Published online Sep 18, 2016. doi: 10.5312/wjo.v7.i9.592
Peer-review started: April 22, 2016
First decision: June 6, 2016
Revised: June 22, 2016
Accepted: July 14, 2016
Article in press: July 18, 2016
Published online: September 18, 2016
Processing time: 144 Days and 12.2 Hours
To systematically review the currently available literature concerning the application of biologic agents such as platelet-rich plasma (PRP) and stem cells to promote anterior cruciate ligament (ACL) healing.
A systematic review of the literature was performed on the use of biologic agents (i.e., PRP or stem cells) to favor ACL healing during reconstruction or repair. The following inclusion criteria for relevant articles were used: Clinical reports of any level of evidence, written in English language, on the use of PRP or stem cells during ACL reconstruction/repair. Exclusion criteria were articles written in other languages, reviews, or studies analyzing other applications of PRP/stem cells in knee surgery not related to promoting ACL healing.
The database search identified 394 records that were screened. A total of 23 studies were included in the final analysis: In one paper stem cells were applied for ACL healing, in one paper there was a concomitant application of PRP and stem cells, whereas in the remaining 21 papers PRP was used. Based on the ACL injury pattern, two papers investigated biologic agents in ACL partial tears whereas 21 papers in ACL reconstruction. Looking at the quality of the available literature, 17 out of 21 studies dealing with ACL reconstruction were randomized controlled trials. Both studies on ACL repair were case series.
There is a paucity of clinical trials investigating the role of stem cells in promoting ACL healing both in case of partial and complete tears. The role of PRP is still controversial and the only advantage emerging from the literature is related to a better graft maturation over time, without documenting beneficial effects in terms of clinical outcome, bone-graft integration and prevention of bony tunnel enlargement.
Core tip: There has been a growing interest in the past years on regenerative approaches to stimulate healing of musculo-skeletal tissues. The present systematic review focuses on the clinical application of biologic agents [platelet-rich plasma (PRP) and stem cells] to favor anterior cruciate ligament healing during procedures of reconstruction or repair. We show that there is inconclusive evidence to support the use of biologic augmentations, also due to the paucity of trials currently available, especially concerning stem cells. Looking at PRP, positive findings in terms of promotion of graft maturation were documented, but no beneficial influence was observed in terms of clinical outcome, bone-graft integration and prevention of tunnel enlargement.
- Citation: Di Matteo B, Loibl M, Andriolo L, Filardo G, Zellner J, Koch M, Angele P. Biologic agents for anterior cruciate ligament healing: A systematic review. World J Orthop 2016; 7(9): 592-603
- URL: https://www.wjgnet.com/2218-5836/full/v7/i9/592.htm
- DOI: https://dx.doi.org/10.5312/wjo.v7.i9.592
Anterior cruciate ligament (ACL) injuries are among the most common conditions treated in everyday orthopaedic practice, with an increasing incidence in the past years due to a concurrent increase of sports activities among the general population[1,2]. ACL lesions can be distinguished in complete tears, generally treated by reconstruction, and partial tears (i.e., incomplete tears of one or both ACL bundles). Their management can be very challenging, ranging from non-operative treatment to surgery (augmentation or traditional reconstruction), depending on the patients’ symptoms and functional needs[3]. Epidemiological studies reveal an average incidence of 30 ACL injuries per 100000 people annually. Every year 175000 patients undergo ACL reconstruction in the United States[4]. These numbers highlight the social and economical impact of ACL injuries, and therefore justify the large interest in optimizing the treatment strategies for this particular injury. Several reconstructive techniques have been proposed in the past decades, mainly differing in terms of graft selection and graft fixation. The overall results are quite satisfactory, even at long-term evaluation, without a difference in terms of outcome among different surgical techniques[5,6]. Nevertheless, a documented failure rate of up to 14% for ACL reconstruction[7], stimulates scientific efforts to find solutions that could promote better graft maturation and healing to minimize the risk of failure and to allow a faster recovery for patients. Beyond maximizing the results of ACL reconstruction, there is an increasing demand for minimally invasive options to enhance intrinsic ACL healing in case of partial ruptures. These injuries represent a substantial amount of ACL injuries, whose treatment algorithm is still controversial. The possibility of promoting ACL healing without reconstruction is regarded as an attractive perspective, due to the inherent lower surgical morbidity and the faster return to physical activities.
The recent progress in the field of regenerative medicine has led to the application of biologic agents (platelet-derived growth factors and stem cells), which could provide a positive stimulus to tissue healing. Platelet-rich plasma (PRP) is currently the most exploited biological augmentation used in orthopaedic practice, both for the treatment of degenerative disease (like osteoarthritis and tendinopathies), and sports-related injuries[8,9]. It is an autologous blood derived product which is obtained by centrifugation or filtration of peripheral blood in order to concentrate platelets, which are a reservoir of several growth factors and bioactive molecules involved in tissue homeostasis and anabolism[10,11]. Several in vitro and animal studies demonstrated that intra-ligamentary administration of PRP determines an increase in cellular density and neovascularization of the ACL. This results in a better organization of collagen fibers for superior tensile resistance and biomechanical properties[12-14]. In light of these promising pre-clinical data, and also due to the easy preparation modalities, platelet-rich products are used more and more by clinicians from all over the world. In more recent times, mesenchymal stem cells from different sources have been proposed to augment ACL reconstruction or repair. In this case, flourishing pre-clinical literature suggests that stem cell administration could stimulate tissue maturation, improve histological appearance, and favor bone-to-tendon integration[15,16]. Therefore, the implementation of experimental techniques in the clinical practice seems reasonable. A relevant number of clinical reports have been published investigating the actions of these fashionable biological strategies so far. The aim of the present paper is to systematically review the available literature concerning the application of PRP or stem cells to stimulate ACL healing, and to trace the state of the art in the use of biologic agents in this particular field of sports medicine. Moreover, this paper reveals if these novel strategies could really play a beneficial role in promoting graft maturation and healing and provide a better clinical outcome in the treatment of complete and partial ACL lesions.
A systematic review of the literature was performed on the use of biologic agents (i.e., PRP or stem cells) to promote ACL healing during surgical reconstruction or repair. The search was conducted on the PubMed database on March 31, 2016 using the following formula: [(PRP or platelet concentrate or platelet gel or platelet rich plasma or ACP or autologous conditioned plasma or PRGF or PRF or platelet lysate or platelet derived growth factors or plasma growth factors or platelet rich fibrin) or (stem cell or mesenchymal or mesenchymal stem cell or bone marrow concentrate or bone marrow aspirate or adipose derived or peripheral blood)] and (ACL or anterior cruciate ligament).
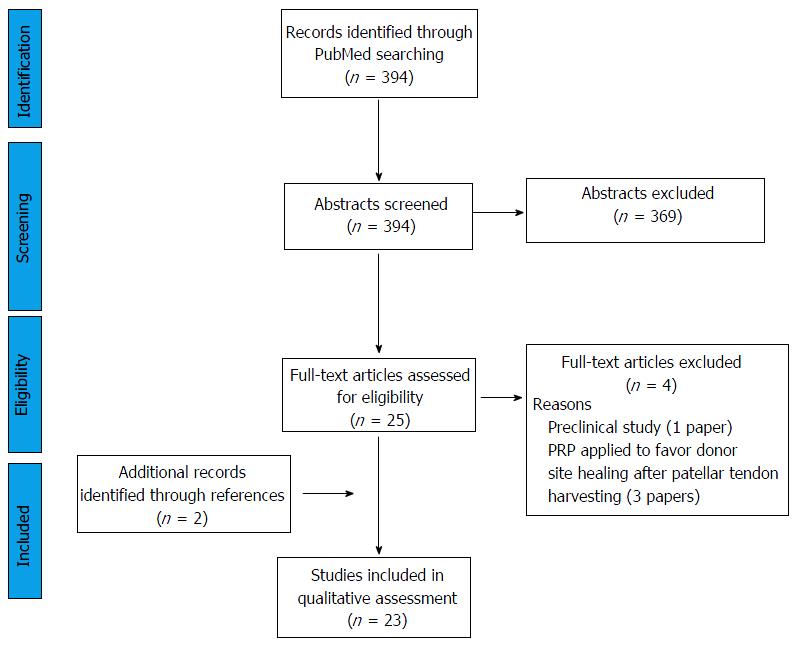
Screening process and analysis were conducted separately by two independent observers (Di Matteo B and Andriolo L). First, the articles were screened by title and abstract. The following inclusion criteria for relevant articles were used during the initial screening of titles and abstracts: Clinical reports of any level of evidence, written in English language, published in the past 20 years (1996-2016), on the use of PRP or stem cells during ACL reconstruction/repair. Exclusion criteria were articles written in other languages, reviews, or studies analyzing other applications of PRP/stem cells in knee surgery not related to promoting ACL healing. In the second step, the full texts of the selected articles were screened, with further exclusions according to the previously described criteria. Moreover, the articles not reporting clinical, magnetic resonance imaging (MRI), or histologic results were excluded. Reference lists from the selected papers were also screened. A flowchart of the systematic review is provided in Figure 1. Relevant data were then extracted and collected in a unique database, with the consensus of the two observers, to be analyzed for the purposes of the present manuscript.
The database search identified 394 records, and the abstracts were screened and selected according to the inclusion/exclusion criteria. As shown in Figure 1, a total of 27 full-text articles were assessed for eligibility. Four articles did not fulfill the inclusion criteria and were further excluded, leading to a total of 23 studies included in the final analysis (Figure 1). Only one paper dealt with the application of stem cells for ACL healing[17], one paper investigated the concurrent action of PRP and stem cells[18], whereas the remaining 21 papers focused on PRP application solely (one in partial rupture and 20 in ACL reconstruction)[19-39]. Based on the ACL injury pattern, two papers investigated biologic agents in ACL partial tears[18,19] whereas 21 papers in ACL reconstruction[17,20-39].
Looking at the quality of the available literature, 17 out of 21 studies dealing with ACL reconstruction were randomized controlled trials (RCTs) (Table 1), whereas only case series are available for ACL repair.
| Ref. | Study design | Methods | Results | |
| Clinical results | ||||
| + | Del Torto et al[21] | Prospective comparative study (PRFM vs control) 28 patients (14 vs 14) | ACL reconstruction with hamstring tendon graft fixed in the femoral tunnel through the RIGIDFIX system (DePuy) and in the tibial tunnel through the Bio-INTRAFIX system (DePuy) PRFM was prepared using Cascade Medical Enterprises 2 tube kit (Cascade Medical Enterprises, Wayne, NJ). PRFM clot was sutured in the proximal graft loop and it reaches the proximal tunnel once the graft is pulled in place. Distally, the PRFM clot was inserted between the four strands of the G-ST graft before the interference screw system was applied | PRFM-augmented patients showed a statistically significant higher clinical improvement at 24 mo follow-up (P = 0.032) Objective clinical evaluation both through IKDC score and with Rolimeter arthromether did not show any difference between the two groups |
| Magnussen et al[20] | Retrospective comparative study (PRP vs control) 58 patients (29 vs 29) | ACL reconstruction with allograft tibial tendon, fixed with absorbable cross pin in femoral tunnel and absorbable interference screw in tibial tunnel After graft positioning, intra-articular portion was coated with PRP prepared with GPS II Platelet Concentrate Separation Kit (Biomet) | Decreased effusions at 10 ± 4 d was noted in the PRP group, but this difference disappeared by 8 ± 4 wk No differences in patient-reported outcomes were noted in the 58 patients with two-year outcome data | |
| Darabos et al[22] | Randomized trial (ACS vs control) 62 patients (31 vs 31) | ACL reconstruction with hamstring (30) or patellar tendon (32), fixed with BioTransFix (Arthrex) or RigidFix (Mytek) at femoral side, and with an interference screw at the tibial side ACS was produced drawing venous blood into syringes containing pre-treated glass beads, and after a period of incubation serum is extracted through centrifugation. ACS was administered with an injection regime of 4 injections on day 0 (day of surgery), day 1, day 6, and day 10 | Clinical outcomes were consistently better in patients treated with ACS at all data points and for all outcome parameters, with statistically significant differences in the WOMAC stiffness subscale after 1 yr Decrease in IL-1b synovial fluid concentration was more pronounced in ACS group, with statistically significant lower values in the ACS group at day 10 | |
| Vogrin et al[23] | Randomized trial (PRP vs control) 45 patients (22 vs 23) | ACL reconstruction with double-looped semitendinosus and gracilis tendon graft fixed with two bioabsorbable cross pins in the femoral tunnel and one bioabsorbable interference screw in the tibial tunnel PRP was produced with Magellan system (Medtronic) and applied into the femoral and tibial tunnels as well as onto the graft itself | PRP group demonstrated significantly better anteroposterior knee stability than control group: Calculated improvements in knee stability at 6 mo were 1.3 ± 1.8 mm in the control group and 3.1 ± 2.5 mm in the platelet gel group (P = 0.011) | |
| - | Valentí Azcárate et al[24] | Randomized trial (PRP vs PRGF vs control) 150 patients (50 vs 50 vs 50) | ACL reconstruction using a patellar tendon allograft transtibial technique fixed with a RigidFix technique (DePuy Mitek,) with two biodegradable cross pins at the femoral bone and a tibial biodegradable (Byocril) interference screw PRP was produced with a double-spin procedure using a standard centrifuge and applied covering the ligament and suturing it over itself with gel in its interior, and introducing the gel obtained after activating the poor platelet concentration after implantation of the graft inside the tibial tunnel PRP was produced following Anitua’s technique (PRGF-Endoret Technology) and applied injecting it into the graft before implantation, with the biocompatible fibrin applied into the tibial tunnel at the end of surgery | No significant differences in functional results at the final follow-up of 24 m No statistically significant differences between the three groups in CRP 1 and VAS 24 h after surgery No significant differences in the range of knee motion, muscle torque, KT-1000 or IKDC score The PRGF group showed a statistically significant improvement in swelling scores 24 h after surgery compared with the PRP and control groups |
| Vadalà et al[25] | Randomized trial (PRP vs control) 40 patients (20 vs 20) | ACL reconstruction with hamstrings (Out-In technique), fixed with Swing-Bridge device on femoral side and Evolgate screw on tibial side PRP was produced with Fast Biotech kit (MyCells PPT-Platelet Preparation Tube) and applied as follows: 5 mL between peripheral part of the graft and the femoral tunnel wall; 5 mL in semisolid pattern above the graft; 5 mL of liquid and semisolid PRP on the tibial side | Physical examination as well as the evaluation scales used showed no differences between the two groups at 14.7 mo of follow-up | |
| Nin et al[26] | Randomized trial (PRP vs control) 100 patients (50 vs 50) | ACL reconstruction with patellar tendon allograft, fixed with two biodegra-dable cross pins in femur and a tibial biodegradable interference screw Ligament was covered with PRP (produced with standard centrifuge) and sutured over itself with PRP in its interior. The rest of the gel was introduced after implantation of the graft inside the tibial tunnel | The results did not show any statistically significant differences between the groups for inflammatory parameters, magnetic resonance imaging appearance of the graft, and clinical evaluation scores after 18 mo | |
| Ventura et al[27] | Randomized trial (PRP vs control) 20 patients (10 vs 10) | ACL reconstruction with quadruple hamstring tendon graft, with a femoral transcondylic fixation (BioTransFix) and tibial interference screw (BioRCI, Smith and Nephew) PRP was obtained according to the GPS Biomet-Merck technique (Biomet) and applied in femoral and tibial tunnels | There were no significant differences concerning clinical score and examination between the two groups 6 mo after ACL surgery | |
| Tunnel enlargement | ||||
| + | Starantzis et al[28] | Randomized trial (PRP vs control) 51 patients (25 vs 26) | ACL reconstruction with hamstring tendons (Semitendinosus and Gracilis) as a quadrupled graft, using distal fixation in the femur (Crosspin Linvatec or Endobutton Linvatec) and tibial fixation with a biodegradable interference screw (Linvatec) plus bone bridge suture anchoring Half of the PRP (produced using the Biomet GPS III kit) was added between the strands of the graft and left to form a clot before the graft fixation. Then, the remaining 3 mL was injected into the femoral tunnel using an introducer | The morphology of the dilated tunnels was conical in both groups There was a statistical significant difference in the mid distance of the tunnels between the two groups 1 yr after surgery No significant difference of the Lysholm scores between the two groups during the observation period was detected |
| Darabos et al[22] | Randomized trial (ACS vs control) 62 patients (31 vs 31) | ACL reconstruction with hamstring (30) or patellar tendon (32), fixed with BioTransFix (Arthrex) or RigidFix (Mytek) at femoral side, and with an interference screw at the tibial side ACS was produced drawing venous blood into syringes containing pre-treated glass beads, and after a period of incubation serum was extracted through centrifugation. ACS was administered with an injection regime of four injections on day 0 (day of surgery), day 1, day 6, and day 10 | Bone tunnel enlargement measured with CT scans was significantly less (6 mo: 8%, 12 mo: 13%) in ACS group than in control group (6 mo: 31%, 12 mo: 38%) | |
| - | Vadalà et al[25] | Randomized trial (PRP vs control) 40 patients (20 vs 20) | ACL reconstruction with hamstrings (Out-In technique), fixed with Swing-Bridge device on femoral side and Evolgate screw on tibial side PRP was produced with Fast Biotech kit (MyCells PPT-Platelet Preparation Tube) and applied as follows: 5 mL between peripheral part of the graft and the femoral tunnel wall; 5 mL in semisolid pattern above the graft; 5 mL of liquid and semisolid PRP on the tibial side | The use of PRP did not seem to be effective in preventing tunnel enlargement at 14.7 mo of follow-up |
| Mirzatolooei et al[29] | Randomized trial (PRP vs control) 46 patients (23 vs 23) | ACL reconstruction with single-bundle quadrupled autograft of hamstrings, fixed with a cross-pin in femoral tunnel and a bio-absorbable interference screw in tibial tunnel Graft was immersed in the PRP solution (produced with Double syringe system, Arthrex) for five minutes before implantation; 2 mL of PRP was injected into the femoral tunnel and 1.5 mL into the tibial tunnel at the end of the surgery | Despite slightly less tunnel widening in the PRP group, there were no significant differences at any of the sites of measurement between immediately after surgery and three months post-operatively | |
| Silva et al[30] | Randomized trial (4 groups) 40 patients (10 control vs 10 PRP in FT vs 10 PRP in FT and intra-articular at 2- and 4 wk vs 10 PRP activated with thrombin in FT) | Double-bundle arthroscopic ACL reconstruction with autologous hamstring tendons, fixed with two Endobuttons for the AMT and PLT in the femur and two bioabsorbable interference screws in the tibia PRP (produced with GPS III Kit, Biomet) was placed between the strands of the graft in each femoral tunnel | At 3 mo postoperatively, all tunnels had enlarged compared to the diameter of the drill and most tunnels enlarged more in the midsection than at the aperture in a fusiform manner The use of growth factors during and after surgery did not show any influence in the tunnel enlargement (P = 0.563) | |
| Orrego et al[31] | Randomized trial (4 groups) 108 patients (27 control vs 26 PC vs 28 BP vs 27 PC + BP) | ACL reconstructions with quadruple semitendinosus-gracilis graft, fixed with a biodegradable transfixing pin proximally and a biodegradable inter-ference screw distally; BP was placed by interference fit at the femoral tunnel Five milliliter PRP (produced with Biomet GPS II kit, Biomet) was added between the graft strands before passing it into the tunnel. After fixation, 1 mL of PRP was injected into the femoral tunnel between the graft strands | The use of PC did not show any significant effect in the tunnel widening evolution at 6 mo follow-up The use of a BP effectively prevented tunnel widening The BP and PC combination did not show a synergic effect as compared to PC or BP individually | |
| ACL graft-bone interface/integration | ||||
| + | Rupreht et al[32] | Randomized trial (PRP vs control) 41 patients (21 vs 20) | ACL reconstruction with double-looped semitendinosus and gracilis tendon autograft, fixed with two bioabsorbable cross pins in femoral tunnel and one bioabsorbable interference screw in tibial tunnel. PRP was applied after autograft positioning, into the femoral and tibial tunnels (1 mL in each of them), and onto the graft itself (3 mL) | A gradual increase in the percentage of the tunnel wall consisting of tunnel wall cortical bone (TCB) during the follow-up was observed. At 6 mo the mean percentage of TCB was significantly higher (P = 0.003) in the PRP group than in the control group |
| Vogrin et al[33] | Randomized trial (PRP vs control) 41 patients (21 vs 20) | ACL reconstruction with double-looped semitendinosus and gracilis tendon graft fixed with two bioabsorbable cross pins in the femoral tunnel and one bioabsorbable interference screw in the tibial tunnel PRP was produced with Magellan system (Medtronic) and applied into the femoral and tibial tunnels as well as onto the graft itself | After 4-6 wk, there was a significantly higher level of vascularization in the osteoligamentous interface in PRP group (0.33 ± 0.09 vs 0.16 ± 0.09, P < 0.001) In the intra-articular part of the graft, there was no evidence of revascularization in either group | |
| - | Del Torto et al[21] | Prospective comparative study (PRFM vs control) 28 patients (14 vs 14) | ACL reconstruction with hamstring tendon graft fixed in the femoral tunnel through the RIGIDFIX system (DePuy) and in the tibial tunnel through the Bio-INTRAFIX system (DePuy) PRFM was prepared using Cascade Medical Enterprises 2 tube kit (Cascade Medical Enterprises). PRFM clot was sutured in the proximal graft loop and it reaches the proximal tunnel once the graft is pulled in place. Distally, the PRFM clot was inserted between the four strands of the G-ST graft before the interference screw system was applied | MRI evaluation considering graft–tunnel interface and graft signal intensity provided similar results between the two examined groups, without any statistically significant difference. In the majority of the cases, a good signal quality of the graft and a scarce film of synovial fluid at the graft–tunnel interface were observed |
| Silva et al[17] | Randomized trial (BMC vs control) 43 patients (20 vs 23) | ACL reconstruction with double-looped semitendinosus and gracilis tendon autograft fixed with a Toggleloc Ziploop (Biomet) in femoral tunnel and a bioabsorbable interference screw (Biomet) in tibial tunnel Bone marrow was harvested from the iliac crest and concentrated to obtain 3 mL BMC. 1.5 mL of BMC concentrate was injected inside the femoral end of the graft itself before graft positioning, and the remaining 1.5 mL BMC injected within the tunnel around the graft, from the bottom down to the entrance of the tunnel | Adult non-cultivated BMC did not seem to accelerate graft-to-bone healing in ACL reconstruction: No difference in the signal-to-noise ratio of the inter-zone on MRI between the experimental and the control group 3 mo after surgery | |
| Valentí Azcárate et al[24] | Randomized trial (PRP vs PRGF vs control) 150 patients (50 vs 50 vs 50) | ACL reconstruction using a patellar tendon allograft transtibial technique fixed with a RigidFix technique (DePuy Mitek) with two biodegradable cross pins at the femoral bone and a tibial biodegradable (Byocril) interference screw PRP was produced with a double-spin procedure using a standard centrifuge and applied covering the ligament and suturing it over itself with gel in its interior, and introducing the gel obtained after activating the poor platelet concentration after implantation of the graft inside the tibial tunnel PRP was produced following Anitua’s technique (PRGF-Endoret Technology) and applied injecting it into the graft before implantation, with the biocompatible fibrin applied into the tibial tunnel at the end of surgery | No statistically significant differences were noted between groups in intensity, thickness, or uniformity of graft at 6 mo MRI | |
| Figueroa et al[34] | Comparative study (PRP vs control) 50 patients (30 vs 20) | ACL reconstruction with hamstring tendons fixed with a cross-pin in femoral tunnel and a bio-absorbable interference screw in tibial tunnel PRP was produced with Magellan Magellan system (Medtronic) and applied under arthroscopy in both the tibial (3 mL) and femoral (3 mL) tunnels with a long needle syringe, and directly applied in the intra-articular graft portion (4 mL) | No statistically significant benefit in the PRP group in terms of integration assessment at 6 mo follow-up | |
| Silva et al[35] | Randomized trial (4 groups) 40 patients (10 control vs 10 PRP in FT vs 10 PRP in FT and intra-articular at 2- and 4 wk vs 10 PRP activated with thrombin in FT) | Double-bundle arthroscopic ACL reconstruction with autologous hamstring tendons, fixed with two Endobuttons for the AMT and PLT in the femur and two bioabsorbable interference screws in the tibia PRP (produced with Mini GPS III Kit, Biomet) was placed between the strands of the graft in each femoral tunnel | The graft integration was not complete at 3 mo after surgery in the PL and AM femoral tunnel, and the use of PRP isolated or with thrombin seemed not to accelerate tendon integration | |
| Orrego et al[31] | Randomized trial (4 groups) 108 patients (27 control vs 26 PC vs 28 BP vs 27 PC + BP) | ACL reconstructions with quadruple semitendinosus-gracilis graft, fixed with a biodegradable transfixing pin proximally and a biodegradable interference screw distally; BP placed by interference fit at the femoral tunnel 5 mL PRP (produced with GPS II kit, Biomet) was added between the graft strands before passing it into the tunnel. After fixation, 1 mL of PRP was injected into the femoral tunnel between the graft strands | The use of PC did not show any significant effect in the osteoligamentous interface at 6 mo follow-up | |
| ACL graft remodeling | ||||
| + | Seijas et al[36] | Randomized trial (PRP vs control) 98 patients (49 vs 49) | ACL reconstruction with autologous patellar tendon grafts with 9 mm bone plugs and fixed with hydroxyapatite screws in femur and tibia 8 mL of PRP was produced with PRGF technique (BTI Systems Vitoria, Spain) and percutaneously injected into the suprapatellar joint after portal suture | More patients in the PRP group than controls attained higher stages of remodeling at month 4 (P = 0.003), month 6 (P = 0.0001), and month 12 (but NS P = 0.354) |
| Rupreht et al[37] | Randomized trial (PRP vs control) 41 patients (21 vs 20) | ACL reconstruction with double-looped semitendinosus and gracilis tendon autograft, fixed with two bioabsorbable cross pins in femoral tunnel and one bioabsorbable interference screw in tibial tunnel PRP was applied after autograft positioning, into the femoral and tibial tunnels (1 mL in each of them), and onto the graft itself (3 mL) | MRI measurements indicated a reduced extent of edema during the first postoperative month as well as an increased vascular density and microvessel permeability in the proximal tibial tunnel at 1 and 2.5 postoperative months as the effect of the application of PRP | |
| Radice et al[38] | Comparative study (PRP vs control) 50 patients (25 vs 25) | ACL reconstructions with BPTB autograft (15 vs 10) or Harmstring (10 vs 15). Fixation in BPTB autograft with metallic interference screws, in hamstring autograft with metallic or bioabsorbable cross-pin in the femur and a bioabsorbable screw with a metallic staple in the proximal tibia PRP (produced with GPS III Kit, Biomet) was administered with the help of a sutured and compressed Gelfoam; 5 mL PRP was added homogeneously so as to completely cover the graft | ACL reconstruction with the use of PRPG achieved complete homogeneous grafts assessed by MRI, in 179 d compared with 369 d for ACL reconstruction without PRPG. This represented a time shortening of 48% with respect to ACL reconstruction without PRPG | |
| Sánchez et al[39] | Comparative study (PRP vs control) 37 patients (22 vs 15) | ACL reconstruction with hamstring tendons, fixed with transcondylar screw proximally and PRGF-treated bone plug and two metal staples distally 6 mL PRP was produced with BTI System II (BTI Biotechnology Institute) and injected within the tendon graft fascicles with several punctures performed along the graft length, graft soaked in PRP until implantation and the remaining aliquots applied at the portals during suturing | PRGF resulted in more mature tissue than controls at histology (P = 0.024) Histologically evident newly formed connective tissue enveloping the graft was present in 77.3% of PRGF-treated grafts and 40% of controls Overall, arthroscopic evaluations were not statistically different between PRGF and control groups (P = 0.051) | |
| Orrego et al[31] | Randomized trial (4 groups) 108 patients (27 control vs 26 PC vs 28 BP vs 27 PC + BP) | ACL reconstructions with quadruple semitendinosus-gracilis graft, fixed with a biodegradable transfixing pin proximally and a biodegradable inter-ference screw distally; BP placed by interference fit at the femoral tunnel 5 mL PRP (produced with Biomet GPS II kit, Biomet) was added between the graft strands before passing it into the tunnel. After fixation, 1 mL of PRP was injected into the femoral tunnel between the graft strands | The use of PC had an enhancing effect on the graft maturation process evaluated only by MRI signal intensity at 6 mo follow-up | |
| Ventura et al[27] | Randomized trial (PRP vs control) 20 patients (10 vs 10) | ACL reconstruction with quadruple hamstring tendon graft, with a femoral transcondylic fixation (BioTransFix, Arthrex) and tibial interference screw (BioRCI, Smith and Nephew) PRP was obtained according to the GPS Biomet-Merck technique (Biomet) and applied in femoral and tibial tunnels | CT highlighted a significant difference (P < 0.01) between ACL density of the two groups and showed that ACL density was similar to that of the posterior cruciate ligament in GF-treated group at 6 mo follow-up | |
| - | Figueroa et al[34] | Comparative study (PRP vs control) 50 patients (30 vs 20) | ACL reconstruction with hamstrings fixed with a femoral cross-pin and a tibial bio-absorbable interference screw PRP was produced with Magellan Magellan system (Medtronic, Minneapolis, MN) and applied under arthroscopy in both the tibial (3 mL) and femoral (3 mL) tunnels with a long needle syringe, and directly applied in the intra-articular graft portion (4 mL) | No statistically significant benefit in the PRP group in terms of graft maturation (ligamentization) at 6 mo of follow-up |
| Vogrin et al[33] | Randomized trial (PRP vs control) 41 patients (21 vs 20) | ACL reconstruction with double-looped semitendinosus and gracilis tendon graft fixed with two bioabsorbable cross pins in the femoral tunnel and one bioabsorbable interference screw in the tibial tunnel PRP was produced with Magellan system (Medtronic, Minneapolis, MN) and applied into the femoral and tibial tunnels as well as onto the graft itself | After 4-6 wk, significantly higher level of vascularization in the osteoligamentous interface in PRP group (0.33 ± 0.09 vs 0.16 ± 0.09, P < 0.001). No evidence of revascularization in the intra-articular part in either group | |
| ACL repair | ||||
| + | Centeno et al[18] | Prospective study (BMC, PRP, PL) 10 patients | Pre-injection of hypertonic dextrose solution into the ACL using fluoroscopic guidance 2-5 d prior to BMC injection in order to prompt a brief inflammatory response in the ACL Intra-ligamentous injection of autologous BMC (harvested from iliac crest and isolated through | Patients included had ACL laxity on exam, and MRI evidence of grade 1, 2, or 3 ACL tears < 1 cm retraction 7/10 patients showed improvement in objective measures of ACL integrity in their post-procedure MRIs |
| centrifugation), PRP and PL (prepared from venous blood via centrifugation and recentrifugation after freezing) using fluoroscopic guidance. Remaining BMCs were injected into the joint | The mean VAS change was a decrease of 1.7 (P = 0.25), the mean LEFS change was an increase of 23.3 (P = 0.03), and mean reported improvement was 86.7% | |||
| Seijas et al[19] | Retrospective study (PRGF-Endoret) 19 patients | PRGF-Endoret was produced using the technique described by Anitua and applied with a spine needle in both the proximal origin of the bundle and in the middle portion thereof in an amount of about 4 cc At the end of the surgery another injection of PRGF-Endoret was administered (6 cc) in the articular space. | 16/19 professional soccer players with partial ACL tears returned to the same level Normal KT-1000 values in all operated cases Time to return to play: 16.2 ± 1.4 wk for Tegner 9 pts, 12.3 ± 1.1 for Tegner 10 | |
Concerning delivery methods of biologic agents, several different strategies were tested by authors, sometimes in combination (Table 1): Simple intra-articular injection, intra-ligamentary injection, local application onto the surface of the tendon graft, local injection within the bony tunnels, multiple intra-tendinous depots, or even selective administration through a spongy membrane soaked in PRP and sutured around the graft.
The different application methods will be discussed separately according to the specific ACL treatment analyzed in the clinical trials, i.e., repair (2 studies) or reconstruction (21 studies). A detailed description of the trial features is presented in Table 1.
Two papers investigated the contribution of biologic agents applied to promote healing of ACL in case of partial rupture. The first one, a retrospective case series, was published by Seijas et al[19] in 2014. They analyzed a small cohort of 19 football players who were treated by arthroscopic intra-ligamentary injection of 4 mL of leukocyte-poor PRP, followed by a 6 mL intra-articular injection at the end of surgery. The results were pretty satisfactory and 16 out of 19 patients were able to return to previous sports activity level with stable knees (evaluated by KT-1000), and in particular patients with Tegner Score 10 achieved the fastest return to sports. In the prospective trial published by Centeno et al[18], ten patients in total were treated by fluoroscopic guided intra-ligamentary injection of PRP + platelet lysate + bone marrow derived stem cells (harvested from the iliac crest and concentrated in the operating room). Overall results were satisfactory, with seven out of ten patients presenting signs of ACL healing at MRI evaluation performed at average 3 mo from the procedure. This evidence suggested a potential clinical usefulness of this approach to be confirmed by a larger trial.
Twenty-one papers[17,20-39] investigated the role of biologic agents during ACL reconstruction; only one paper dealt[17] with the use of bone marrow concentrate, whereas 19 focused on PRP. With regards to the graft used for reconstruction, in 14 trials authors employed hamstrings, in three patellar tendon, in two trials ACLs were either reconstructed with hamstrings or patellar tendon, and just one paper documented the use of allograft. The papers published analyzed the following main outcomes after biologic agents administration (Table 1): (1) clinical results evaluated by functional scores, objective measurements and time to return to sports; (2) bony tunnel enlargement over time [evaluated by computed tomography (CT)]; (3) ACL graft-bone interface integration (evaluated by MRI); and (4) ACL graft maturation/remodeling (evaluated by MRI).
Looking at clinical outcomes and functional scores, eight papers[20-27] analyzed this specific aspect, seven of which were RCTs. Overall results were controversial: The RCTs authored by Del Torto et al[21], Darabos et al[22] and Vogrin et al[23] showed significant advantage of PRP administration over the control group. In particular, Del Torto et al[21] documented superior clinical outcome (IKDC subjective score) for the PRP group at any follow-up evaluation up to 24 mo; similar results were reported by Darabos et al[22], who documented less swelling and better performance in functional tests at 6 mo, and also a significant difference in WOMAC Stiffness subscale at 12 mo. Interestingly, also a reduced synovial fluid concentration of IL-1b was found in the PRP group after 10 d from the procedure. Vogrin et al[23] reported a significant difference in favor of PRP when comparing KT-2000 values at 3 and 6 mo after surgery. It was suggested that growth factors played a substantial role in enhancing knee stability. Conversely, four RCTs[24-27] were not able to document any clinical benefit from PRP administration. In particular the trial authored by Valentí Azcárate et al[24] included 150 patients divided into three treatment groups and evaluated up to 24 mo. Two different PRP formulations (PRGF and lab made PRP) were tested without demonstrating any clinical benefit over control groups, either in terms of objective or subjective measurements, with the exception of swelling, which was observed to be less 24 h after surgery in the PRGF group compared to PRP and control groups.
The issue of prevention of bony tunnels enlargement was addressed in six trials, all of which were RCTs[22,25,28-31]. Only in two of them[22,28] it was demonstrated that PRP, injected locally into the tunnels[28] or intra-articularly[22], could prevent their enlargement over time. Conversely, four papers revealed no significant difference with regard to this specific outcome between treatment groups. Additionally, one paper[31] revealed that the only positive effect in preventing tunnel widening was achieved by implanting a bone plug into the tunnel.
Concerning the graft-bone tunnel integration, eight papers (six RCTs)[17,21,24,31-35] investigated this specific issue. In only two RCTs the use of PRP provided beneficial effects in terms of better corticalization of the tunnel walls[32] or higher vascularization at the graft-bone interface[33], whereas in the remaining trials[17,21,24,31,34,35] no inter-group difference was observed over time. Among the papers reporting negative results for PRP in this specific parameter, there is also only one study[17] documenting the role of bone marrow concentrate, which was not able to provide beneficial effects.
Diverging results were reported when analyzing the graft maturation over time after ACL reconstruction. Eight papers in total (five RCTs)[27,31,33,34,36-39] assessed this specific parameter, and six of them (four RCTs) documented a positive influence of PRP administration[27,31,36-39], whereas just two papers (one RCT) failed to reveal any inter-group difference[33,34]. In the vast majority of cases graft maturation was evaluated by imaging assessment, revealing a helpful role of PRP in stimulating a faster and better MRI or CT appearance of the graft. Signal of the graft was more similar to the posterior cruciate ligament, which points out an overall positive modulation of the ligamentization process. One paper[39] reported also histologic evaluation of the grafts, which were biopsied during second look arthroscopies performed after a mean of 15 mo from primary ACL reconstruction. In the PRP group, the graft presented a significantly better enveloping by mature connective tissue, with signs of improved graft maturation, depending on the time passed from surgery.
The main findings of the present systematic review are that: (1) there is a paucity of clinical trials investigating the role of stem cells in promoting ACL healing, both in the treatment of partial and complete ACL tears. Therefore, no conclusive statement can be issued regarding the efficacy of this treatment approach; (2) despite the high number of RCTs, the role of PRP in ACL healing is still controversial, and it is not possible to fully endorse this biologic strategy to this particular indication; and (3) the use of biologic agents in partial ACL rupture is very limited, with just two clinical trials published, and therefore, the possibility of treating this kind of injuries by regenerative approaches remains an open question.
Biologic approaches to enhance healing of musculo-skeletal injuries are for sure a fashionable topic in the field of regenerative medicine. The overall brilliant results documented by in vitro and in vivo trials have stimulated their use in clinical practice, with different indications and targets, ranging from degenerative conditions (such as osteoarthritis and tendinopathies) to muscle and ligament injuries[8,9]. The first product that has encountered a large clinical application (together with a large commercial success) is PRP, whose use has been documented in papers published more than a decade ago. Despite being no more a “novel” treatment option, there is still uncertainty about its real effectiveness and, up to the present moment, there is no clear recommendation for its use in any of the clinical conditions. Therefore, we underline the fact once again, that the positive findings from pre-clinical studies cannot be reproduced in the in vivo model, whose complexity cannot be fully mirrored in laboratory experiments[40].
Looking at PRP potential in ACL healing, the literature is not conclusive with regards to the benefit of PRP application in providing a faster recovery and better functional outcome after ACL reconstruction. However, the evidence is currently limited to short term evaluations (up to 24 mo), whereas no trial has yet pointed out if the administration of platelet-derived growth factors could be effective at longer follow-up, providing more stable results or lower rate of recurrent injury. Interestingly, most of the studies documented a superior graft maturation over time after PRP administration. These findings were achieved by imaging evaluation, and in one case also by histology[39]. A better ligamentization of the graft may be related to superior and longer lasting mechanical properties that could impact clinical outcome at middle-to-long term evaluation. In light of that, further studies correlating imaging, histology and functional scores are expected to better clarify the role of PRP. Considering that patients after ACL reconstruction have high expectations in terms of durability of results, the possibility of reducing re-injury rate and providing a more stable clinical outcome seems very attractive and could justify the use of biologic agents. With regards to aspects such as bone-graft integration and prevention of bony tunnel enlargement, results are controversial and, overall, the majority of trials fail to support the use of PRP for this indication. By the way, research is moving forward in the attempt to optimize PRP technology to obtain the best possible results[41]. The main limiting factor that scientists and clinicians have to overcome is the great inter-product variability and the absence of a well defined therapeutic protocol[42]. When so many variables come into play, there is an intrinsic difficulty in finding the right way to move on. First of all, there are plenty of different PRP products that could be used. All of them differ in many fundamental aspects, such as preparation procedures, cellular content, platelet concentration rate, physical properties and eventual use of activators to enhance growth factors’ release. Authors have been using either lab made products or commercial kits from several companies, all characterized by different preparation protocols that rendered different PRP formulations[11,43,44]. In particular, the aspect of cellularity is currently the most debated one and there are controversial findings regarding the role of the different components of PRP, especially leukocytes whose presence has been deemed as detrimental based on laboratory findings that have not been confirmed in the clinical setting, leaving many questions open to investigation[45,46].
Another fundamental issue regards the application modalities of PRP during ACL reconstruction or repair. Several different approaches have been proposed, ranging from simple intra-articular injections to intra-ligamentary deposition, graft coating, topical use into the bony tunnels, or at the bone-graft interface. Even more complex strategies, such as suturing PRP clots or PRP-soaked sponges directly onto the graft have been suggested. In any case, based on the available data, it is impossible to endorse an ideal product or an ideal therapeutic modality to stimulate ACL healing.
Looking at the application of mesenchymal stem cells, they have been introduced into clinical practice more recently than PRP, and they have been tested mainly in the field of cartilage regeneration/osteoarthritis[47]. With regards to ACL healing, the current evidence is strongly affected by the paucity of literature available that prevents any indications for the use of such biological augmentation. As pointed out previously, only two papers in total (one RCT and one case series) investigated the potential efficacy of bone marrow concentrate in this particular field of sports medicine[17,18]. One of these studies applied the stem cell concentrate to treat partial ACL tears together with PRP, which prevents any clear understanding of the real contribution of each biological product. The lack of data is a severe flaw, also considering that stem cell therapy is characterized by the same great variability described in the case of PRP products. Many factors should be taken into account. First of all, the source of stem cells should be considered, since it is possible to obtain them from different anatomical sites (bone marrow, adipose tissue, synovial tissue, peripheral blood and so on), and this peculiar aspect could play a major role in determining clinical outcome. Furthermore, stem cells could be used as a concentrate, or could be expanded in vitro and then applied during surgery[48]. By the way, the application of cellular therapy in orthopaedics is under strict surveillance and clinicians have to face regulatory burdens that are currently limiting the number of ongoing clinical trials[49]. For this reason, there is such a great dichotomy between the flourishing pre-clinical literature and the very limited data coming from clinical studies. This explains the fact that mainly bone marrow concentrate has been tested for ACL healing at the moment, since it is the easiest and safest way to collect and administer stem cells in a surgical setting. In light of these remarks, both in the field of PRP and stem cell augmentation for ACL healing, there is a need of further basic science studies to better understand the mechanisms of action of these powerful biological agents. Moreover, there is also a need of more high-level comparative trials that could clarify if some specific “products” or applicative modalities are truly better than others.
A further consideration should be issued on the ACL injury patterns that have been treated with biologic agents. The large majority of papers (21) were focused on their application during ACL reconstructive surgery, whereas only two trials, both case series, have investigated their potential in partial ACL tears (Table 1). The possibility of enhancing ACL healing in case of partial rupture is very attractive, because it can contribute to functional recovery and avoid the higher surgical stress of traditional reconstruction. However, even in this case, the current lack of data prevents a reliable assessment of the efficacy of biologic agents, when applied for this specific purpose. The technique described in the literature to deliver PRP or stem cells into the injured ACL (intra-ligamentary injection under fluoroscopic or arthroscopic check) is feasible[18,19] and results seem to be encouraging. Again further high quality trials, with higher number of patients and longer follow-up, are needed to confirm these preliminary findings. Furthermore, considering that partial ACL tears may have variable features, it would also be clinically relevant to introduce and validate a classification system, with the aim of understanding whether the biological approach could be more effective in specific lesion patterns.
In conclusion, based on the available clinical evidence, there is a lack of data about the efficacy of stem cells in ACL healing, whereas the data concerning the role of PRP are not conclusive to understand if it could provide a faster recovery and better functional outcome during ACL repair/reconstruction. Despite some positive findings in terms of graft maturation and clinical outcome, further long-term studies are needed to identify whether the administration of PRP could truly play a beneficial role during ACL reconstruction. Lastly, in contrast to the large number of trials dealing with ACL reconstruction, the treatment of partial ACL tears with biologic agents has been poorly investigated, and therefore there is need of more high quality data to understand the efficacy of biologic agents in this particular application.
Complete and partial anterior cruciate ligament (ACL) tears are among the most common injuries treated by orthopaedic surgeons every day. Currently, there are several surgical approaches that have been proposed to reconstruct/repair torn ACL and, despite overall satisfactory clinical outcome at medium-long term, there is still a failure rate up to 14% as documented by some studies. The current trend of research is aimed at finding solutions that could provide better and longer lasting results by stimulating ligament regeneration through the use of powerful biologic agents, such as platelet-rich plasma (PRP) and stem cells. The main goal is to achieve a tissue quality which is more similar to the one of the native ligament. The aim of the present paper is to systematically review the state of art regarding the application of biologic agents to promote ACL healing.
Tissue engineering and regenerative approaches are currently widely applied in orthopaedics to stimulate healing of several tissues, from bone to cartilage and ligaments. In particular, PRP and stem cells are the most exploited strategies tested in clinical practice. Their application in the field of ACL healing (both for reconstruction and repair) represents the current cutting-edge technology to stimulate ligament regeneration in the attempt to improve clinical outcomes.
A total of 23 studies were included in the final analysis. In one paper stem cells were applied for ACL healing, in one paper there was a concomitant application of PRP and stem cells, whereas in the remaining 21 papers PRP was used. The main findings of the present systematic review are that: (1) there is a paucity of clinical trials investigating the role of stem cells in promoting ACL healing, both in the treatment of partial and complete ACL tears. Therefore, no conclusive indication can be issued regarding the efficacy of this treatment approach; (2) despite the high number of randomized controlled trials, the role of PRP in ACL healing is still controversial, and it is not possible to fully endorse this biologic strategy to this particular indication; and (3) the use of biologic agents in partial ACL rupture is very limited, with only two clinical trials published, and therefore, the possibility of treating this kind of injuries by regenerative approaches remains an open question.
The application of PRP and stem cells in the field of ACL repair/reconstruction is technically feasible and safe. Several different approaches have been proposed, ranging from simple intra-articular injections to intra-ligamentary deposition, graft coating, topic use into the bony tunnels, or at the bone-graft interface. Even more complex strategies, such as suturing PRP-clots or PRP-soaked sponges directly onto the graft have been suggested. However, at present moment, it is impossible to endorse an ideal biologic product or an ideal therapeutic modality to stimulate ACL healing. There is a need of further basic studies to better understand the mechanisms of action of these powerful biological agents and also more high-level comparative trials are required to clarify if some specific “products” or applicative modalities are truly better than others.
PRP is an autologous blood derivative which contains a higher concentration of platelets with respect to whole blood. The platelets act as a resorvoir of a milieau of growth factors that could play a fundamental role in stimulating tissue healing and regeneration. Stem cells in orthopaedics are usually mesenchymal stem cells obtained from bone marrow. They can be concentrated or expanded in lab for being used as a biologic augmentation during surgical procedures or as an injective approach for treating a wide range of musculo-skeletal disorders.
This is a very interesting review article on biologic agents for ACL injury healing. It is well-written and has a correct methodology and structure.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bicanic G, Hernandez-Sanchez S, Kulshrestha V S- Editor: Gong XM L- Editor: Wang TQ E- Editor: Li D
| 1. | Spindler KP, Wright RW. Clinical practice. Anterior cruciate ligament tear. N Engl J Med. 2008;359:2135-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 336] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 2. | Prodromos CC, Han Y, Rogowski J, Joyce B, Shi K. A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury-reduction regimen. Arthroscopy. 2007;23:1320-1325.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 536] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 3. | Sonnery-Cottet B, Colombet P. Partial tears of the anterior cruciate ligament. Orthop Traumatol Surg Res. 2016;102:S59-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Bollen S. Epidemiology of knee injuries: diagnosis and triage. Br J Sports Med. 2000;34:227-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Järvelä T. Double-bundle versus single-bundle anterior cruciate ligament reconstruction: a prospective, randomize clinical study. Knee Surg Sports Traumatol Arthrosc. 2007;15:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 237] [Article Influence: 13.2] [Reference Citation Analysis (1)] |
| 6. | Kongtharvonskul J, Attia J, Thamakaison S, Kijkunasathian C, Woratanarat P, Thakkinstian A. Clinical outcomes of double- vs single-bundle anterior cruciate ligament reconstruction: a systematic review of randomized control trials. Scand J Med Sci Sports. 2013;23:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Salmon L, Russell V, Musgrove T, Pinczewski L, Refshauge K. Incidence and risk factors for graft rupture and contralateral rupture after anterior cruciate ligament reconstruction. Arthroscopy. 2005;21:948-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 380] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 8. | Filardo G, Kon E, Roffi A, Di Matteo B, Merli ML, Marcacci M. Platelet-rich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc. 2015;23:2459-2474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 9. | Di Matteo B, Filardo G, Kon E, Marcacci M. Platelet-rich plasma: evidence for the treatment of patellar and Achilles tendinopathy--a systematic review. Musculoskelet Surg. 2015;99:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28:429-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 384] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 11. | Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 1190] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 12. | Xie X, Wu H, Zhao S, Xie G, Huangfu X, Zhao J. The effect of platelet-rich plasma on patterns of gene expression in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;180:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Mastrangelo AN, Vavken P, Fleming BC, Harrison SL, Murray MM. Reduced platelet concentration does not harm PRP effectiveness for ACL repair in a porcine in vivo model. J Orthop Res. 2011;29:1002-1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Joshi SM, Mastrangelo AN, Magarian EM, Fleming BC, Murray MM. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med. 2009;37:2401-2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Hao ZC, Wang SZ, Zhang XJ, Lu J. Stem cell therapy: a promising biological strategy for tendon-bone healing after anterior cruciate ligament reconstruction. Cell Prolif. 2016;49:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Hirzinger C, Tauber M, Korntner S, Quirchmayr M, Bauer HC, Traweger A, Tempfer H. ACL injuries and stem cell therapy. Arch Orthop Trauma Surg. 2014;134:1573-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Silva A, Sampaio R, Fernandes R, Pinto E. Is there a role for adult non-cultivated bone marrow stem cells in ACL reconstruction? Knee Surg Sports Traumatol Arthrosc. 2014;22:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Centeno CJ, Pitts J, Al-Sayegh H, Freeman MD. Anterior cruciate ligament tears treated with percutaneous injection of autologous bone marrow nucleated cells: a case series. J Pain Res. 2015;8:437-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Seijas R, Ares O, Cuscó X, Alvarez P, Steinbacher G, Cugat R. Partial anterior cruciate ligament tears treated with intraligamentary plasma rich in growth factors. World J Orthop. 2014;5:373-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Magnussen RA, Flanigan DC, Pedroza AD, Heinlein KA, Kaeding CC. Platelet rich plasma use in allograft ACL reconstructions: two-year clinical results of a MOON cohort study. Knee. 2013;20:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Del Torto M, Enea D, Panfoli N, Filardo G, Pace N, Chiusaroli M. Hamstrings anterior cruciate ligament reconstruction with and without platelet rich fibrin matrix. Knee Surg Sports Traumatol Arthrosc. 2015;23:3614-3622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Darabos N, Haspl M, Moser C, Darabos A, Bartolek D, Groenemeyer D. Intraarticular application of autologous conditioned serum (ACS) reduces bone tunnel widening after ACL reconstructive surgery in a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2011;19 Suppl 1:S36-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Vogrin M, Rupreht M, Crnjac A, Dinevski D, Krajnc Z, Recnik G. The effect of platelet-derived growth factors on knee stability after anterior cruciate ligament reconstruction: a prospective randomized clinical study. Wien Klin Wochenschr. 2010;122 Suppl 2:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Valentí Azcárate A, Lamo-Espinosa J, Aquerreta Beola JD, Hernandez Gonzalez M, Mora Gasque G, Valentí Nin JR. Comparison between two different platelet-rich plasma preparations and control applied during anterior cruciate ligament reconstruction. Is there any evidence to support their use? Injury. 2014;45 Suppl 4:S36-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Vadalà A, Iorio R, De Carli A, Ferretti M, Paravani D, Caperna L, Iorio C, Gatti A, Ferretti A. Platelet-rich plasma: does it help reduce tunnel widening after ACL reconstruction? Knee Surg Sports Traumatol Arthrosc. 2013;21:824-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Nin JR, Gasque GM, Azcárate AV, Beola JD, Gonzalez MH. Has platelet-rich plasma any role in anterior cruciate ligament allograft healing? Arthroscopy. 2009;25:1206-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Ventura A, Terzaghi C, Borgo E, Verdoia C, Gallazzi M, Failoni S. Use of growth factors in ACL surgery: Preliminary study. J Orthopaed Traumatol. 2005;6:76-79. [DOI] [Full Text] |
| 28. | Starantzis KA, Mastrokalos D, Koulalis D, Papakonstantinou O, Soucacos PN, Papagelopoulos PJ. The Potentially Positive Role of PRPs in Preventing Femoral Tunnel Widening in ACL Reconstruction Surgery Using Hamstrings: A Clinical Study in 51 Patients. J Sports Med (Hindawi Publ Corp). 2014;2014:789317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Mirzatolooei F, Alamdari MT, Khalkhali HR. The impact of platelet-rich plasma on the prevention of tunnel widening in anterior cruciate ligament reconstruction using quadrupled autologous hamstring tendon: a randomised clinical trial. Bone Joint J. 2013;95-B:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Silva A, Sampaio R, Pinto E. Femoral tunnel enlargement after anatomic ACL reconstruction: a biological problem? Knee Surg Sports Traumatol Arthrosc. 2010;18:1189-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Orrego M, Larrain C, Rosales J, Valenzuela L, Matas J, Durruty J, Sudy H, Mardones R. Effects of platelet concentrate and a bone plug on the healing of hamstring tendons in a bone tunnel. Arthroscopy. 2008;24:1373-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Rupreht M, Vogrin M, Hussein M. MRI evaluation of tibial tunnel wall cortical bone formation after platelet-rich plasma applied during anterior cruciate ligament reconstruction. Radiol Oncol. 2013;47:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Vogrin M, Rupreht M, Dinevski D, Hašpl M, Kuhta M, Jevsek M, Knežević M, Rožman P. Effects of a platelet gel on early graft revascularization after anterior cruciate ligament reconstruction: a prospective, randomized, double-blind, clinical trial. Eur Surg Res. 2010;45:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Figueroa D, Melean P, Calvo R, Vaisman A, Zilleruelo N, Figueroa F, Villalón I. Magnetic resonance imaging evaluation of the integration and maturation of semitendinosus-gracilis graft in anterior cruciate ligament reconstruction using autologous platelet concentrate. Arthroscopy. 2010;26:1318-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Silva A, Sampaio R. Anatomic ACL reconstruction: does the platelet-rich plasma accelerate tendon healing? Knee Surg Sports Traumatol Arthrosc. 2009;17:676-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 36. | Seijas R, Ares O, Catala J, Alvarez-Diaz P, Cusco X, Cugat R. Magnetic resonance imaging evaluation of patellar tendon graft remodelling after anterior cruciate ligament reconstruction with or without platelet-rich plasma. J Orthop Surg (Hong Kong). 2013;21:10-14. [PubMed] |
| 37. | Rupreht M, Jevtič V, Serša I, Vogrin M, Jevšek M. Evaluation of the tibial tunnel after intraoperatively administered platelet-rich plasma gel during anterior cruciate ligament reconstruction using diffusion weighted and dynamic contrast-enhanced MRI. J Magn Reson Imaging. 2013;37:928-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Radice F, Yánez R, Gutiérrez V, Rosales J, Pinedo M, Coda S. Comparison of magnetic resonance imaging findings in anterior cruciate ligament grafts with and without autologous platelet-derived growth factors. Arthroscopy. 2010;26:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 39. | Sánchez M, Anitua E, Azofra J, Prado R, Muruzabal F, Andia I. Ligamentization of tendon grafts treated with an endogenous preparation rich in growth factors: gross morphology and histology. Arthroscopy. 2010;26:470-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 40. | Di Matteo B, Kon E, Filardo G. Intra-articular platelet-rich plasma for the treatment of osteoarthritis. Ann Transl Med. 2016;4:63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 41. | Kon E, Filardo G, Di Matteo B, Marcacci M. PRP for the treatment of cartilage pathology. Open Orthop J. 2013;7:120-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Tschon M, Fini M, Giardino R, Filardo G, Dallari D, Torricelli P, Martini L, Giavaresi G, Kon E, Maltarello MC. Lights and shadows concerning platelet products for musculoskeletal regeneration. Front Biosci (Elite Ed). 2011;3:96-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Mautner K, Malanga GA, Smith J, Shiple B, Ibrahim V, Sampson S, Bowen JE. A call for a standard classification system for future biologic research: the rationale for new PRP nomenclature. PM R. 2015;7:S53-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 212] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 44. | Loibl M, Lang S, Brockhoff G, Gueorguiev B, Hilber F, Worlicek M, Baumann F, Grechenig S, Zellner J, Huber M. The effect of leukocyte-reduced platelet-rich plasma on the proliferation of autologous adipose-tissue derived mesenchymal stem cells. Clin Hemorheol Microcirc. 2016;61:599-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Assirelli E, Filardo G, Mariani E, Kon E, Roffi A, Vaccaro F, Marcacci M, Facchini A, Pulsatelli L. Effect of two different preparations of platelet-rich plasma on synoviocytes. Knee Surg Sports Traumatol Arthrosc. 2015;23:2690-2703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 46. | Kreuz PC, Krüger JP, Metzlaff S, Freymann U, Endres M, Pruss A, Petersen W, Kaps C. Platelet-Rich Plasma Preparation Types Show Impact on Chondrogenic Differentiation, Migration, and Proliferation of Human Subchondral Mesenchymal Progenitor Cells. Arthroscopy. 2015;31:1951-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016;24:1307-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 48. | Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 541] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 49. | Chirba MA, Sweetapple B, Hannon CP, Anderson JA. FDA regulation of adult stem cell therapies as used in sports medicine. J Knee Surg. 2015;28:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |