Published online Jul 18, 2016. doi: 10.5312/wjo.v7.i7.418
Peer-review started: January 22, 2016
First decision: March 1, 2016
Revised: March 26, 2016
Accepted: May 10, 2016
Article in press: May 11, 2016
Published online: July 18, 2016
Processing time: 170 Days and 23.8 Hours
AIM: To analyse bone remodeling in regard to the age of scaphoid non-unions (SNU) with immunohistochemistry.
METHODS: Thirty-six patients with symptomatic SNU underwent surgery with resection of the pseudarthrosis. The resected material was evaluated histologically after staining with hematoxylin-eosin (HE), tartrate resistant acid phosphatase (TRAP), CD 68, osteocalcin (OC) and osteopontin (OP). Histological examination was performed in a blinded fashion.
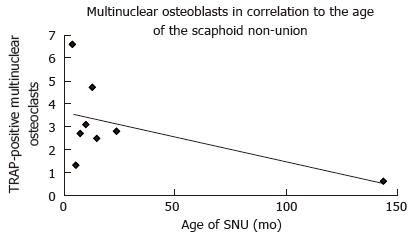
RESULTS: The number of multinuclear osteoclasts in the TRAP-staining correlated with the age of the SNU and was significantly higher in younger SNU (P = 0.034; r = 0.75). A higher number of OP-immunoreactive osteoblasts significantly correlated with a higher number of OC-immunoreactive osteoblasts (P = 0.001; r = 0.55). Furthermore, a greater number of OP-immunoreactive osteoblasts correlated significantly with a higher number of OP-immunoreactive multinuclear osteoclasts (P = 0.008; r = 0.43). SNU older than 6 mo showed a significant decrease of the number of fibroblasts (P = 0.04). Smoking and the age of the patients had no influence on bone remodeling in SNU.
CONCLUSION: Multinuclear osteoclasts showed a significant decrease in relation to the age of SNU. However, most of the immunhistochemical findings of bone remodeling do not correlate with the age of the SNU. This indicates a permanent imbalance of bone formation and resorption as indicated by a concurrent increase in both osteoblast and osteoclast numbers. A clear histological differentiation into phases of bone remodeling in SNU is not possible.
Core tip: The bone remodeling in regard to the age of scaphoid non-union is investigated with immunohistochemistry. Multinuclear osteoclasts showed a significant decrease in relation of the age of scaphoid non-union, but smoking and the age of the patients had no influence on bone remodeling. Most of the immunhistochemical findings of bone remodeling do not correlate with the age of the scaphoid non-unions, which indicates a permanent imbalance of bone formation and resorption.
- Citation: Rein S, Hanisch U, Schaller HE, Zwipp H, Rammelt S, Weindel S. Evaluation of bone remodeling in regard to the age of scaphoid non-unions. World J Orthop 2016; 7(7): 418-425
- URL: https://www.wjgnet.com/2218-5836/full/v7/i7/418.htm
- DOI: https://dx.doi.org/10.5312/wjo.v7.i7.418
The scaphoid is the most commonly fractured carpal bone[1-3]. Scaphoid non-union (SNU) occurs in approximately 5% to 13% of treated scaphoid fractures and in an unknown number of unrecognized fractures[2,4-6]. The reasons for this high non-union rate are multifactorial including fracture location and vascularity, failure of recognizing the fracture and inadequate initial treatment[7,8]. Additionally, scaphoid fractures heal by intramembranous ossification, which leaves the scaphoid without protective fracture callus against potentially disruptive forces. Progressive osteoarthritis, so called scaphoid non-union advanced collapse, inevitably develops in all cases with untreated SNU over time[9].
Fracture healing consists of a regulatory circuit, which requires the proliferation and differentiation of osteoblasts and osteoclasts for bone regeneration and remodeling, together with formation of new blood vessels for bone vascularisation and a myriad of intercellular interactions and molecular communications to coordinate this complex process[10]. Osteoblasts produce organic components of the extracellular matrix, regulate the mineralisation of the osteoid and therefore are essential for bone formation[11,12]. Osteoclasts are responsible for bone resorption by removing mineralized matrix and breaking up the organic bone. Both osteoclasts and activated macrophages show a high expression of tartrate resistant acid phosphatase (TRAP) and glycoprotein CD 68. TRAP is synthesized as latent proenzyme and activated by proteolytic cleavage and reduction[13,14]. Osteocalcin (OC) is an extracellular matrix protein produced by osteoblasts, which constitutes 2% of the total protein content in bone. It is distributed in cement lines of both cortical and trabecular bone[15,16]. OC is thought to have a role in the early stages of bone healing and is a marker for bone formation[17,18]. Osteopontin (OP) is a non-collagenous extracellular matrix protein and is biosynthesized by osteoblasts, osteoclasts, osteocytes, activated fibroblasts, hypertrophic chondrocytes and cemented lines[16]. It is a multifunctional protein that is involved in several aspects of bone turnover and remodeling as well as fracture healing[16,19].
A recent study found significant less bone remodeling in SNU older than a mean age of 45 mo[20]. However, conventional histological investigation is not sufficient to analyze bone remodeling, because staining of the tissue is unspecific. Immunohistochemistry (IHC) using specific markers of bone resorption and bone formation is helpful to shed further light on the process of bone remodeling in SNU. It is hypothesized that there would be differences in numbers of immunohistochemically stained cells that would correlate with the age of the SNU. This difference in staining may lead to the identification of more bone formation (increase in osteoblasts) or bone resorption (increase in number of osteoclasts) over time and may generate more information about the development of SNU. Therefore the aim of this study was to evaluate bone remodeling of SNU with immunohistochemical markers in regard to the age of the fracture.
The study was conducted in accordance with the Helsinki Declaration. The local ethics committee review board approved the study (367/2007A).
Thirty six male patients with a mean age of 26 (SD 12; range: 12-56) years at the time of injury were included in this study. Sixteen right and 20 left wrists were injured. The mean time between injury and surgery for non-union was 22 (SD 27; range: 4-144) mo. Six SNU were localised in the proximal third, 27 in the middle third, and one in the distal third of the scaphoid, respectively. In two patients, exact localisation of SNU was not defined. No additional surgery was performed during the follow-up period in 25 cases. However, one patient received a vascularised bone graft from the distal radius, one patient a four corner fusion, one patient a denervation of the wrist, and four patients another kind of wrist surgery in the postoperative follow-up. The data of the longer postoperative period in four patients were not available. Seventeen patients were non-smokers, 16 patients were smokers, but in three patients it was unclear, whether they are smokers or non-smokers.
Only patients, who stated a defined date of trauma having a symptomatic SNU, were included in this study. Exclusion criteria were unclear date of trauma, prior surgical treatment or associated adjacent injuries of the wrist as well as relevant underlying clinical diseases as diabetes mellitus or vascular disorders. Delayed fracture healing was defined between 4 to 6 mo. If no stable ossification was seen after 6 mo, the term non-union was used[21].
During surgery the SNU was resected completely, whereas resection sides showed healthy bone verified by macroscopic bleeding. Autologous cancellous bone was interposed in the former SNU gap and compression osteosynthesis using a Herbert screw was performed[22]. Specimens were immediately fixed in 4% neutral buffered (pH = 7.4) formaldehyde solution for 24 h at 4 °C, decalcified with diaminoethanetetraacetic acid and embedded in paraffin.
Sections of 2 μm were cut on a Leica rotation microtome (RM2055, Wetzlar, Germany) and mounted on silane-coated slides for conventional staining, enzyme- and IHC. HE-staining was performed in all specimens for morphological evaluation. Subsequently, the tissue sections were stained with TRAP, CD 68 (working dilution: 1:150, monoclonal, clone: KP-1, mouse anti-human, Dako, Glostrup, Denmark), OC (working dilution: 1:250, monoclonal, clone: OCG-3, mouse anti-human, Zytotec, Berlin, Germany) and OP (working dilution: 1:300, polyclonal, rabbit antisera, Chemicon, Temecula, Canada). Blocks and slides were stored at room temperature.
The mounted sections were dehydrated beginning with xylol in decreasing concentrations. Sections were then rehydrated with distilled water and pretreated according to the individual instructions from the suppliers of the used primary antibodies.
No special pretreatment was necessary for CD 68 and OC. For OP, specimens were treated with trypsine (pH = 6.0) for 30 min. After washing in phosphate-buffered saline solution (PBS, pH = 7.4), endogenous peroxidase activity was blocked in all sections with 1% hydrogen peroxide for 5 min. Nonspecific electrostatic protein charging was blocked with blocking reagent (Dako, Glostrup, Denmark) for 10 min at room temperature. Sections were incubated with respective normal sera (Linaris, Wertheim, Germany) for an hour at room temperature and then incubated overnight at 37 °C with primary antibodies. Biotinylated secondary antibodies were added for 30 min at 37 °C, followed by an avidin-biotin-enzyme complex for 30 min at 37 °C (Vectastain ABC-HRP kit, Linaris, PK-4000, Wertheim-Bettingen, Germany) at room temperature. The peroxidase activity was visualized with 3’-3’-diaminobenzidine. Then counterstaining with hematoxylin was performed. Sections were washed thoroughly three times in PBS for 5 min after each step. Finally, sections were dehydrated and covered with Entellan (Merck, Darmstadt, Germany). Control procedures, i.e., identical staining without adding primary antibodies, were performed in parallel. Then counterstaining with hematoxylin was performed.
Histopathological examination of the stained tissue sections was performed using an Olympus BHS light microscope in the transmitted mode at final magnifications of 40 ×, 100 ×, 200 × and 400 ×. One section in each staining per subject was analysed. Total cell counts were counted at an original magnification of 100 × in 10 subsequent adjacent visual fields, representing the whole width of the non-union. Only fibroblasts were counted in 5 subsequent visual fields, because the volume of fibroblast tissue was not big enough for 10 visual fields in most cases. All specimens were blinded for cell counts.
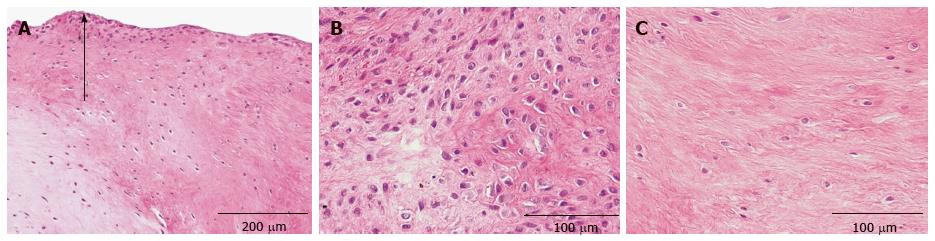
Histopathological analysis was centered on osteopathological criteria including determination of chondrocytes and extracellular matrix (ECM) at the non-union gap, osteoblasts, osteoclasts, osteocytes, osteoid and cement lines of the underlying bone, mesenchymal cells and ECM in resorptive bone cysts as well as cysts containing fibrous or fibrocartilage tissue and typical hyaline cartilage in the OC and OP staining[23].
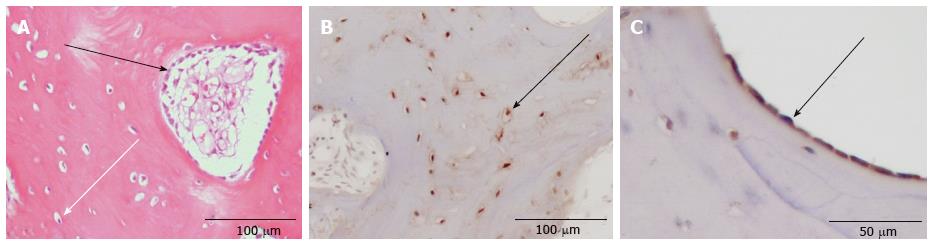
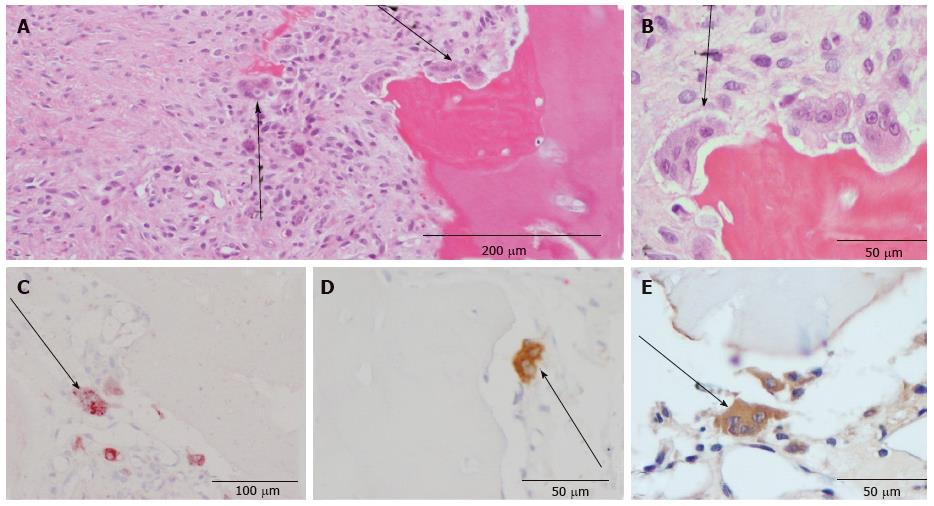
Morphological analysis was first performed with the HE-staining. With the help of the following criteria the different cell types were identified: Osteoblasts, which are mononuclear cells, were counted if they lined up the external surface of bone trabeculae and the surface of Haversian canals (Figure 1)[23,24]. Osteocytes were counted if they were embedded into the mineralized bone matrix (Figure 1). Cells with two to fifteen nuclei in small resorptive excavations (Howship’s lacunae) on the bone surface were counted as multinuclear osteoclasts (Figure 2).
Mononuclear osteoclast precursors could only be identified in the TRAP and CD 68 staining and were counted as mononuclear positive-stained cells in these two stainings. Fibroblasts were counted as cells located in the non-union gap in the HE-staining (Figure 3).
Multinuclear osteoclasts were counted in the TRAP staining, with CD 68 and OP-IHC. Osteoblasts and osteocytes were counted with OP- and OC-IHC, respectively.
Statistical analysis was performed with a two sided t test in order to analyse the occurrence of OC- and OP-positive cells in the different parts of the SNU with a level of significance of P≤ 0.05.
The two-sided Pearson correlation analysis was used to investigate the linear relationship with regard to age of SNU and patients, and counted cell numbers. Correlation analysis was performed with Spearman’s rho coefficient with a significance level of P≤ 0.05. The influence of smoking has been investigated with the Kruskal-Wallis test followed by the Mann-Whitney test with a level of significance of P≤ 0.05. Statistical analysis was performed with the computer program SPSS (Version 11.5, Chicago, United States).
Table 1 gives an overview over the markers that could reliably and reproducibly be detected. Negative procedures without antibodies showed no staining. OP was immunolocalized within chondrocytes and ECM of the non-union, osteoclasts, osteoblasts, osteoid and osteocytes of the underlying bone as well as the hyaline cartilage. Cement lines of newly formed lamellar bone only stained positively for OP in 8 out of 36 cases (Table 1). OC showed immunoreactivity in cement lines, osteocytes, osteoblasts and hyaline cartilage (Table 1). Resorptive and fibrous bone cysts showed immunoreactivity for OP in most cases but not for OC (Table 1). Enzyme-histochemical staining against TRAP specifically stained osteoclasts and mononuclear precursors indicating bone resorption during the remodeling process (Figure 2C). The macrophage marker CD 68 was detected in mononuclear and multinuclear macrophages or osteoclasts (Figure 2D). Mononuclear macrophages/osteoclast precursors and multinuclear osteoclasts stained positively for CD 68 in 32 out of 36 cases, whereas in only 8 out of 36 cases osteoclasts were stained positive for TRAP.
| Histological feature | Immunohistochemical findings | ||||||||||||||
| Osteocalcin | OP | ||||||||||||||
| Yes(n) | Range of time (mo) | No(n) | Range of time (mo) | Not assessable(n) | Yes(n) | Range of time (mo) | No(n) | Range of time (mo) | Not assessable (n) | ||||||
| Non-union | Chondrocytes | 1 | 12 | 12 | 35 | 4 | 144 | - | 25 | 4 | 144 | 11 | 6 | 60 | - |
| ECM | 0 | - | - | 36 | 4 | 144 | - | 34 | 4 | 144 | 2 | 15 | 24 | - | |
| Underlying bone | Osteoblasts | 31 | 6 | 144 | 4 | 4 | 22 | 1 | 34 | 4 | 144 | 2 | 9 | 15 | - |
| Osteoclasts | 2 | 8 | 29 | 33 | 4 | 144 | 1 | 33 | 4 | 85 | 2 | 9 | 15 | 1 | |
| Osteocytes | 32 | 6 | 144 | 4 | 4 | 15 | - | 34 | 4 | 144 | 2 | 9 | 15 | - | |
| Osteoid | 13 | 7 | 28 | 23 | 4 | 144 | - | 321 | 4 | 85 | 41 | 14 | 144 | - | |
| Cement lines | 33 | 4 | 144 | 3 | 7 | 15 | - | 8 | 7 | 144 | 28 | 4 | 85 | - | |
| Resorptive bone cysts | Mesenchymal Cells | - | - | - | 15 | 4 | 60 | 21 | 11 | 4 | 24 | 5 | 9 | 60 | 20 |
| ECM | - | - | - | 15 | 4 | 60 | 21 | 15 | 4 | 60 | 1 | 15 | 15 | 20 | |
| Fibrous bone cysts | Mesenchymal cells | 1 | 15 | 15 | 12 | 7 | 54 | 23 | 10 | 7 | 54 | 7 | 4 | 28 | 19 |
| ECM | 1 | 15 | 15 | 12 | 7 | 54 | 23 | 12 | 7 | 54 | 5 | 4 | 24 | 19 | |
| Hyaline cartilage | Typical | 33 | 4 | 144 | 2 | 9 | 12 | 1 | 34 | 4 | 144 | 1 | 15 | 15 | 1 |
Osteoid showed immunoreactivity for OP in 32 younger SNU (18.5 SD 17.9 mo) with a range of age between 4 to 85 mo, whereas there was no immunoreactivity for OP in 4 older SNUs (50.5 SD 62.7 mo) with a range of age between 14 to 144 mo. The difference between the two groups was statistical significant (P = 0.02; Table 1).
Single results of the cell counting are presented in Table 2. The number of multinuclear osteoclasts in the TRAP-staining correlated with the age of the SNU and was significantly higher in younger SNU (P = 0.034; r = 0.75; Figure 4). All other correlations in regard to the age of the SNU showed no significant results.
| Single results of the cell counting | |||||
| Histological feature | HE | TRAP (n = 8) | CD 68 (n = 32) | OP (n = 36) | OC (n = 36) |
| Osteoblasts | N/A | N/A | N/A | 23.4 ± 12 | 25.6 ± 19.5 |
| Osteoclasts | |||||
| Uninuclear | N/A | 9 ± 6.5 | 4.6 ± 5.1 | N/A | N/A |
| Multinuclear | N/A | 2.3 ± 0.8 | 1.8 ± 1.2 | 1.1 ± 0.8 | N/A |
| Osteocytes | N/A | N/A | N/A | 38.2 ± 11.2 | 50.8 ± 16.4 |
| Fibroblasts | 285 ± 181 | N/A | N/A | N/A | N/A |
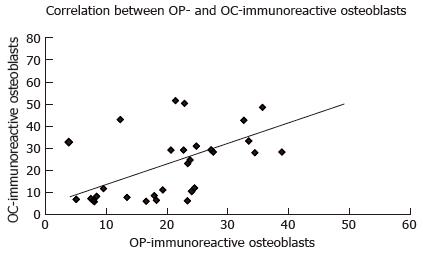
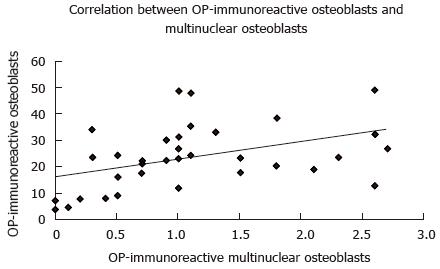
A higher number of OP-immunoreactive osteoblasts significantly correlated with a higher number of OC-immunoreactive osteoblasts (P = 0.001; r = 0.55; Figure 5). Furthermore, a greater number of OP-immunoreactive osteoblasts correlated significantly with a higher number of OP-immunoreactive multinuclear osteoclasts (P = 0.008; r = 0.43; Figure 6).
A mean of 285 (SD 181) fibroblasts were counted in the 36 investigated SNU in the HE staining. A mean of 457 (SD 175) fibroblasts were counted in SNU (n = 4) up to 6 mo old. In contrast, a mean of 264 (SD 173) fibroblasts were measured in SNU (n = 32) older than 6 mo. This was a significant decrease of fibroblasts in SNU, which are older than 6 mo (P = 0.04). However, no significant correlations have been found between the age of the patients and all investigated cell types. Furthermore, no significant differences have been observed between smokers and non-smokers for all investigated cell types.
A recent study has shown that significant less bone remodeling takes place in older SNU with a mean age of 45 mo compared to a mean age of 18 mo[20]. However, these results were based on conventional HE-staining. Several bone-specific extracellular matrix proteins may be used to assess bone remodeling[16]. OC is reportedly the most specific noncollagenous bone matrix protein, being expressed by osteoblasts and osteocytes[25]. In the present study, we demonstrate specific staining of osteoblasts, osteocytes, cement lines, hyaline cartilage and in some cases osteoid (n = 13). OP is reportedly expressed by osteocytes, osteoblasts, and their precursors, osteoclasts, hypertrophic chondrocytes, and cement lines[15,16]. We have seen specific staining of osteoblasts, osteoclasts, osteocytes, osteoid, chondrocytes, ECM of the non-union gap, and hyaline cartilage. OP interacts with osteoclasts, implicating it as a potentially important marker of bone resorption[26].
A greater number of OC-immunoreactive osteoblasts correlated significantly with a greater number of OP-immunoreactive osteoblasts. However, correlation analysis between the two markers showed no time-dependent significant differences. Furthermore, a higher number of OP-immunoreactive osteoblasts correlated significantly with a higher number of OP-immunoreactive multinuclear osteoclasts, indicating a higher bone remodeling in younger SNU. These findings confirm the theory that bone remodeling is a balance between bone formation and bone resorption in which osteoblasts exhibit two opposite phenotypes. There is the osteogenic phenotype, which secretes bone matrix at the bone resorption site, and the osteoclastogenic phenotype, which supports osteoclast differentiation in the old bone area[11]. The close interplay between osteoblasts and osteoclasts during bone repair is well established[10-12,17,23,24].
A recent study has shown that cell viability and mineralization-positive colony forming units were significantly reduced in osteoblasts retrieved from non-union sites. This study identified a set of significantly down-regulated factors in those “non-union osteoblasts” that are involved in the regulation of osteoblast proliferation and differentiation[27]. This indicates that activity of osteoblasts in non-unions is altered, which could explain the lack of time-dependent changes in the OC- and OP-staining. However, another study could demonstrate, that OC-positive osteoblasts, which were taken from SNU, possessed osteogenic capability and could be stimulated by recombinant human bone morphogenetic protein-2 in vitro, resulting in significant increase in osteoblast differentiation and bone production[28].
The number of osteoclasts decreased significantly in older SNU, which could be shown in the TRAP-staining, but not in the CD 68 IHC. This could be explained by the fact that CD 68 is not a specific osteoclast marker but rather a marker for several cells of the monocyte/macrophage lineage.
It is known, that nicotine has a dose dependent negative effect on bone healing, resulting in ischemia, diminished osteoblast function and decreased expression of bone morphogenetic protein[29,30]. However, in this study nicotine abuse had no measurable influence on bone remodeling in SNU.
The high account of fibroblasts reflects a cell rich fibrous tissue in the non-union gap. We have seen a significant decrease of the count of fibroblasts in SNU older than 6 mo. Our explanation is that the instability in the non-union gap induces or provokes an activation of fibroblasts. For that reason, further research on this topic could be the investigation of proliferation with specific immunohistochemical markers, e.g., Ki 67.
A greater number of OP-immunoreactive osteoblasts significantly correlated with a greater number of OC-immunoreactive osteoblasts and OP-immunoreactive multinuclear osteoclasts. Multinuclear osteoclasts show a significant decrease in older SNU. Fibroblasts showed a significant decrease in SNU, which are older than 6 mo. These results indicate a decreased bone remodeling in older SNU. On the other hand, permanent remodeling indicates mechanical instability and imbalance. Therefore most of the immunohistochemical markers of bone remodeling do not correlate with the age of the SNU. Smoking had no influence on bone remodelling in SNU.
The authors thank Ursula Range for her advice with the statistical analysis, Suzanne Manthey and Doreen Küchler for histological preparation as well as Thomas Albrecht for preparation of the photographs.
The scaphoid is the most commonly fractured carpal bone, whereas non-union occurs in approximately 5% to 13% of treated scaphoid fractures.
Conventional histological stainings are insufficient to analyze bone remodeling, because staining of the tissue is unspecific.
Immunohistochemistry using specific markers of bone resorption and bone formation is helpful to shed further light on the process of bone remodeling in scaphoid non-union.
Multinuclear osteoclasts, as a marker for bone resorption, showed a significant decrease in relation of the age of scaphoid non-union, but smoking and the age of the patients had no influence on bone remodeling. Most of the immunhistochemical findings of bone remodeling do not correlate with the age of the scaphoid non-unions (SNU), which indicates a permanent imbalance of bone formation and resorption.
This is a study on the bone remodeling in regard to the age of SNU with immunohistochemistry. The rationale for the study is appropriate and it is an interesting paper with a valuable contribution.
Manuscript source: Invited manuscript
P- Reviewer: Luo XH, Maia LP S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Dunn AW. Fractures and dislocations of the carpus. Surg Clin North Am. 1972;52:1513-1538. [PubMed] |
| 2. | Leslie IJ, Dickson RA. The fractured carpal scaphoid. Natural history and factors influencing outcome. J Bone Joint Surg Br. 1981;63-B:225-230. [PubMed] |
| 3. | Hove LM. Epidemiology of scaphoid fractures in Bergen, Norway. Scand J Plast Reconstr Surg Hand Surg. 1999;33:423-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 175] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Düppe H, Johnell O, Lundborg G, Karlsson M, Redlund-Johnell I. Long-term results of fracture of the scaphoid. A follow-up study of more than thirty years. J Bone Joint Surg Am. 1994;76:249-252. [PubMed] |
| 5. | Eddeland A, Eiken O, Hellgren E, Ohlsson NM. Fractures of the scaphoid. Scand J Plast Reconstr Surg. 1975;9:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 109] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Dias JJ, Brenkel IJ, Finlay DB. Patterns of union in fractures of the waist of the scaphoid. J Bone Joint Surg Br. 1989;71:307-310. [PubMed] |
| 7. | Perlik PC, Guilford WB. Magnetic resonance imaging to assess vascularity of scaphoid nonunions. J Hand Surg Am. 1991;16:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 94] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Pandit S, Wen DY. Scaphoid fractures with unusual presentations: a case series. Cases J. 2009;2:7220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Lindström G, Nyström A. Natural history of scaphoid non-union, with special reference to “asymptomatic” cases. J Hand Surg Br. 1992;17:697-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 85] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Schweiberer L, Baumgart R, Deiler S. The biological reaction in atrophic and hypertrophic pseudarthrosis of diaphysis of long bone. Causes and forms of appearance. Chirurg. 1999;70:1193-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Nakahama K. Cellular communications in bone homeostasis and repair. Cell Mol Life Sci. 2010;67:4001-4009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Owen M. Lineage of osteogenic cells and their relationship to the stromal system. In: Bone and Mineral Research 1985; 1-25. |
| 13. | Ljusberg J, Ek-Rylander B, Andersson G. Tartrate-resistant purple acid phosphatase is synthesized as a latent proenzyme and activated by cysteine proteinases. Biochem J. 1999;343 Pt 1:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 14. | Ljusberg J, Wang Y, Lång P, Norgård M, Dodds R, Hultenby K, Ek-Rylander B, Andersson G. Proteolytic excision of a repressive loop domain in tartrate-resistant acid phosphatase by cathepsin K in osteoclasts. J Biol Chem. 2005;280:28370-28381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Ingram RT, Clarke BL, Fisher LW, Fitzpatrick LA. Distribution of noncollagenous proteins in the matrix of adult human bone: evidence of anatomic and functional heterogeneity. J Bone Miner Res. 1993;8:1019-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Rammelt S, Corbeil D, Manthey S, Zwipp H, Hanisch U. Immunohistochemical in situ characterization of orthopedic implants on polymethyl metacrylate embedded cutting and grinding sections. J Biomed Mater Res A. 2007;83:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Rammelt S, Neumann M, Hanisch U, Reinstorf A, Pompe W, Zwipp H, Biewener A. Osteocalcin enhances bone remodeling around hydroxyapatite/collagen composites. J Biomed Mater Res A. 2005;73:284-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990-1047. [PubMed] |
| 19. | Yamazaki M, Nakajima F, Ogasawara A, Moriya H, Majeska RJ, Einhorn TA. Spatial and temporal distribution of CD44 and osteopontin in fracture callus. J Bone Joint Surg Br. 1999;81:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Rein S, Hanisch U, Rammelt S, Schmidt G, Schaller HE, Zwipp H, Oehmke M, Weindel S. Histopathological, radiological and clinical aspects of the temporal assignment of scaphoid non-union. Arch Orthop Trauma Surg. 2010;130:1243-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Rüter A, Mayr E. Pseudarthrosis. Chirurg. 1999;70:1239-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Herbert TJ, Fisher WE. Management of the fractured scaphoid using a new bone screw. J Bone Joint Surg Br. 1984;66:114-123. [PubMed] |
| 23. | Athanasou N. Injury and repair of skeletal tissues. Clinical, radiological, and pathological correlation of diseases of bone, joint, and soft tissue. Hodder Arnold: Gran Bretagna 2001; . |
| 24. | Lian J, Stein G, Canalis E. Bone formation: osteoblast lineage cells, growth factors, matrix proteins and the mineralization process. Philadelphia: Lippincott-Williams & Wilkins 1999; . |
| 25. | Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. Establishment of an osteocyte-like cell line, MLO-Y4. J Bone Miner Res. 1997;12:2014-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 402] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 26. | Miyauchi A, Alvarez J, Greenfield EM, Teti A, Grano M, Colucci S, Zambonin-Zallone A, Ross FP, Teitelbaum SL, Cheresh D. Recognition of osteopontin and related peptides by an alpha v beta 3 integrin stimulates immediate cell signals in osteoclasts. J Biol Chem. 1991;266:20369-20374. [PubMed] |
| 27. | Hofmann A, Ritz U, Hessmann MH, Schmid C, Tresch A, Rompe JD, Meurer A, Rommens PM. Cell viability, osteoblast differentiation, and gene expression are altered in human osteoblasts from hypertrophic fracture non-unions. Bone. 2008;42:894-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Qu G, von Schroeder HP. The osteogenic potential of pseudoarthrosis tissue and bone from human scaphoid non-unions. J Hand Surg Eur Vol. 2008;33:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Hollinger JO, Schmitt JM, Hwang K, Soleymani P, Buck D. Impact of nicotine on bone healing. J Biomed Mater Res. 1999;45:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Ma L, Zheng LW, Cheung LK. Influence of nicotine on blood perfusion and bone healing during distraction osteogenesis. Ann R Australas Coll Dent Surg. 2008;19:52-54. [PubMed] |