Published online Dec 18, 2016. doi: 10.5312/wjo.v7.i12.832
Peer-review started: June 27, 2016
First decision: August 11, 2016
Revised: September 19, 2016
Accepted: October 25, 2016
Article in press: October 27, 2016
Published online: December 18, 2016
Processing time: 168 Days and 21.6 Hours
To assess whether the surgical apgar score (SAS) is a prognostic tool capable of identifying patients at risk of major complications following lower extremity amputations surgery.
This was a single-center, retrospective observational cohort study conducted between January 2013 and April 2015. All patients who had either a primary transtibial amputation (TTA) or transfemoral amputation (TFA) conducted at our institution during the study period were assessed for inclusion. All TTA patients underwent a standardized one-stage operative procedure (ad modum Persson amputation) performed approximately 10 cm below the knee joint. All TTA procedures were performed with sagittal flaps. TFA procedures were performed in one stage with amputation approximately 10 cm above the knee joint, performed with anterior/posterior flaps. Trained residents or senior consultants performed the surgical procedures. The SAS is based on intraoperative heart rate, blood pressure and blood loss. Intraoperative parameters of interest were collected by revising electronic health records. The first author of this study calculated the SAS. Data regarding major complications were not revealed to the author until after the calculation of SAS. The SAS results were arranged into four groups (SAS 0-4, SAS 5-6, SAS 7-8 and SAS 9-10). The cohort was then divided into two groups representing low-risk (SAS ≥ 7) and high-risk patients (SAS < 7) using a previously established threshold. The outcome of interest was the occurrence of major complications and death within 30-d of surgery.
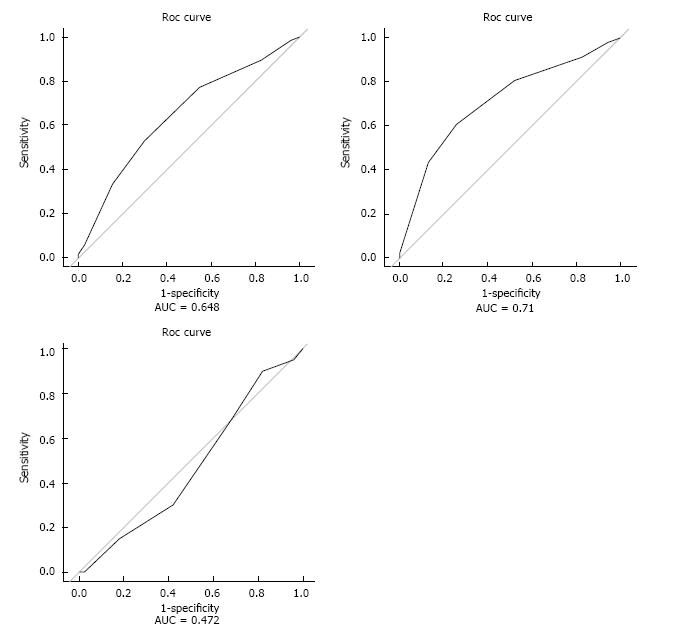
A logistic regression model with SAS 9-10 as a reference showed a significant linear association between lower SAS and more postoperative complications [all patients: OR = 2.00 (1.33-3.03), P = 0.001]. This effect was pronounced for TFA [OR = 2.61 (1.52-4.47), P < 0.001]. A significant increase was observed for the high-risk group compared to the low-risk group for all patients [OR = 2.80 (1.40-5.61), P = 0.004] and for the TFA sub-group [OR = 3.82 (1.5-9.42), P = 0.004]. The AUC from the models were estimated as follows: All patients = [0.648 (0.562-0.733), P = 0.001], for TFA patients = [0.710 (0.606-0.813), P < 0.001] and for TTA patients = [0.472 (0.383-0.672), P = 0.528]. This indicates moderate discriminatory power of the SAS in predicting postoperative complications among TFA patients.
SAS provides information regarding the potential development of complications following TFA. The SAS is especially useful when patients are divided into high- and low-risk groups.
Core tip: This study presents new knowledge regarding the use of Surgical Apgar Score (SAS) in dysvascular lower extremity amputations (LEA) surgery. There is a significant increase in complications with a low SAS after LEA surgery. This is even more pronounced when the transfemoral amputation (TFA) sub-group is analyzed separately. Thus, for a TFA patient with a SAS < 7, the odds of a major complication or death is four times greater than for a patient with a SAS ≥ 7. ROC analysis confirms the discriminatory power of the SAS approach among the TFA patients. However, the SAS model proved to be of no prognostic value in the transtibial amputation group.
- Citation: Wied C, Foss NB, Kristensen MT, Holm G, Kallemose T, Troelsen A. Surgical apgar score predicts early complication in transfemoral amputees: Retrospective study of 170 major amputations. World J Orthop 2016; 7(12): 832-838
- URL: https://www.wjgnet.com/2218-5836/full/v7/i12/832.htm
- DOI: https://dx.doi.org/10.5312/wjo.v7.i12.832
The surgical apgar score (SAS) has a strong correlation with the occurrence of major complications or death within 30-d following general and vascular surgery[1-4]. The scoring system is based on three intraoperative variables: Lowest mean arterial pressure, lowest heart rate and estimated intraoperative blood loss. The score seems to be irrelevant to several orthopedic sub-specialties[5,6]. However, there is a lack of knowledge regarding the utility of the score in the field of lower extremity amputations (LEA). An increasing number of high-risk patients are undergoing amputations[7]. They diverge from standard orthopedic patients due to their high age and many co-morbidities[8]. Studies show that their associated 30-d postoperative mortality is up to 30%[9], a rate unmatched in the orthopedic specialty. Our aim with this study is to assess whether the SAS is a prognostic tool capable of identifying patients at risk of major complications following LEA surgery.
This was a single-center, retrospective observational cohort study conducted between January 2013 and April 2015. All patients who had either a primary transtibial amputation (TTA) or transfemoral amputation (TFA) at our institution during the study period were assessed for inclusion. Exclusion criteria included: Re-amputations, a combination of amputation and removal of intramedullary nails and patients with incomplete data registrations. Patients were identified through our local operation database. All patients eligible for inclusion were included. The decision regarding the amputation level was made by senior consultants taking into account skin perfusion pressure measurement results and patient general condition. All TTA patients underwent a standardized one-stage operative procedure (admodum Persson amputation)[10] performed approximately 10 cm below the knee joint. All TTA procedures were performed with sagittal flaps. The TFA procedures were performed in one stage approximately 10 cm above the knee joint, performed with anterior/posterior flaps. Trained residents or senior consultants performed the surgical procedures. The tourniquet, when used, was inflated around the femur to a pressure of 100 mmHg above systolic blood pressure[11]. Standardized care was provided for all patients including standards for fluid replacement and thromboprophylaxis. Initial postoperative rehabilitation programs were initiated on the first postoperative day if patients could cooperate.
Intraoperative parameters of interest were collected by reviewing electronic health records. The first author of this study calculated the SAS by following the algorithm described in Table 1. Data regarding major complications were not revealed to the author until after the calculation of SAS. SAS results were arranged into four groups (SAS 0-4, SAS 5-6, SAS 7-8 and SAS 9-10) as proposed by Gawande et al[2]. The cohort was then divided into two groups representing low-risk (SAS ≥ 7) and high-risk (SAS < 7) patients using a previously established threshold[5,6,12]. The outcome of interest was the occurrence of major complications and death within 30-d of surgery. The definitions of major complications were in accordance with Gawande et al[2] from their original study of the field[2]. This included the following: Bleeding requiring ≥ 4 units of red cell transfusion within 3 d following the operation, acute renal failure (postoperative creatinine > 200 μmol/L), acute myocardial infarction, X-ray verified pneumonia, pulmonary embolism, stroke, sepsis and death. The data collectors within this group were cautious not to register preexisting diseases as postoperative complications. The findings were double-checked by two independent researchers to avoid over-registration of complications. The outcomes were omitted compared to Gawande’s original work due either to their rare occurrence in amputation surgery or lack of registration in the electronic charts. The study was approved by the local ethics committee and registered at the regional data protection agency (04.12.2012) (j. no. 01975 HVH-2012-053).
| 0 point | 1 point | 2 points | 3 points | 4 points | |
| Estimated blood loss, mL | > 1000 | 601-1000 | 101-600 | < 100 | - |
| Lowest mean arterial pressure, mmHg | < 40 | 40-54 | 55-69 | > 70 | - |
| Lowest heart rate, beats per min | > 85 | 76-85 | 66-75 | 56-65 | < 55 |
Continuous data are presented as median values with interquartile ranges (IQR) or mean values with standard deviations (SD). Comparison between TTA and TFA patients was performed using the t-test or the Wilcoxon rank sum test. Categorical data are presented as numbers with percentages. Comparison between TTA and TFA patients was performed using the chi-squared test or Fisher’s exact test. Logistic regression models analyzed the relationship between complications and SAS. Odds ratios (OR) were estimated both for each group level with SAS 9-10 as a reference level and as an average change between levels in the SAS groups to estimate a linear effect across the groups. Both models were analyzed for all patients as one group and were stratified for TFA and TTA procedures.
The discriminatory accuracy of the SAS was evaluated by ROC analysis. The results were expressed as area under the curve (AUC) and related 95%CI. Logistic regression for complications was made based on the high- and low-risk grouping to evaluate the level of increased risk based on the threshold. This was performed both for all patients as one group and on a stratified basis for the TFA and the TTA procedures. The fits of the logistic regression models were evaluated using the Hosmer-Lemeshow goodness-of-fit test. A p-value of 0.05 was considered statistically significant. All analyses were performed by a biostatistician working with R 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
One hundred seventy out of 228 consecutive patients who underwent LEA surgery during the two-year study period were included in the final analysis. In total, 58 patients were excluded due to re-amputation (n = 37) or missing data (n = 21). The preoperative characteristics are shown in Table 2 and the intraoperative characteristics in Table 3. The overall incidence of major complications and deaths is shown in Table 4 and in relation to the SAS groups in Table 5, which also includes the results from the regression model comparing the individual groups to the reference group. There was a significant linear association demonstrated between SAS group level [OR = 2.00 (1.33-3.03), P = 0.001] and complications or death, Table 5. This effect was statistically significant for the TFA group [OR = 2.61 (1.52-4.47), P < 0.001] but not for the TTA sub-group [OR = 1.12 (CI: 0.55-2.29), P = 0.76]. A significant increase in complications or deaths was observed in the high-risk group (SAS < 7) compared to the low-risk group (SAS ≥ 7) [OR = 2.80 (1.40-5.61), P = 0.004]. Corresponding results when the TFA and TTA groups were analyzed separately were [TFA: OR = 3.81 (1.5-9.42), P = 0.004], [TTA: OR = 1.69 (0.56-5.12), P = 0.35]. The results of the Hosmer-Lemeshow Goodness-of-fit test for the four models all had P-values close to 1, supporting the model assumptions. The results of ROC analysis are shown in Figure 1. The cut-off point of SAS ≥ 7 was found to be optimal when compared to all other cut-off points based on the specificity and sensitivity as evaluated by the Youden index. The AUC from the models were estimated as follows: All patients = 0.648 (0.562-0.733, P = 0.001), TFA = 0.710 (0.606-0.813, P < 0.001) and TTA =0.472 (0.383-0.672, P = 0.528).
| All patients (n = 170) | TTA patients (n = 70) | TFA patients (n = 100) | P-value | |
| Sex (women/men) | 74/96 | 26/44 | 48/52 | 0.21 |
| % | (44/56) | (37/63) | (48/52) | |
| Age, yrs | 74 (12) | 72 (12) | 76 (12) | 0.01 |
| Body mass index | 24.5 (6.5) | 24.9 (5.3) | 24.2 (7.2) | 0.47 |
| Cause for amputation | ||||
| Diabetes/arteriosclerosis/other | 58/105/7 | 30/36/4 | 28/69/3 | 0.03 |
| % | (34/62/4) | (43/51/6) | (28/69/3) | |
| ASA-groups, 1-2/3-4 | 25/142 | 16/54 | 9/88 | 0.03 |
| % | (15/84) | (23/77) | (9/88) | |
| Dementia | 20 (12) | 6 (9) | 14 (14) | 0.34 |
| Cardiovascular disease | 46 (27) | 22 (31) | 24 (24) | 0.30 |
| Pulmonary disease | 30 (18) | 12 (17) | 18 (18) | 1.00 |
| Cerebral apoplexy | 40 (24) | 11 (16) | 29 (29) | 0.07 |
| Kidney disease | 45 (26) | 17 (24) | 28 (28) | 0.72 |
| Diabetes mellitus | 72 (42) | 37 (53) | 35 (35) | 0.02 |
| Diagnosed with cancer | 14 (8) | 6 (9) | 8 (8) | 1.00 |
| Transfused preoperatively (patients) | 34 (20) | 10 (14) | 24 (24) | 0.17 |
| NSAID or acetylsalicylic acid (yes) | 82 (48) | 38 (54) | 44 (44) | 0.21 |
| Clopidogrel (yes) | 35 (21) | 13 (19) | 22 (22) | 0.70 |
| Preoperative hemoglobin, g/L (SD) | 108 (16.1) | 108 (16.1) | 106 (16.1) | 0.48 |
| Preoperative thrombocytes (SD) | 354 (151) | 366 (157) | 345 (146) | 0.46 |
| All patients (n = 170) | TTA patients (n = 70) | TFA patients (n = 100) | P-value | |
| Bilateral amputation procedure | 9 (5) | 4 (6) | 5 (5) | |
| Rank of surgeon, resident/consultant % | 116/54 | 45/25 | 71/29 | 0.40 |
| (68/32) | (64/36) | (71/29) | ||
| Duration of surgery, minutes (SD) | 81 (23) | 85 (26) | 79 (20) | 0.06 |
| Neuraxial/general anesthesia | 121/49 | 56/14 | 65/35 | 0.04 |
| % | (71/29) | (80/20) | (65/35) | |
| Vasopressor agents during surgery (yes) | 106 | 37 (52) | 69 (69) | 0.037 |
| Tourniquet use (yes) | 49 (29) | 35 (50) | 11 (11) | < 0.001 |
| Initial heart rate (beats/min) (SD) | 85 (16) | 84 (15) | 85 (17) | 0.77 |
| Initial blood pressure (mmHg) | Sys: 131 (21) | Sys: 131 (20) | Sys: 131 (21) | 0.83 |
| Dia: 73 (13) | Dia: 74 (13) | Dia: 73 (12) | 0.552 | |
| Lowest heart rate (beats/min) | 72 (SD: 1.2) | 70 (SD: 1.9) | 74 (SD: 1.5) | 0.13 |
| Lowest mean arterial pressure (mmHg) | 64 (SD: 0.9) | 65 (SD: 1.4) | 64 (SD: 1.1) | 0.32 |
| Estimated blood loss (mL), median (IQR) | 300 (IQR: 125-475) | 248 (IQR: 88-408) | 400 (IQR: 231-569) | 0.15 |
| Perioperative blood lactate (SD) | 1.2 (1.2) | 1.0 (0.5) | 1.3 (1.5) | 0.33 |
| Perioperative acid-base balance (SD) | 7.39 (0.01) | 7.41 (0.05) | 7.38 (0.09) | 0.05 |
| Total (n = 170) | TTA group (n = 70) | TFA group (n = 100) | |
| Death | 30 (18) | 9 (13) | 21 (21) |
| Bleeding requiring ≥ 4 units of RBC transfusion within 3 d following operation | 28 (16) | 7 (10) | 21 (21) |
| Sepsis | 12 (7) | 4 (6) | 8 (8) |
| Acute myocardial infarction/acute heart failure | 7 (4) | 0 (0) | 7 (7) |
| Acute renal failure | 6 (4) | 1 (1) | 5 (5) |
| Pneumonia | 18 (11) | 5 (7) | 13 (13) |
| Stroke | 1 (1) | 0 (0) | 1 (1) |
| Pulmonary embolism | 0 (0) | 0 (0) | 0 (0) |
| Score | SAS 9-10 | SAS 7-8 | SAS 5-6 | SAS 0-4 |
| All patients (%) | 5 (3) | 57 (34) | 70 (41) | 38 (22) |
| Complications and deaths within SAS group (%) | 1 (20) | 14 (25) | 29 (41) | 22 (58) |
| Odds Ratio between groups (reference SAS 9-10) | (ref.) | OR = 1.30 CI: 0.13-12.64 P = 0.82 | OR = 2.83 CI: 0.30-26.64 P = 0.36 | OR = 5.50 CI: 0.56-53.99 P = 0.143 |
| Linearity of the model, OR (95%CI), P-value | 2.00 (CI: 1.33-3.03) P = 0.001 | |||
| Transfemoral procedure, n = 100 (%) | 4 (4) | 31 (31) | 38 (38) | 27 (27) |
| Complications and deaths within SAS group (%) | 1 (25) | 8 (26) | 17 (45) | 20 (74) |
| Odds ratio between groups (reference SAS 9-10) | (ref.) | OR = 1.04 CI: 0.10-11.52 P = 0.97 | OR = 2.43 CI: 0.23-25.51 P = 0.46 | OR = 8.57 CI: 0.76-96.52 P = 0.08 |
| Linearity of the model, OR (95%CI), P-value | 2.61 (CI: 1.52-4.47) P < 0.001 | |||
| Transtibial procedure, n = 70 (%) | 1 (1) | 26 (37) | 32 (46) | 11 (16) |
| Complications and deaths within SAS group (%) | 0 (0) | 6 (23) | 12 (38) | 2 (18) |
| Odds Ratio between groups (reference SAS 7-8) | 1 | (ref.) | OR = 0.74 CI: 0.13-4.41 P = 0.74 | OR = 2.00 CI: 0.63-6.38 P = 0.24 |
| Linearity of the model, OR (95%CI), P-value | 1.12 (CI: 0.55-2.29) P = 0.76 | |||
This study presents new knowledge regarding the use of the SAS in dysvascular LEA surgery. There was a statistically significant increase in complications with a low SAS after LEA surgery. This is even more pronounced when the TFA sub-group is analyzed separately. Thus, for a TFA patient with a SAS < 7, the odds of a major complication or death are four times larger than for a patient with a SAS ≥ 7. ROC analysis confirms the discriminatory power of the SAS approach among the TFA patients. However, the SAS model proved to be of no prognostic value in the TTA group.
The population requiring LEA is old, fragile and has several co-morbidities, as clearly shown by this study. When compared to the cohort of general and vascular surgery patients[1,2,12], our average LEA patient is approximately 20 years older and 84% of patients have an ASA score classifications > 2 (possibly one of the highest reported in any orthopedic cohort), which compares to 34% in the study by Gawande et al[2]. The mortality rate in the present study is also high, with 18% of patients dead within the first 30 postoperative days. This clearly shows how vulnerable LEA patients are and how an easily applied risk stratification tool would be of great value for individualizing postoperative monitoring and care. Such a score was suggested in 2007[2,3] and the SAS has proved to be useful in several surgical specialties including gynecologic, urology and colorectal surgery[1,2,13,14].
However, only recently has the predictive value of the SAS on patients undergoing orthopedic surgery been evaluated[6,15]. Furthermore, only in spine surgery was the SAS model found to be of value[6]. Despite these findings, it was expected that the SAS could prove useful in the LEA cohort due to the similarities in demographics shared with patients undergoing general and vascular surgery[1]. To some extent, the results from this study did confirm our expectations, although it was surprising to see how poorly the score predicted outcome for the TTA patients. In the TFA group, however, approximately four times as many patients with complications were SAS high-risk patients compared to low-risk and the specificity as analyzed by the ROC model confirmed the difference. It is reasonable to conclude that the SAS model has discriminatory power on the TFA sub-group. The TFA group is significantly different from the TTA group in several important variables regarding the SAS model, which increases the risk of postoperative complications or death. For example, TFA patients are significantly older, more often classified as ASA 3-4, more frequently amputated due to severe arteriosclerosis, have procedures more frequently performed under general anesthesia and more frequently require the aid of vasopressor agents such as Ephedrine or Phenylephrine to secure a stable mean arterial pressure compared to TTA patients (Tables 1 and 2). These differences signal that the TTA group is in markedly better pre-operative condition and therefore less exposed to postoperative complications. Another important matter regarding the low specificity of TTA analysis could be superior hemostatic control in the TTA group where 50% had a pneumatic tourniquet applied during surgery. This lower intraoperative blood loss significantly[16] and potentially reduces the risk of intraoperative tachycardia[17]. Most acute LEA procedures are performed with a TFA approach[18], which to some extent is backed by the results from registration of the blood lactate and acid-base balance. Perioperative blood lactate is 0.3 higher in the TFA group with a markedly higher standard deviation pointing out several high outliers. Furthermore, the blood acid-base balance was found to be lower. In the event of an acute amputation, the operating staff would be challenged to maintain intact vital parameters during surgery. A larger drop in mean arterial pressure or a sudden rise in heart rate or intraoperative bleeding would affect the SAS and the outcome.
Limitations of this cohort study include those associated with its retrospective design and the missing data. Since there are many co-morbidities within this study, there is a risk that some post-operative complications could already have been present before amputation. However, the data collectors within this group have been highly aware of this matter. The study was performed on a unique group of patients often considered poor candidates for intensive care treatment with high post-operative morbidity and mortality. Randomized controlled trials and prospective studies can be a dubious task with this population, and we found the retrospective design sufficient to answer our research question of the study. The study provides some evidence of the value of the SAS in the post-operative treatment. However, further prospective studies examining the performance of the SAS seem warranted.
In conclusion, it seems warranted that the SAS approach provides the medical staff with information regarding the potential postoperative course after TFA surgery, especially when the patients are divided into high- and low-risk groups. The scoring system could prove useful in guiding preventive strategies such as optimizing intraoperative blood pressure or heart rate. The previously established threshold of SAS < 7 to define high-risk patients and SAS ≥ 7 to define low-risk patients was confirmed to be the optimal cut-point by ROC analysis within this study. The SAS showed no discriminatory power in the TTA sub-group, most likely due to optimized hemostatic control, fewer acute amputations and overall better condition compared to TFA patients.
There is an increasing number of high-risk elderly and severely comorbid patients scheduled for dysvascular lower extremity amputations. An easily applied risk stratification tool would be of great value for individualizing postoperative monitoring and care. The surgical apgar score (SAS, 0-10 points) has a strong correlation with the occurrence of major complications or death within 30-d following general and vascular surgery. However, a similar correlation has not been demonstrated in general orthopedic surgery. The primary aim of this study is to assess whether the SAS is a prognostic tool capable of identifying patients vulnerable to major complications (including death) following lower extremity amputations surgery.
The authors have reported the first series in the literature of patients with lower extremity amputation who were evaluated with the SAS. The scoring system could prove useful in guiding preventive strategies such as optimizing intraoperative blood pressure or heart rate.
When divided into four groups (SAS: 0-4, 5-6, 7-8 and 9-10), a logistic regression model shows a significant linear association between decreasing SAS and increasing postoperative complications in transfemoral amputation patients. This effect is even more pronounced when the patients were compared in high-risk and low-risk SAS groups.
The SAS should be considered during postoperative care of transfemoral amputation patients.
The paper is well-written and this observational study is informative for readers.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country of origin: Denmark
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lazzarini PA, Monteiro-Soares M, Turner AP S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Ohlsson H, Winsö O. Assessment of the Surgical Apgar Score in a Swedish setting. Acta Anaesthesiol Scand. 2011;55:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Gawande AA, Kwaan MR, Regenbogen SE, Lipsitz SA, Zinner MJ. An Apgar score for surgery. J Am Coll Surg. 2007;204:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 347] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 3. | Regenbogen SE, Lancaster RT, Lipsitz SR, Greenberg CC, Hutter MM, Gawande AA. Does the Surgical Apgar Score measure intraoperative performance? Ann Surg. 2008;248:320-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Haddow JB, Adwan H, Clark SE, Tayeh S, Antonowicz SS, Jayia P, Chicken DW, Wiggins T, Davenport R, Kaptanis S. Use of the surgical Apgar score to guide postoperative care. Ann R Coll Surg Engl. 2014;96:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Thorn CC, Chan M, Sinha N, Harrison RA. Utility of the Surgical Apgar Score in a district general hospital. World J Surg. 2012;36:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Urrutia J, Valdes M, Zamora T, Canessa V, Briceno J. Can the Surgical Apgar Score predict morbidity and mortality in general orthopaedic surgery? Int Orthop. 2012;36:2571-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the United States. South Med J. 2002;95:875-883. [PubMed] |
| 8. | Varma P, Stineman MG, Dillingham TR. Epidemiology of limb loss. Phys Med Rehabil Clin N Am. 2014;25:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 9. | Kristensen MT, Holm G, Kirketerp-Møller K, Krasheninnikoff M, Gebuhr P. Very low survival rates after non-traumatic lower limb amputation in a consecutive series: what to do? Interact Cardiovasc Thorac Surg. 2012;14:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Persson BM. Sagital incision for below-knee amputation in ischaemic gangrene. J Bone Joint Surg Br. 1974;56:110-114. |
| 11. | Ishii Y, Matsuda Y. Effect of tourniquet pressure on perioperative blood loss associated with cementless total knee arthroplasty: a prospective, randomized study. J Arthroplasty. 2005;20:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Regenbogen SE, Ehrenfeld JM, Lipsitz SR, Greenberg CC, Hutter MM, Gawande AA. Utility of the surgical apgar score: validation in 4119 patients. Arch Surg. 2009;144:30-36; discussion 37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Reynolds PQ, Sanders NW, Schildcrout JS, Mercaldo ND, St Jacques PJ. Expansion of the surgical Apgar score across all surgical subspecialties as a means to predict postoperative mortality. Anesthesiology. 2011;114:1305-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Haynes AB, Regenbogen SE, Weiser TG, Lipsitz SR, Dziekan G, Berry WR, Gawande AA. Surgical outcome measurement for a global patient population: validation of the Surgical Apgar Score in 8 countries. Surgery. 2011;149:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Wuerz TH, Regenbogen SE, Ehrenfeld JM, Malchau H, Rubash HE, Gawande AA, Kent DM. The Surgical Apgar Score in hip and knee arthroplasty. Clin Orthop Relat Res. 2011;469:1119-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Choksy SA, Lee Chong P, Smith C, Ireland M, Beard J. A randomised controlled trial of the use of a tourniquet to reduce blood loss during transtibial amputation for peripheral arterial disease. Eur J Vasc Endovasc Surg. 2006;31:646-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Hartmann B, Junger A, Röhrig R, Klasen J, Jost A, Benson M, Braun H, Fuchs C, Hempelmann G. Intra-operative tachycardia and peri-operative outcome. Langenbecks Arch Surg. 2003;388:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Aulivola B, Hile CN, Hamdan AD, Sheahan MG, Veraldi JR, Skillman JJ, Campbell DR, Scovell SD, LoGerfo FW, Pomposelli FB. Major lower extremity amputation: outcome of a modern series. Arch Surg. 2004;139:395-399; discussion 399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 324] [Article Influence: 15.4] [Reference Citation Analysis (0)] |