and in a total of 70% (n = 35) in the digital group. The hip offset was scheduled ≤ 5 mm in 50% of patients (n = 25) of both groups (both with analogue and digital planning).
The mean inclination angle of the acetabular cup was 44.5° (SD ± 4.2°) in group zero and 45° (SD ± 5.8°) in the digital planning group, and there was no significant difference between the two groups.
A total of 88% of the prostheses (n = 44) in the analogue planning group and 92% (n = 46) in the digital planning group were implanted at angles between 30° und 50°.
The mean postoperative leg length difference (LLD) was 4.6 mm in the analogue planning group (SD ± 5.0 mm) and 2.7 mm in the digital planning group (SD ± 3.4 mm). A total of 80% of patients in group zero and 90% in the digital planning group had postoperative leg length differences of < 10 mm.
This study covers multiple aspects of preoperative planning: Although several studies of analogue planning have been previously reported[11,12], our study offers an additional direct comparison with digital planning. Few studies have compared analogue and digital planning procedures. Surprisingly, their results varied: González Della Valle et al[13] demonstrated that analogue planning resulted in a higher planning accuracy for both cup and femoral stem. However, The et al[1] concluded that digital planning was superior to analogue planning in regard to both components. In contrast, Gamble et al[6] found a significantly higher accuracy only for the acetabular cup when digital planning was used, whereas identical results were achieved with femoral stems. The results of the latter study are similar to ours: We also found an equally low exact precision (32%) for the femoral stem with both analogue and digital planning (when only a pelvic overview was used). However, in total, our data clearly showed that analogue planning offered the lowest levels of results for both exact precision and deviation by one size.
However, digital planning of the acetabular cup resulted in a clearly higher exact size determination (54%) in our study when compared to the results of other recent studies that reported an accuracy of only 34% to 42%[4,6,10]. Nevertheless, this result is still not satisfactory for several reasons: First, whether the scaling sphere was actually placed in the correct plane cannot be retrospectively evaluated[14]. Accordingly, inaccurate positioning and an inappropriately rotated femur have detrimental effects on X-ray imaging quality and, thus, on planning precision. The femoral stem component is more prone to such effects than the cup[15]. This may explain the lower planning accuracy with regard to the stem component. In our study, it was more common that a smaller-than-needed size of the femoral stem component was selected (though the difference was only one size) when compared to a larger-than-needed size (40% vs 20%). Kniesel et al[16] reported similar results. In their evaluation of different calibration methods, Franken et al[17] also found that there was a tendency to underestimate the real dimensions when the reference sphere was placed in the centre between the patient’s legs. Furthermore bone density is a crucial criterion when selecting the stem component: It is common that larger components are selected for patients with lower bone density[4].
Second, we were able to demonstrate for the first time that the exact correlation between planning and eventually implanted components (cup and stem) can be significantly increased to more than 2/3 of the cases with an additional a.p. hip view. When a size deviation of ± one size is also taken into account, an accuracy level of above 90% can be achieved for both the cup and the stem. Hip view with central X-ray beam targeting the proximal femur results in the minimisation of parallax shifts with reduction of rotational deviations[7], which may explain the higher planning precision. However, there are no data currently available with regard to an additional centred hip view for component planning.
In additional to component selection, it is clear that leg length difference is another very important preoperative planning parameter, even though a maximum clinical difference of 10 mm is generally considered to be acceptable[18]. The validation of different measurement methods for leg length differences has been the subject of multiple studies, with various results. Meermans et al[19] found that the horizontal line through the teardrops offers a more accurate reference marker when compared to the line between the two ischial tuberosities. However, Tripuraneni et al[20] concluded that the teardrop line is most commonly prone to measurement errors and that the obturator line would be the most accurate reference. In our study, the biischial line was used as an anatomical landmark for LLD assessment. We found a significant difference in planning accuracy in favour of the digital method: 90% of the patients showed a postoperative leg length difference of less than 10 mm, which is in line with the results reported in the studies of Unnanuntana et al[10] or González Della Valle et al[21]. However, it must be emphasised that complete compensation of leg length differences is not always practical and necessary, particularly in elderly patients with scoliotic deformities.
With regard to offset, analogue and digital procedures were found to be equivalent in terms of planning and correlation with implant positions. The digital method was significantly superior to the analogue method in terms of planning vertical and horizontal positions of the rotation centre. Good results were achieved for both cup planning and implantation when the inclination angle was within the “safe zone” (30°-50°) according to Lewinnek et al[22]. This was observed in both groups. Implantation outside of this range is known to promote abrasion and prosthesis loosening in the mid and long term[23].
Finally, it is necessary to address the weaknesses of the present study: Digital planning was performed by the senior surgeon who then also operated the patients. Hence, it is possible that the use of the initially scheduled component size was “enforced” during surgery. However, severe post-surgery complications, e.g., hip dislocation, periprosthetic fractures or stem loosening were reported in none of the 100 patients with digital planning and implantation. Conversely, a common sentiment in the literature is that planning should be performed by the operating surgeon[1,18], and this procedure has been propagated by the authors in those studies.
Moreover, an advancement of the digital two-dimensional planning is already underway using a three-dimensional CT. However, this method can expose patients to high levels of radiation and is probably not necessary for most THA patients with osteoarthritis[24]. In contrast, the addition of the a.p hip view confers a negligible radiation exposure of only 0.05 mSv. Because our study exclusively investigated cementless total hip arthroplasties, the results cannot be completely transferred to cemented THA or to other components. To date, it also remains unclear if the commonly occurring minor differences between planning and surgery cause long-term clinical consequences. Studies on this aspect are not available yet and are needed.
In conclusion, the digital planning of cementless THA performed by the surgeon based on additional antero-posterior hip view significantly increases the correlation between preoperative planning and eventual implant sizes. Therefore, we recommend that it should be implemented as a standard in preoperative planning.
Digital preoperative planning is an essential practice in total hip arthroplasty (THA). However, the accuracy is variable and often insufficient.
The current research hotspot is the analysis and the improvement of preoperative planning in THA.
This case-control study could represent that digital THA planning performed by the operating surgeon and based on additional antero-posterior hip view increases the accuracy of preoperative planning in THA.
An additional antero-posterior hip view should be implemented as a standard in preoperative planning.
The authors present a nice prospective study about accuracy in digital planning of cementless total hip replacement.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Anand A, Zak L S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
| 1. | The B, Verdonschot N, van Horn JR, van Ooijen PM, Diercks RL. Digital versus analogue preoperative planning of total hip arthroplasties: a randomized clinical trial of 210 total hip arthroplasties. J Arthroplasty. 2007;22:866-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Knight JL, Atwater RD. Preoperative planning for total hip arthroplasty. Quantitating its utility and precision. J Arthroplasty. 1992;7 Suppl:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Bono JV. Digital templating in total hip arthroplasty. J Bone Joint Surg Am. 2004;86-A Suppl 2:118-122. [PubMed] |
| 4. | Efe T, El Zayat BF, Heyse TJ, Timmesfeld N, Fuchs-Winkelmann S, Schmitt J. Precision of preoperative digital templating in total hip arthroplasty. Acta Orthop Belg. 2011;77:616-621. [PubMed] |
| 5. | The B, Diercks RL, van Ooijen PM, van Horn JR. Comparison of analog and digital preoperative planning in total hip and knee arthroplasties. A prospective study of 173 hips and 65 total knees. Acta Orthop. 2005;76:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Gamble P, de Beer J, Petruccelli D, Winemaker M. The accuracy of digital templating in uncemented total hip arthroplasty. J Arthroplasty. 2010;25:529-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Bunke J, Delorme S, Kamm KF, Kooijman H, Lorenz A. Physical-technical principles of image creation. Manual of diagnostic radiology. Radiation physics, radiation biology, radiation protection. Heidelberg: Springer 2003; 1-159. [DOI] [Full Text] |
| 8. | Bertin KC, Röttinger H. Anterolateral mini-incision hip replacement surgery: a modified Watson-Jones approach. Clin Orthop Relat Res. 2004;248-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 245] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Dastane M, Dorr LD, Tarwala R, Wan Z. Hip offset in total hip arthroplasty: quantitative measurement with navigation. Clin Orthop Relat Res. 2011;469:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 10. | Unnanuntana A, Wagner D, Goodman SB. The accuracy of preoperative templating in cementless total hip arthroplasty. J Arthroplasty. 2009;24:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Eggli S, Pisan M, Müller ME. The value of preoperative planning for total hip arthroplasty. J Bone Joint Surg Br. 1998;80:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 153] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Miashiro EH, Fujiki EN, Yamaguchi EN, Chikude T, Rodrigues LH, Fontes GM, Rosa FB. Preoperative planning of primary total hip arthroplasty using conventional radiographs. Rev Bras Ortop. 2004;49:140-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | González Della Valle A, Comba F, Taveras N, Salvati EA. The utility and precision of analogue and digital preoperative planning for total hip arthroplasty. Int Orthop. 2008;32:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Kirschner S, Hamann C, Handreka S, Günther KP, Hartmann A. [Templating and radiological outcome monitoring for elective total hip arthroplasty. Applied quality management principles for safe patient care]. Unfallchirurg. 2011;114:776-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | White SP, Bainbridge J, Smith EJ. Assessment of magnification of digital pelvic radiographs in total hip arthroplasty using templating software. Ann R Coll Surg Engl. 2008;90:592-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Kniesel B, Konstantinidis L, Hirschmüller A, Südkamp N, Helwig P. Digital templating in total knee and hip replacement: an analysis of planning accuracy. Int Orthop. 2014;38:733-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Franken M, Grimm B, Heyligers I. A comparison of four systems for calibration when templating for total hip replacement with digital radiography. J Bone Joint Surg Br. 2010;92:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Bertz A, Indrekvam K, Ahmed M, Englund E, Sayed-Noor AS. Validity and reliability of preoperative templating in total hip arthroplasty using a digital templating system. Skeletal Radiol. 2012;41:1245-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Meermans G, Malik A, Witt J, Haddad F. Preoperative radiographic assessment of limb-length discrepancy in total hip arthroplasty. Clin Orthop Relat Res. 2011;469:1677-1682. [PubMed] |
| 20. | Tripuraneni KR, Archibeck MJ, Junick DW, Carothers JT, White RE. Common errors in the execution of preoperative templating for primary total hip arthroplasty. J Arthroplasty. 2010;25:1235-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | González Della Valle A, Slullitel G, Piccaluga F, Salvati EA. The precision and usefulness of preoperative planning for cemented and hybrid primary total hip arthroplasty. J Arthroplasty. 2005;20:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217-220. [PubMed] |
| 23. | Kennedy JG, Rogers WB, Soffe KE, Sullivan RJ, Griffen DG, Sheehan LJ. Effect of acetabular component orientation on recurrent dislocation, pelvic osteolysis, polyethylene wear, and component migration. J Arthroplasty. 1998;13:530-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 456] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 24. | Steinberg EL, Shasha N, Menahem A, Dekel S. Preoperative planning of total hip replacement using the TraumaCad™ system. Arch Orthop Trauma Surg. 2010;130:1429-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |