INTRODUCTION
From an anthropologic point of view, the upper extremity in humans has evolved into an instrument capable of achieving a large range of motion in order to perform highly complex tasks. This “open kinetic chain” demands different anatomic structures in comparison to the “closed kinetic chain” of the lower extremity.
Consequently, pathological conditions differ between the two extremities. Pathology of the lower extremity generally results in reduced mobility of the patient. In the upper extremity however, pathologies here restrict the patient from performing simple activities in daily life. In this situation, the problem cannot be managed by the help of external aids (e.g., wheelchairs or crutches)[1].
The elbow is a complex joint, consisting of three independent joints which cooperate together to move in multiple axes while maintaining a high level of stability[2]. The humero-ulnar joint permits a flexion/extension motion and is additionally stabilized by the olecranon and coronoid process in extreme flexion and extension. The combination of the proximal and distal radio-ulnar joint allows a pronation/supination movement, which is restricted by ligaments to a certain degree. The flexion of the elbow is important in allowing the hand to reach above and at the level of the head in order to achieve simple, yet important day-to-day activities, such as eating and the washing of hair and face. The combination of these movements, as well as shoulder rotations, allows versatile positioning of the hand in space and is a prerequisite for the fulfillment of complex tasks.
A decreased range of motion in the elbow joint can be directly due to pathology, i.e., primary osteoarthritis, or trauma. Pain, usually secondary to pathology such as rheumatoid arthritis, is another factor that may restrict elbow function as well. A total elbow arthroplasty (TEA) can improve the range of motion and can also relieve pain in selected cases. Therefore, TEA can considerably improve function of the upper limb and increase the quality of life.
Though the use of TEA has almost doubled between 1998 and 2011 in the United States, it is still a relatively uncommon orthopedic procedure. It is performed more often in women than in men[3] and is also used in relatively young patients[4,5]. The number of of TEA performed annually is 1.4 in 100000 of the population, considerably less than the 70 to 99 in 100000 of the population for total hip replacement[4,6].
The expanding practice of TEA leads to a new field in orthopedic surgery. We believe it is necessary to understand the history of the development of TEA in order to accomplish further improvements. In this review we will focus on the evolution of the elbow arthroplasty, from a historic overview to the present and address issues that could improve the clinical outcome in today’s practice.
THE PAST
The first salvage surgery by excising infected humeral and ulnar bone was performed by Ambroise Pare in the sixteenth century to prevent amputation due to an infected elbow joint[7]. In the nineteenth century, as more advanced surgical and post-operative care could be provided, creating a pseudoarthrosis by resecting the distal humerus became an option for incapacitating elbow disease. Following the developments in hip surgery, instead of resecting the joint, the idea of replacing the diseased elbow joint became a concept. It resulted in two streams; the anatomical arthroplasty, aimed to recreate native anatomical structures, and the functional arthroplasty, which covers the functionality of the elbow joint but does not resemble normal anatomical structures.
In 1925, the first attempt to replace an elbow joint by prosthetic materials was documented, when Robineau inserted an anatomically correct elbow prosthesis, consisting of metal and vulcanized rubber. In 1941, Boerema used a hinged non-anatomical prosthesis completely made of metal[7].
In 1952, Venable[8] published a case-report of a custom-made anatomical prosthesis after a comminutive fracture of the distal humerus which was not amendable for proper osteosynthesis. A short-term follow-up of 15 mo was reported with a good outcome[8].
The promising results of experimental elbow surgery led to a rush on patents for elbow arthroplasties by several inventive doctors. In 1954, a functional prosthetic elbow joint was patented by Prevo[9], but did not reach a widespread use due to frequent loosening. In 1972, Dee[10] reported his treatment of 12 patients using a functionally designed TEA. This publication initiated an increase in various TEA models in the 1970’s, ranging from stemmed devices to anatomy-resembling resurfacing models[9,11-17]. However, overall post-operative complication rates including; loosening, deep infection, and ulnar nerve neuropathy were high; ranging up to 57%[18].
It has been a challenge to design a TEA, which copies the native function and stability of all three articulations in the elbow joint. A drawback of anatomical arthroplasties was the lack of intrinsic stability. The anatomical, unlinked resemblance requires the integrity of ligaments and muscles. However, these structures often become insufficient in long-standing disease such as rheumatoid arthritis. Therefore, the unlinked anatomical design has lead to a high dislocation rate[19,20].
During flexion and extension of the elbow, some degrees of valgus and varus laxity is normal[21]. However, the linked “first generation” TEA’s did not offer this laxity, which resulted in frequent loosening due to stress at the implant-bone transition[18]. This problem was overcome by the “second generation” TEA, introducing sloppy hinges, which allow some varus-valgus laxity due to their semi-constrained design.
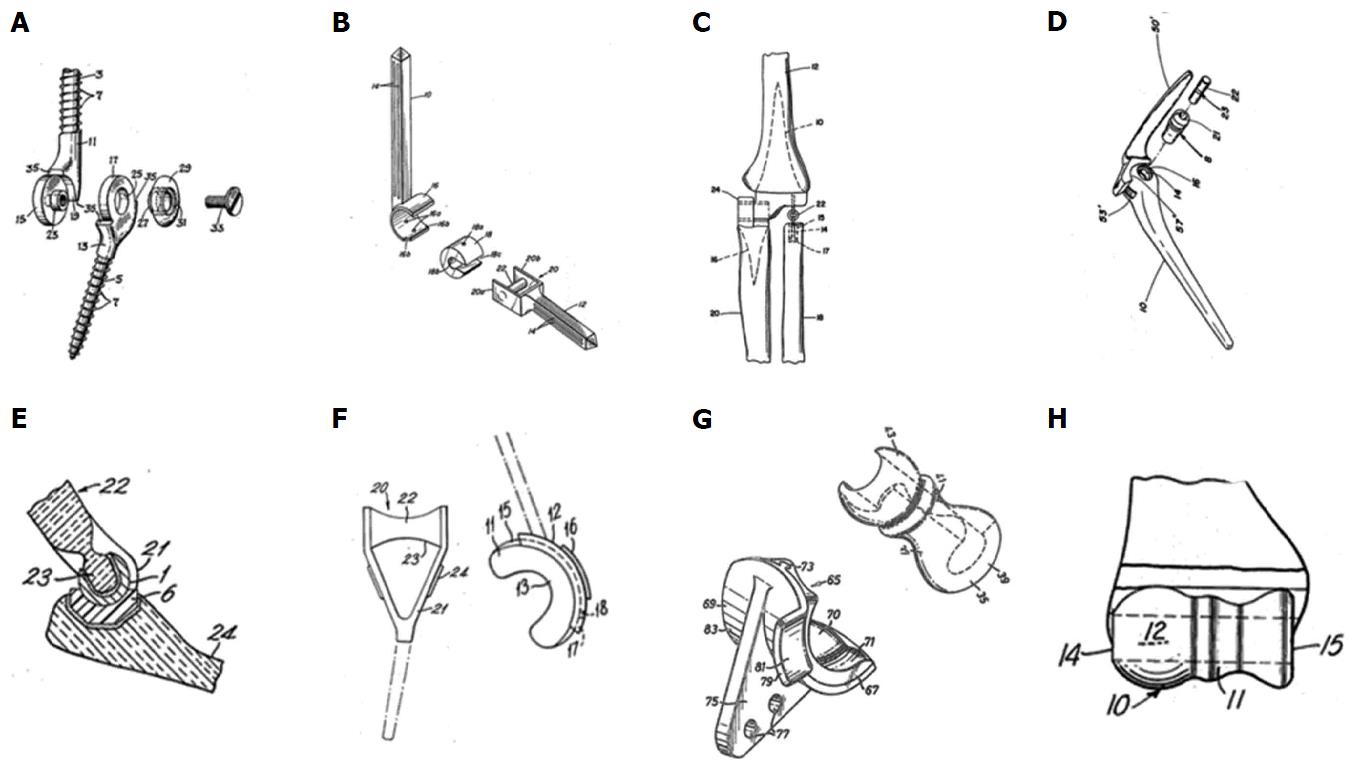
Fixation of the prosthesis proved to be challenging too, resulting in the application of a wide range of methods: Prevo[9] designed screw-threaded stems, Stevens a slide-on self-locking resurfacing arthroplasty, Schlein[11], Pritchard et al[13] and Dee[10] used smooth cemented stems, Roper et al[14] used a cemented humeral component and Amis and Miller[16] used screw fixation for the ulnar component[9,11,13-17]. Harmon[12] used two rings as a radiocapitellar joint. These models are presented in Figure 1.
Figure 1 Historical, distinctive types of total elbow arthroplasty.
A: Prevo[9] (1954); B: Schlein[11] (1974); C: Harmon[12] (1978); D: Pritchard[13] (1976); E: Roper[14] (1975); F: Dee[15] (1974); G: Amis[16] (1981); H: Stevens[17] (1970).
Beside improvements in materials and models, different operative techniques have arisen, each with their own advantages. In general, two approaches can be distinguished; the triceps sparing and non-triceps sparing approach. The non-triceps sparing approach, entails the triceps tendon to be split longitudinally or reflected from its insertion at the olecranon and at the end of surgery, needs to be repaired, yielding good results[22].
In the triceps sparing approach, a Chevron osteotomy of the olecranon is performed, distal to the triceps insertion, which is turned aside en-bloc with the triceps tendon attached. After insertion of the TEA this Chevron osteotomy is repaired. A study showed the triceps-sparing approach may result in better range of motion and a lower chance of infection compared to the triceps-detaching approach[23].
The human factor of gained experience on TEA surgery, together with improved materials, has led to positive results regarding clinical outcome and revision rates. Also larger trials and level 4 follow-up data coming from registries have enabled more thorough research on TEA, contributing to evidence-based patient care.
THE PRESENT
In today’s practice, the indications for elbow arthroplasty include all kinds of incapacitating elbow diseases, such as primary osteoarthritis, post-traumatic osteoarthritis, rheumatoid arthritis, comminutive elbow fractures, post-traumatic deformities and oncologic disease. However, unlike in hip and knee arthroplasty, the main indication is not primary osteoarthritis. In 1997, the main indication for TEA in the State of New York, United States, was rheumatoid arthritis. However, in 2006 a shift was seen to trauma as the main indication for TEA[5].
Today, both the linked sloppy hinged and unlinked TEA’s are available. Fixed hinge models are not used contemporarily. According to the patient’s pathology and surgeons’ preferred choice of the type of implant is often made pre-operatively. A “third generation” type of TEA is currently available, which allows the surgeon to decide during surgery to place a linked or unlinked implant.
Survival rates of different types of TEA have improved in the past four decades to around 90% after 5 years[24,25]. Cumulative revision rates after four to five years for fixed-hinge models is 13%, for sloppy hinge models 11%, and for unlinked models 13%[25]. In the short term, the main cause of failure is infection, while in the long term, the main cause is aseptic loosening by prosthetic wear[25,26]. When compared per group, the fixed-hinge models have a loosening rate of 11%, the sloppy hinged models 5% and the unlinked TEA’s 10%[25].
Deep, periprosthetic infection is a serious complication in arthroplasty surgery, since it requires aggressive treatment in order to preserve the implant without removing it, as well as other problems to patients. To counter the infection rate, the use of per-operative antibiotics has become standard and maximum aseptic measures are taken during surgery, such as double gloving and laminar flow[27]. Use of antibiotic-containing bone cement has lowered the deep infection rate to around 8%[4,28].
The use of bone cement might play a role in the aseptic loosening rate. A comparative study of cemented and uncemented ulnar components showed a lower rate of loosening in cemented components[29]. To avoid the use of bone cement and still achieve a firm bone-implant interface, several prosthetic coatings are available. These use the concept of bone ingrowth or osseointegration. The prosthesis is coated with hydroxyapatite, the molecular equivalent of bone. Human osteoclasts can dissolve the coating and attract osteoblasts to replace the coating with human bone[30]. A different concept is the ability to host osteoblasts in an optimal environment to enhance the intertwining of bone and implant. This can be accomplished with tantalum mesh or titanium beads[31-33].
To prevent metallosis, which might occur in metal-on-metal articulations, and to minimize shear stress between components, a plastic inlay is used. Depending on the type of arthroplasty, the inlay is either a polyethylene layer between unlinked components (iBP) or a bushing (Discovery, Coonrad-Morrey). These inlays are made of different materials, which aim to minimize wear of the prosthesis.
Wear debris can trigger “particle disease”, which in turn leads to arthroplasty component loosening and eventually failure[34]. Analysis of loosened TEA’s showed presence of wear debris (predominantly bone cement, polyethylene and metal) in surrounding tissue, due to wear of the polyethylene interface[35]. The inlay wear can be lowered by either crosslinking the polymers or adding substances, such as vitamin E[36,37]. However, no long-term follow-up results are published for elbow arthroplasty.
Patient-reported outcome scales nowadays have a more prominent role in assessing elbow function. Outcome measures have shifted from solely surgeon-opinion, to patient-oriented questionnaires, which focus on activities of daily life[38]. In a review on outcomes after TEA, the patient-reported outcomes were good or excellent in 78% of cases[25]. The function assessed by improvement of range of motion, was better in fixed-hinge models and sloppy hinged models (38 degrees and 35 degrees, resp.) than for unlinked models (20 degrees)[25].
THE FUTURE
Considering the present issues of aseptic loosening and infective complications of elbow arthroplasty, there is obvious room for improvement. Ongoing insights in elbow kinematics might guide implant designers in refining TEA, not only by design but also by choice of material[21]. The previously mentioned third generation TEA models might provide a good choice when a pre-operative decision on linked or unlinked TEA is not yet clear. Also, restoring the radiocapitellar joint by inserting a radial head prosthesis is possible.
Because of the increasing use of elbow arthroplasties, an inevitable problem occurs; revision arthroplasty. Because of good results, orthopedic surgeons may perform TEA’s with less difficulty in incapacitated patients than several decades ago. Besides, treatment of systemic diseases, such as rheumatoid arthritis has improved, with an overall increase in the quality of life, exposing TEA to a longer period of use. Results on TEA revision are promising; in a recent study revision led to pain relief and improved range of motion after failure of primary TEA[39].
The improved overall results might also question the need of post-operative functional restrictions, such as restricted lifting activities. These movements lead to shear distracting forces on the bone-implant junction and are therefore theoretical risk factors for implant loosening. In linked TEA types the pulling forces during lifting are transferred more to the humeral component than in unlinked TEA, since unlinked TEA requires ligaments and muscles to remain stable in this situation and is not connected to the ulnar component. However, no studies on post-operative rehabilitation are published, yet high-demanding patients show worse overall implant survival compared to low-demanding patients[40]. Therefore, research on post-operative management should be conducted to determine both mechanical factors influencing implant survival and optimal functional improvement.
Furthermore, several aspects on TEA research itself should be addressed. By setting up large implant registries, trends in the long-term can be studied. In Scotland, Sweden, Norway, the Netherlands and New Zealand, data on elbow arthroplasties are reported on a routine basis[4,40,41]. If this could be expanded to more countries, larger cohort studies with better follow-ups are possible[42]. Large registries also raise the possibility to assess practical questions, for example, a recommended minimum of annual cases to retain optimal surgical results. The Scottish and Finnish arthroplasty registers show that high-volume specialized centers yield better implant survival[4,40].
Use of pre-operative plain radiographs allows to plan implant size on beforehand, to optimize concordance between the pre-operative native elbow joint and the arthroplasty. Concerning the planning of the implant size, a radiograph-based planning tool is available, with good results in hip and knee arthroplasty. However, even though the intra-observer variability is good, the predictive value of this form of planning is insufficient[43]. A three-dimensional planning tool would possibly give more accurate information on TEA placement and sizing[44].
Another question is the use of three-dimensional guiding. Creating three-dimensional structures can be seen in two ways, creating the implant itself or re-creating the diseased elbow. Firstly, unlike Venable described in 1952, patient-specific implants could be made without preceding surgery, according to preoperative CT-scans. However, on a large scale, this might be too labor-intensive to plan and too expensive to fabricate. Therefore, patient-specific implants could be used in cases, where usual implants are not suitable.
Secondly, re-creating the diseased elbow could be of beneficial use in complex cases with severe deformation, e.g., the surgeon practicing on a model beforehand. This is already a method used in maxillofacial surgery[45]. In knee arthroplasty, patient-specific cutting guides, based on pre-operative MRI- or CT-scans, are available for difficult cases[46-49].
CONCLUSION
The knowledge on elbow arthroplasty has improved greatly in the past seven decades. With more encouraging results and a more widespread awareness, further improvements can be made. By setting up databases on implants, a structured analysis on adverse factors can be made to identify further improvable factors. Advances in materials and technical aids, such as three-dimensional printers, might improve postoperative outcomes.
ACKNOWLEDGMENTS
We would like to thank Robin L Peckitt for his review on English grammar.
P- Reviewer: Murata A, Smith GCS
S- Editor: Ji FF L- Editor: A E- Editor: Li D